Asthma guidelines allow antileukotriene medications to be used as an alternative to inhaled corticosteroids (ICSs) in second-step intensity therapy. The aim of this study was to determine whether asthma control can be maintained after reducing treatment from low-dose ICS to montelukast.
MethodsIn this prospective, real-life 12-week trial, 84 young patients with asthma (7–18 years) controlled by low-dose ICS, had treatment switched to montelukast. Symptoms and PEF were monitored daily; exhaled nitric oxide (eNO) and spirometry every four weeks; sputum eosinophil (sEo) and bronchial hyperreactivity (BHR) assessed at the beginning and at the end of the study. The primary endpoint was number of patients discontinued from the study due to asthma exacerbations.
ResultsEleven patients (13.1%) were discontinued due to asthma exacerbations. At the beginning, patients with elevated percentage of sEo had increased risk of exacerbations (relative risk RR, 6.6; 95% CI, 1.2–35.6), as well as those with augmented BHR (RR, 4.24; 95% CI, 1.1–16.2) as compared to patients who completed the study. An intensification of symptoms and increased use of beta-adrenergics were observed during the last visit before exclusion from the study, but not changes in spirometry, PEF, and eNO. No change in clinical parameters, inflammatory markers or BHR was observed in patients remaining in the study.
ConclusionsAfter treatment switch from low-dose ICS to montelukast, asthma control was maintained in the majority of patients during the 12-week observation period. Sputum eosinophilia or BHR before the treatment switch was exacerbation risk factor.
Mild asthma is the most common form of asthma in children. It is estimated to affect 50–70% of all asthma patients.1 In school-age children and adolescents, mild asthma is more symptomatic and less well-controlled than in adults.2 Clinical remission is quite often achieved in pubertal age, while reduction in allergic inflammation is less common.3 Despite the mild course of the disease, asthma exacerbations are relatively common and amount to 30–40% of asthma exacerbations requiring emergency consultation.2 Patients, especially those in adolescence, often want to discontinue chronic treatment1,4 and express concern regarding steroid side effects, which could reduce compliance.
GINA 2006 guidelines have allowed the possibility to use antileukotriene drugs as an alternative second-line treatment as part of second-step intensity asthma therapy.5 The PRACTALL consensus on the treatment of paediatric patients recommends leukotriene receptor antagonists (LTRAs) as an alternative first-line treatment equivalent to inhaled corticosteroids (ICSs).6 In both recommendations, the choice of the asthma controller medication pertains to patients who have hitherto not received any anti-inflammatory treatment.
However, few studies address the issue of step-down treatment in mild persistent asthma.7–9 Moreover, current asthma treatment recommendations contain only recommendations on step-down ICS treatment, not including LTRAs.5,6 The available literature lacks data on effects of step-down therapies from low-dose ICS to montelukast in paediatric populations, particularly including inflammatory markers analysis (i.e. sputum eosinophils, sEos).
The aim of our study was to determine whether asthma control could be maintained after replacing low-dose ICS with montelukast in a group of children and adolescents with well-controlled asthma.
Materials and methodsThe study was designed as prospective, real-life, single-site, observational trial, single-blinded for the study physician with regard to inflammatory markers and hyperreactivity. The study consisted of a 2-week run-in period with clinical observation during low-dose ICS treatment as used hitherto, and of a 12-week observation following a switch in the anti-inflammatory treatment from ICS to montelukast (main study).
Patients aged 7–18 years, meeting the eligibility criteria were included in the study. All the patients had been diagnosed with mild persistent asthma for at least two years, with the activity of their asthma within the previous two years confirmed by assessments of bronchial hyperreactivity (BHR) following hyperosmolar saline challenge after a four-week cessation of ICS treatment.10 Patients qualified for the study had good asthma control,5 maintained for at least six months by the lowest dose ICS (budesonide 100mcg once daily from Turbuhaler® – or equivalent), without any exacerbations requiring systemic corticosteroids within the last year, without respiratory tract infection within one month and with FEV1 values above 80% of predicted.
The study was approved by a Bioethics Committee, and parents/guardians of all patients and all patients over 15 years of age gave written informed consent before the study's commencement.
During the run-in period, patients completed diaries of diurnal and nocturnal symptoms, including the use of beta-adrenergics and medical interventions. PEF measurements (Personal Best, Philips Respironics) were recorded twice daily. Clinical assessment was based on a 6-point clinical scale (0–5, where 5 is the maximum symptom intensity).11
At the visit held at the end of the 2-week run-in period, asthma control was evaluated using GINA criteria. Next, determination of nitric oxide in the exhaled air (eNO; Hypair FeNO, Medi-Soft S.A., Belgium) and spirometry (Easy One, Medizin Technik, Switzerland) were performed. Then, BHR and sputum induction were simultaneously assessed by the combined method.12 The assessment included eight cycles of inhalations of 4.5% saline, lasting 0.5, 0.5, 1, 2, and four times four minutes respectively, with FEV1 measurements and attempts to cough up sputum one minute after each cycle. The assessment was completed after the last inhalation or earlier if FEV1 was reduced by more than 20%. Sputum was analysed according to the method described by Popov et al.13
After the above measurements, anti-inflammatory treatment with ICS was switched to montelukast 5mg (children under the age of 15) or 10mg (children over the age of 15).
During the 12-week observation period, patients continued daily completion of diaries (symptoms, beta-adrenergic use, PEF). Every four weeks, the study physician assessed the patient's asthma control (GINA 2006 criteria and ACT), eNO measurement and spirometry were also performed. Induced sputum and hyperreactivity assessments were repeated after 12 weeks of montelukast treatment. The eNO and sEos measurement results remained unknown to the clinician until the end of the observation.
The primary endpoint of the study was the number of patients discontinued from the study due to asthma deteriorations. Study participation was discontinued in cases of severe exacerbation or three mild exacerbations, and the patients received ICS. At the time of severe or third mild exacerbation patients were seen by a physician, to evaluate discontinuation criteria. Severe exacerbations were diagnosed by PEF drops of more than 30% below the baseline value on two consecutive days and/or by the need to use physician-prescribed systemic steroids (oral prednisone 1mg/kg for seven days) in addition to beta-adrenergics. Mild exacerbations were diagnosed by PEF drops of 20–30% below the baseline and/or by the need to use more than three additional beta-adrenergics inhalations per day (as compared with the run-in period) and/or nocturnal symptoms on two consecutive days.14 In such cases, rescue beta-adrenergics were administered, accompanied by ICS (budesonide 2× 200mcg for seven days) in the first two episodes.
Secondary endpoints were clinical symptoms, beta-adrenergic use, lung functions, BHR and inflammatory markers.
Based on the 2-week run-in period, median and interquartile ranges of baseline clinical, spirometric and inflammatory parameters, ACT scores and PEF (in % predicted) were calculated. The eNO cut-off point was established at 25ppb15 and abnormal sputum cytology was defined as an eosinophilic ratio of above 2.5%.16 Significant bronchial hyperreactivity was defined as dose–response slope values (DRS, the ratio of the percentage reduction in FEV1 and volume of saline administered in mL) of above 0.55%/mL.10
In patients who experienced exacerbations leading to study exclusion, baseline parameters were compared with those measured in patients who completed the study. Next, the values of the last measurements prior to exclusion from the study were compared to the baseline values.
In further analysis of the patients who completed the study, values measured in the 2-week run-in period were compared to the parameters measured within the 2-week periods preceding the visits in Weeks 4, 8 and 12 of montelukast treatment (i.e. in treatment Weeks 2–4, 6–8 and 10–12).
Comparisons between patients discontinued and continuing participation in the study were analysed using the Mann–Whitney test. Paired data were compared using the Wilcoxon signed ranked test and repeated measurements analysed using the Friedman test. Data were analysed using the STATISTICA package, version 5.5A (StatSoft Inc.). Group population distribution was compared using the chi-square (χ2) test.
ResultsThe study was started in December 2008 and completed in May 2009. Eighty-six mild asthma patients aged 7–17 years (median 14.0 (interquartile range, IQR 5.0)), were preliminarily qualified to the study. Only two (2.4%) patients had no atopy, while 77 (89.5%) patients were allergic to house dust mites. The group consisted of 63 (73.3%) boys and 23 (26.7%) girls. Disease duration was 3 to 16 years (9.0 (4.8)), while the duration of ICS treatment ranged from 1 to 13 years (5.5 (3.8)).
Two patients withdrew from the study. One patient was discontinued due to side effects of the treatment (insomnia and anxiety probably related to montelukast); another one was lost for unknown reasons. The following analysis includes the results of 84 patients in the per-protocol population.
There were no asthma exacerbations during the 2-week run-in period. Median values of FEV1 (in % predicted), FEV1/FVC and FEF25–75 (in % predicted) were 97.5 (13.0), 85.5 (13.0) and 87.0 (27.25), while PEF was 104.8 (21.0) of the predicted.
Median ACT was 25.0 (0.0). One patient (1.2%) was found to have an ACT value suggestive of poor asthma control (17 pts.). Median eNO level was 21.0 (15.0), while values above 25ppb were observed in 28 (33.0%) patients. Median DRS value was 0.27 (0.32)%/mL, with elevated values in 17 (20.2%) patients. Adequate sputum was achieved in 72 (85.7%) patients. Median sEos ratio was 1.0% (4.0%); values above 2.5% were observed in 26 (31.0%) patients.
During the 12-week study, five patients (6.0%) experienced severe exacerbations. Thirty-nine episodes of mild exacerbations were observed: six patients with three episodes (resulting in study discontinuation), two patients with two episodes, and 17 patients with one mild exacerbation episode. The median times to the 1st, 2nd, and 3rd mild exacerbation were 3 weeks, 6 weeks, and 11 weeks, respectively.
In effect, 11 patients (13.1% of the study population; seven boys and four girls) were discontinued from further montelukast treatment. One patient was discontinued within the first four weeks of the study, two patients were discontinued between Week 4 and Week 8, and eight patients after eight weeks of the study. Median time to severe exacerbation or to third mild exacerbation was 11.0 (2.0) weeks. The full, 12-week period of montelukast treatment including all scheduled evaluations was completed by 73 patients (86.9%).
Patients who discontinued the study due to exacerbations were found to have significantly shorter disease duration compared to the group of patients who completed the study (6.0 (3.0) vs. 9.0 (4.0), p=0.005).
Eight out of 11 discontinued patients had adequate sputum in the baseline assessment. They had significantly higher median percentage of eosinophils in the baseline assessment compared to patients continuing participation in the study (5.5% (IQR 5.0%) vs. 1.0 (IQR 3.1%), p=0.002). In this group, sEos were elevated in six (out of eight) patients, including all five patients with severe exacerbations, while in the completers group, elevated sEos were observed in 20 out of 64 patients (31.3%) in whom adequate sputum was achieved (p=0.04, χ2; RR, 6.60; 95% CI, 1.22–35.60).
In addition, the baseline DRS values were higher in the patients who discontinued due to exacerbations (0.38 (IQR 0.58) vs. 0.24 (IQR 0.30), p=0.024). Baseline DRS values were elevated (above 0.55) in five patients in this group, and in 12 patients who completed the study (p=0.026, χ2; RR, 4.24, 95% CI, 1.11–16.15).
There were no significant differences between groups in the baseline values of patient age [14.0 (5.0) and 12.0 (4.5), p=0.14], treatment duration [6.0 (4.0) and 5.0 (3.5), p=0.14] and of the clinical parameters (p values between 0.27 and 0.87), spirometry (i.e. p=0.64 for FEV1) and eNO (p=0.94).
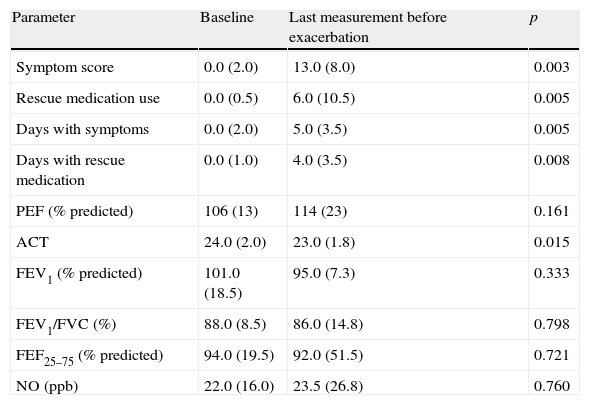
In the group of patients discontinued from the study, significantly higher intensity of symptoms and higher use of beta-adrenergics compared to the baseline were observed during the 2-week period preceding the exclusion. In addition, the median number of symptom days and rescue medication days were higher (Table 1). Five patients had elevated eNO levels in the last measurements prior to exacerbation, including all patients with severe exacerbations.
Baseline and pre-exacerbation values in 10 patients discontinued from the study due to exacerbations (one patient was discontinued prior to the Week 4 visit).
| Parameter | Baseline | Last measurement before exacerbation | p |
| Symptom score | 0.0 (2.0) | 13.0 (8.0) | 0.003 |
| Rescue medication use | 0.0 (0.5) | 6.0 (10.5) | 0.005 |
| Days with symptoms | 0.0 (2.0) | 5.0 (3.5) | 0.005 |
| Days with rescue medication | 0.0 (1.0) | 4.0 (3.5) | 0.008 |
| PEF (% predicted) | 106 (13) | 114 (23) | 0.161 |
| ACT | 24.0 (2.0) | 23.0 (1.8) | 0.015 |
| FEV1 (% predicted) | 101.0 (18.5) | 95.0 (7.3) | 0.333 |
| FEV1/FVC (%) | 88.0 (8.5) | 86.0 (14.8) | 0.798 |
| FEF25–75 (% predicted) | 94.0 (19.5) | 92.0 (51.5) | 0.721 |
| NO (ppb) | 22.0 (16.0) | 23.5 (26.8) | 0.760 |
Data are shown as median (interquartile range).
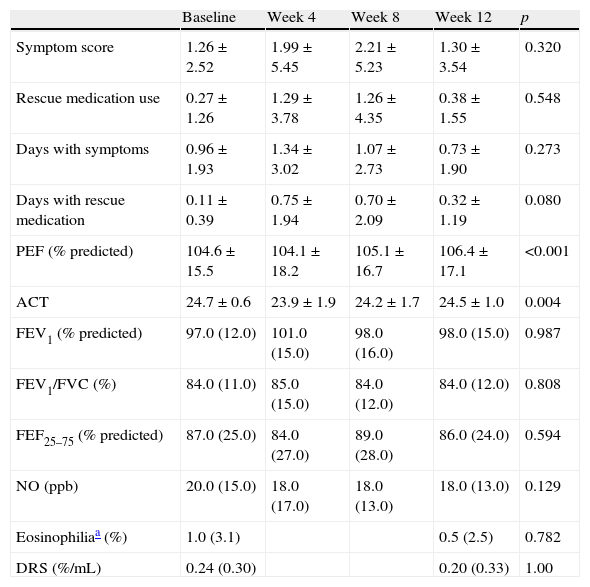
In patients who completed the 12-week period of treatment with montelukast, there were no statistically significant changes of the symptom score (p=0.32), asthma symptom days (p=0.27), rescue medication days (p=0.08) and beta-adrenergic use (p=0.55). The only significant changes in the PEF and ACT were observed, but they were small and clinically not important (Table 2). No statistically significant changes of the remaining outcomes, i.e. spirometric, inflammatory and hyperreactivity measurements were observed.
Average changes in asthma markers in completers population (n=73, Friedman test).
| Baseline | Week 4 | Week 8 | Week 12 | p | |
| Symptom score | 1.26±2.52 | 1.99±5.45 | 2.21±5.23 | 1.30±3.54 | 0.320 |
| Rescue medication use | 0.27±1.26 | 1.29±3.78 | 1.26±4.35 | 0.38±1.55 | 0.548 |
| Days with symptoms | 0.96±1.93 | 1.34±3.02 | 1.07±2.73 | 0.73±1.90 | 0.273 |
| Days with rescue medication | 0.11±0.39 | 0.75±1.94 | 0.70±2.09 | 0.32±1.19 | 0.080 |
| PEF (% predicted) | 104.6±15.5 | 104.1±18.2 | 105.1±16.7 | 106.4±17.1 | <0.001 |
| ACT | 24.7±0.6 | 23.9±1.9 | 24.2±1.7 | 24.5±1.0 | 0.004 |
| FEV1 (% predicted) | 97.0 (12.0) | 101.0 (15.0) | 98.0 (16.0) | 98.0 (15.0) | 0.987 |
| FEV1/FVC (%) | 84.0 (11.0) | 85.0 (15.0) | 84.0 (12.0) | 84.0 (12.0) | 0.808 |
| FEF25–75 (% predicted) | 87.0 (25.0) | 84.0 (27.0) | 89.0 (28.0) | 86.0 (24.0) | 0.594 |
| NO (ppb) | 20.0 (15.0) | 18.0 (17.0) | 18.0 (13.0) | 18.0 (13.0) | 0.129 |
| Eosinophiliaa (%) | 1.0 (3.1) | 0.5 (2.5) | 0.782 | ||
| DRS (%/mL) | 0.24 (0.30) | 0.20 (0.33) | 1.00 |
Data are shown as median (interquartile range) or as mean±SD.
As shown by a post hoc, week-by-week analysis conducted according to GINA 2006 criteria, good asthma control was achieved in individual weeks in 59.6–78.6% of patients receiving montelukast treatment for 12 weeks.
DiscussionIn the mild asthma patients, we have analysed the efficiency of asthma control after anti-inflammatory treatment step-down to montelukast. This was a real-life, prospective, single-blind study without a control group in patients managed by the same physician from the onset of the disease. To our knowledge, this is the first analysis exploring the clinical and inflammatory outcomes (particularly sputum eosinophilia) after step-down therapy switch from ICS to montelukast in schoolchildren and adolescents with mild persistent asthma. Patients with asthma controlled by the lowest ICS dose and without severe exacerbations within one year prior to the study start were enrolled. Within 12 weeks of montelukast treatment, exacerbations of asthma leading to study discontinuation were experienced by 13% of patients. This group consisted of patients with shorter disease duration, frequently with higher sEos or BHR, with lower baseline ACT values before the start of the treatment. Prior to exacerbations, an intensification of symptoms was observed, but no changes in lung function or eNO levels, suggesting higher sensitivity of clinical outcomes as predictors of impending asthma destabilisation. Among patients remaining in the study, the inflammatory markers did not rise at the end of the 12-week observation.
Despite good disease control with low ICS doses in the run-in period, 1/3 of patients had elevated sEos. This finding is consistent with earlier reports regarding subclinical eosinophilic inflammation in well-controlled asthma in children.16–18
Within 12 weeks of montelukast treatment, severe exacerbations requiring administration of prednisone were experienced by 6.0% of patients. The only reference available in literature is the LOCCS study of fluticasone to montelukast step-down treatment in mild persistent asthma.7 This study included 166 patients; children accounted for 16% of the study population and were not subject to separate analysis. Only 53% of the patients had received ICS in the past, while all the patients received a relatively higher controlling dose of inhaled corticosteroid (fluticasone 2× 100mcg) in the 4-week run-in period. Good control criteria were much more liberal – for example, up to 15 SABA doses per week were allowed. The number of severe exacerbations requiring prednisone treatment in the 16-week period of the LOCCS study was similar and amounted to 7.8% of patients. In other studies comparing 8-week montelukast treatment with fluticasone19 or placebo,20 the ratios of severe exacerbations in patients receiving LTRAs were 6.9% and 12.1%, respectively.
Overall, exacerbations of any severity were experienced by 30 patients (35.7%). This means that only 64.3% of patients treated with montelukast were free from disease exacerbations in the 12-week period. In the LOCCS study, 30.3% of patients treated with montelukast experienced severe or moderate exacerbations (“treatment failures”).7 The authors underlined that the risk associated with the step-down montelukast treatment was small (regardless of the significantly higher frequency of treatment failures) and the number of clinically significant exacerbations did not differ significantly between groups treated with montelukast and fluticasone.7
All the patients eligible for step-down anti-inflammatory treatment had stable disease within the preceding year, with good asthma control confirmed by detailed analysis in the run-in period. In particular, there were no differences in the clinical outcomes measured during the run-in period, in baseline lung function assessments and eNO levels between patients discontinued later due to disease exacerbations, and patients remaining in the study. This means that the baseline clinical outcomes, lung function assessments or eNO levels were not predictive of the risk of exacerbation before the treatment switch in the study population.
Subclinical eosinophilic inflammation before the treatment switch was a significant risk factor of exacerbation. It was observed in six out of eight patients discontinued from the study (in whom induced sputum was collected), including all patients with severe exacerbations. In the group of patients discontinued from the study, mean baseline sEos values were significantly higher. Elevated sEos increased the risk of exacerbation during the 12-week treatment more than sixfold, which was consistent with the previous studies.21 Similar significant differences were found between the groups in the assessment of non-specific BHR following hyperosmolar saline challenge. Bronchial hyperreactivity in the run-in period assessment increased the risk of exacerbations during the montelukast treatment period by a factor of four. Prior studies confirmed greater usefulness of indirect BHR assessment methods compared to direct challenge tests in monitoring of anti-inflammatory asthma treatment.22
We have observed a significant intensification of symptoms and use of rescue medication within two weeks preceding exacerbations that led to montelukast discontinuation. This is consistent with findings reported by Tattersfield23 and highlights the role of clinical symptom monitoring in assessing the actual asthma control. Contrary to the above-cited work, we observed no reduction in PEF values prior to exacerbation, which confirms the limited usefulness of this parameter in asthma patients.24
Despite the fact that half of the patients had elevated eNO levels in the last measurements prior to exacerbation (including all patients with severe exacerbations), the mean values were not significantly different from those measured in the run-in period. Although one might expect that frequent assessments of eNO would increase the usefulness of this index as a factor predictive of exacerbation, Dutch researchers have observed only a statistically insignificant trend towards reduction in the number of exacerbations, while monitoring the treatment by daily home-based monitoring of eNO levels.25 In addition, in the recent study by Cabral, eNO assessed every two weeks was not a clinically useful predictive factor of future exacerbations.26
The final analysis of clinical outcomes in patients who completed the 12-week study did not show significant worsening of the parameters. Although this might be related to the low patient number and the relatively short study period, these observations are in line with the results of the only study available in literature regarding step-down treatment from ICS to montelukast.7 According to Boushey, identification of change in clinical outcomes requires the monitoring of large patient populations, since symptoms are occasional in many patients with asthma of this severity.9 On the other hand, studies comparing montelukast with ICS in study periods similar to that of our study gave contradictory results: no clinical differences27 or significantly better clinical parameters in patients treated with fluticasone compared to montelukast.28
Lack of significant difference in lung function measurements in our 12-week montelukast study is, according to O’Byrne, typical of mild asthma, where the baseline parameters are usually normal and are not subject to significant changes in the course of the treatment.4 However, in a cross-over study in children with mild-to-moderate asthma, comparing 8-week treatments with montelukast and fluticasone, Zeiger and Szefler19,28 observed better improvements in functional assessments during fluticasone treatment compared to montelukast treatment.
Reports regarding PEF values are contradictory. In the study by Peters et al.,7 no PEF changes were observed in the course of a 16-week ICS to montelukast step-down treatment. However, Zeiger et al. observed lower improvement in PEF variability and morning PEF values in the montelukast group in a cross-over study comparing 8-week treatments with montelukast and fluticasone.19 This is indicative of the difficulties in finding sensitive indicators of asthma destabilisation.
In patients without exacerbations, no significant increases in sEos or eNO levels were observed after ICS to montelukast step-down treatment during the 12-week study. This may confirm the anti-inflammatory effect of montelukast, sufficient in most patients hitherto controlled on low-dose ICSs, although it is significantly less effective than that of 200–300mcg/day of beclomethasone, according to literature reports.29
Although elevated baseline DRS values were exacerbation risk factors, non-specific BHR also did not rise significantly in the 12-week montelukast treatment period. Few literature reports confirm the beneficial effect of LTRA on non-specific BHR assessed by indirect methods.30
The main limitation of our study was the lack of a control group, which could result in overestimation of the montelukast efficacy. Our study was conducted at a single site, without blinding of researchers and subjects, which limits the results generalisability. However, this was a real-world study and for this reason compliance with the study medication was not calculated.
Another limitation of the study was perhaps too short an observation period of 12 weeks. This could be confirmed by the growing number of patients excluded from the study due to exacerbations in consecutive 4-week periods between scheduled visits, although there were only two patients with two exacerbations at the end of the 12-week observation period (i.e. patients at highest risk of being discontinued from the study). In the TREXA study,31 49% of young patients, previously well-controlled on low dose ICS and included in placebo arm for 44 weeks, had exacerbations requiring oral corticosteroids. In our study there was no seasonal control for exacerbations, which could possibly have been related to viral infections or allergen exposures.
In summary, good control of asthma achieved on low-dose ICSs was maintained in the majority of patients following the switch to montelukast in the 12-week observation period. Exacerbations caused 13% of patients to be discontinued from the study. The factors that predicted exacerbations were bronchial hyperresponsiveness and presence of sputum eosinophils. Exacerbations were preceded by an intensification of clinical symptoms, without any drops in PEF or spirometry values. Exhaled nitric oxide levels did not have any predictive value. No rise in inflammatory markers or bronchial hyperreactivity was observed in the patients remaining in the study. The above observations warrant being investigated further in larger, longer-term studies.
The results of the study suggest that in young patients with well-controlled asthma it is advisable to determine hyperreactivity and sputum eosinophilia before switching the treatment from low-dose inhaled steroids to montelukast. The monitoring of clinical symptoms is important following the treatment reduction.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
FundingThis research project was conducted without any external financial assistance.
Conflict of interestH. Mazurek received honoraria from GSK, travel grant and honoraria from AstraZeneca. J. Ciółkowski received travel grant from UCB. B. Stasiowska reported no conflict of interest.
We thank all the patients and their families for their participation and support during the study; Celina Kabaj for assistance with the sputum induction and Łukasz Ciółkowski for technical assistance.