The role of osteopontin (OPN) has not been elucidated in childhood asthma.
ObjectiveOur purpose was to investigate whether OPN levels change due to allergic inflammation in pre-school and school-age children.
MethodsIn this prospective, cross-sectional study, 42 healthy children and a total of 51 children with asthma were recruited. OPN levels and its association with clinical and laboratory parameters were investigated in the study population. The asthma group were divided into two groups with respect to age, ≤5-years (n=23) and >5-years (n=28), and labelled Asthma Group 1 and Asthma Group 2, respectively. OPN levels were compared between subgroups.
ResultsSerum OPN levels were significantly higher in the asthma group when compared to the control group (p=0.004). OPN levels were similar in Asthma Group 1 and control groups, whereas it was found to be higher in Asthma Group 2 (p>0.025, p=0.001, respectively). In the >5-years age asthmatic group, OPN levels of the patients with allergic rhinitis (n=15) were higher than those of the patients (n=13) without allergic rhinitis (p=0.021).
ConclusionThe study underscores the relationship between childhood asthma and OPN as the first study in the literature. In this study we found that OPN, which plays a role in Th2 mediated inflammation, may also play a role in childhood asthma. The fact that OPN levels do not increase in preschool-age children with asthma might be due to the transient wheezing in this group.
Asthma is a chronic inflammatory disease of the airways characterised by Th2-mediated inflammation and bronchial hyperreactivity. It typically presents with wheezing and dyspnoea in children. In preschool age, wheezing and asthma are heterogeneous disorders with many phenotypic and variable expressions. Although almost half of the children are reported to have wheezing in the first six years of life, only 40% of these toddlers will experience persistent wheezing symptoms in later childhood.1 Asthma in children older than school age shows typical features of Th2-mediated inflammation. However, it is not the same for preschool children with asthma. The progression of clinical features of preschool and school-age children with asthma might not be the same due to possible pathophysiological differences between these two groups. However, there is not a definitive biomarker to identify children with high-risk phenotypes who will go on to have persistent asthma.
Osteopontin (OPN) is identified in many cell types in the immune system. It has been shown to be produced especially by T-cells, B-cells, macrophages, neutrophils, eosinophils, natural killer cells, CD11c-positive dendritic cells (DCs) and bronchial epithelium. OPN is a protein expressed during the inflammatory processes related to Th2 lymphocyte activity.2 It was demonstrated in previous studies that OPN plays role in asthma,3–7 allergic rhinitis,8 allergic conjunctivitis9 and response to venom immunotherapy.10 However, the role of OPN in paediatric asthma has not been studied.
In this study, we aimed to investigate whether OPN levels were affected by allergic inflammation in preschool and school-age children. The relationship between OPN and clinical and laboratory parameters along with inflammatory cytokines for pre-school and school-age children with asthma was examined.
Materials and methodsStudy subjectsThis prospective, cross-sectional study was conducted with 93 children, 51 of whom suffered from asthma and 42 of whom were healthy controls, presenting to the Fatih University paediatric allergy and well-child outpatient clinics between March 2011 and April 2012. The asthma group included children of ages ≤5 years, with at least four wheezing attacks during the previous year, and ages >5 years who were diagnosed clinically and functionally according to the GINA criteria (Asthma Group 1 and Asthma Group 2, respectively). Treatment of the children with asthma was arranged according to the updated GINA guidelines.11 Children of similar age and gender with no history of allergic disease or wheezing were selected as the control group. The control group was divided into two groups according to their ages, ≤5 and >5 years (Control Group 1 and Control Group 2, respectively). Those with chronic diseases (e.g. malnutrition, anatomic malformation of the respiratory system, chronic lung disease, heart disease, gastro-oesophageal reflux disease, cystic fibrosis) and those with a history of chronic drug use (e.g. antiepileptics, immune suppressives) were excluded from the study.
Venous blood samples were collected into Vacuette tubes (Greiner Bio-One, Monroe, NC, USA) and centrifuged at 3000g for 15min at 4°C. Serum samples were stored at −80°C for no more than six months. Levels of IL-4, IL-6 and IL-10 were measured by the EASIA (Enzyme Amplified Sensitivity Immunoassay) method using a ELX-800 system (DIAsource, Nivelles, Belgium). Levels of IL-13, IL-17, transforming growth factor beta (TGF-β) and osteopontin were measured by the ELISA (Enzyme Linked Immunosorbent Assay) method using a ELX-800 system (RayBiotech, Norcross, GA, USA). Levels of high sensitive C reactive protein (hsCRP) were measured by turbidimetric assay method using a Roche P 800 modular system (Hitachi, Tokyo, Japan). Levels of eosinophil cationic protein (ECP) were measured by the chemiluminescent assay method using an Immulite 2000 systems (Simens, Llanberis, Gwynedd, UK). The eosinophil counts were measured by LH-780 system (Beckman Coulter, Mervue, Galway, Ireland). Levels of total serum IgE were measured by the ECLIA (Electrochemiluminescence) method using an ELX-800 system.
Atopy in the asthma group was investigated using a skin prick test (SPT) and specific IgE (sIgE) measurements. A test was considered positive if the SPT results demonstrated a wheal with a mean diameter of at least 3mm greater than that of a saline control. Each child was tested with a core battery of allergens (e.g. dust mite, cockroach, cat, dog, mould, grass, tree, weed, milk, egg, and peanut) and a clinic-specific battery of locally relevant allergens (ALK Abelló, Hørsholm, Denmark). Spirometry (Vmax encore; VIASYS Healthcare Inc., Conshohocken, PA, USA) and bronchodilator reversibility were defined as greater than a 12% or 200ml change from baseline FEV1. These parameters were measured according to the GINA criteria.11
The study was initiated upon approval by the Local Ethics Committee of Fatih University in accordance with the Helsinki Declaration. The written informed consent of the parent(s) of each subject was also obtained before the study.
Statistical analysisData analysis was performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). Whether the continuous variables were normally distributed was determined by using Shapiro–Wilk test. Homogeneity of variance was evaluated by the Levene test. Data were shown as mean±SD or median (min–max), where applicable. When there were two independent groups, medians were compared using the Mann–Whitney U-test. Differences between the medians of more than two groups were evaluated by using the Kruskal–Wallis test. When the p-value from the Kruskal–Wallis tests were found to be statistically significant, Conover's non-parametric multiple comparison test was used to identify which group differed from the other. The mean ages between groups were compared using the t test. Nominal data were analysed using Pearson's Chi-Square or Fisher's exact tests, where appropriate. Degrees of association between continuous variables were calculated by the Spearman's correlation coefficient. A p value of less than 0.05 was considered statistically significant. For all possible multiple comparisons, the Bonferroni Correction was applied for controlling Type I error.
Power analysisIn ≤5-years age group, we planned to enrol 23 patients with asthma and 21 control subjects. The mean values and standard deviation of OPN were 6.16±1.52. We calculated power of OPN as 0.88, whereas in >5-years age group, 28 patients with asthma and 21 control subjects were enrolled with the mean values and standard deviation of OPN of 6.8±1.6. We calculated the power of OPN as 0.94 in the latter group. p values were accepted to be 0.025 in both groups.
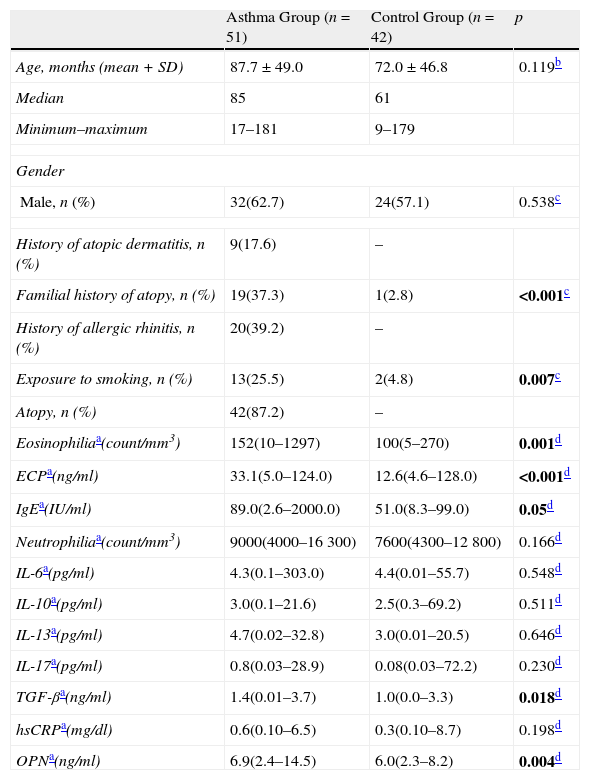
ResultsThe study was conducted with 93 children, 51 of whom suffered from asthma, with ages ranging between 17 and 181 months (mean age: 87.7±49.0 months, 32 males), and 42 of whom were healthy controls with ages ranging between 9 and 179 months (mean age: 72.0±46.8 months, 24 males). The demographic features and laboratory parameters of the population are presented in Table 1. Atopy was found to be 87.2% in the asthma group. When the two groups were compared, the eosinophil count, total serum IgE, ECP, and TGF-β levels were found to be higher in the asthma group, than those of the control group (p=0.001, p=0.05, p<0.001, and p=0.018, respectively). No difference was determined between serum interleukin levels (IL-6, IL-10, IL-13, and IL-17) of the two groups (p>0.05). Serum IL-4 levels of the two groups could not be measured due to technical problems in the laboratory. The hsCRP levels were checked to exclude a possible infection, and were found to be similar between the groups (p>0.05). The serum OPN level of the asthma group was determined to be statistically higher than that of the control group (p=0.004).
Demographic and laboratory characteristics of the study population.
| Asthma Group (n=51) | Control Group (n=42) | p | |
| Age, months (mean+SD) | 87.7±49.0 | 72.0±46.8 | 0.119b |
| Median | 85 | 61 | |
| Minimum–maximum | 17–181 | 9–179 | |
| Gender | |||
| Male, n (%) | 32(62.7) | 24(57.1) | 0.538c |
| History of atopic dermatitis, n (%) | 9(17.6) | – | |
| Familial history of atopy, n (%) | 19(37.3) | 1(2.8) | <0.001c |
| History of allergic rhinitis, n (%) | 20(39.2) | – | |
| Exposure to smoking, n (%) | 13(25.5) | 2(4.8) | 0.007c |
| Atopy, n (%) | 42(87.2) | – | |
| Eosinophiliaa(count/mm3) | 152(10–1297) | 100(5–270) | 0.001d |
| ECPa(ng/ml) | 33.1(5.0–124.0) | 12.6(4.6–128.0) | <0.001d |
| IgEa(IU/ml) | 89.0(2.6–2000.0) | 51.0(8.3–99.0) | 0.05d |
| Neutrophiliaa(count/mm3) | 9000(4000–16300) | 7600(4300–12800) | 0.166d |
| IL-6a(pg/ml) | 4.3(0.1–303.0) | 4.4(0.01–55.7) | 0.548d |
| IL-10a(pg/ml) | 3.0(0.1–21.6) | 2.5(0.3–69.2) | 0.511d |
| IL-13a(pg/ml) | 4.7(0.02–32.8) | 3.0(0.01–20.5) | 0.646d |
| IL-17a(pg/ml) | 0.8(0.03–28.9) | 0.08(0.03–72.2) | 0.230d |
| TGF-βa(ng/ml) | 1.4(0.01–3.7) | 1.0(0.0–3.3) | 0.018d |
| hsCRPa(mg/dl) | 0.6(0.10–6.5) | 0.3(0.10–8.7) | 0.198d |
| OPNa(ng/ml) | 6.9(2.4–14.5) | 6.0(2.3–8.2) | 0.004d |
ECP: eosinophil cationic protein; TGF-β: transforming growth factor beta; hsCRP: high sensitive C reactive protein; OPN: osteopontin.
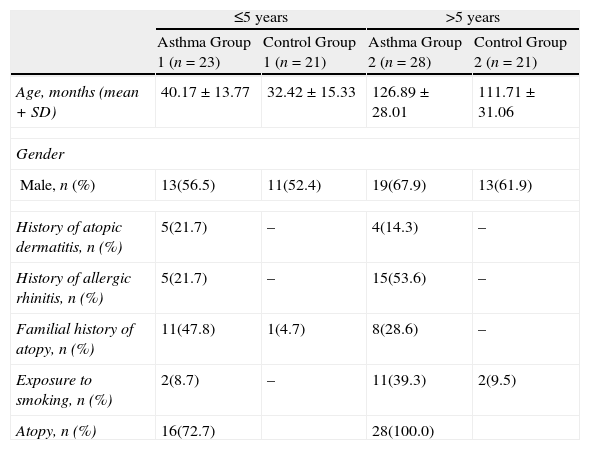
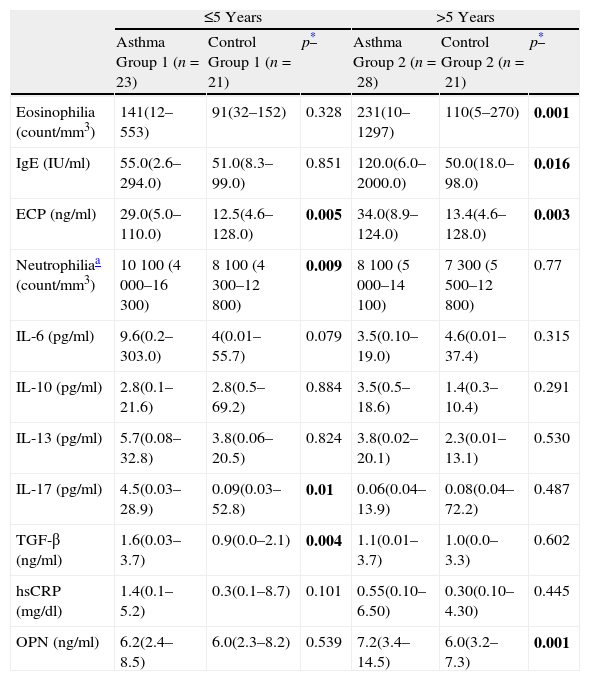
Patients were divided into two groups according to their ages: ≤5-years and >5-years. The demographic features of the four groups are presented in Table 2. When Asthma Group 1 was compared to Control Group 1; ECP, IL-17, TGF-β levels, and neutrophil count in the asthma group were higher (p=0.005, p=0.01, p=0.004, and p=0.009, respectively). Eosinophil count along with the IgE, IL-6, IL-10, IL-13 and hsCRP levels of the two groups were found to be similar (p>0.025).
Demographic characteristics of study population according to age groups.
| ≤5 years | >5 years | |||
| Asthma Group 1 (n=23) | Control Group 1 (n=21) | Asthma Group 2 (n=28) | Control Group 2 (n=21) | |
| Age, months (mean+SD) | 40.17±13.77 | 32.42±15.33 | 126.89±28.01 | 111.71±31.06 |
| Gender | ||||
| Male, n (%) | 13(56.5) | 11(52.4) | 19(67.9) | 13(61.9) |
| History of atopic dermatitis, n (%) | 5(21.7) | – | 4(14.3) | – |
| History of allergic rhinitis, n (%) | 5(21.7) | – | 15(53.6) | – |
| Familial history of atopy, n (%) | 11(47.8) | 1(4.7) | 8(28.6) | – |
| Exposure to smoking, n (%) | 2(8.7) | – | 11(39.3) | 2(9.5) |
| Atopy, n (%) | 16(72.7) | 28(100.0) | ||
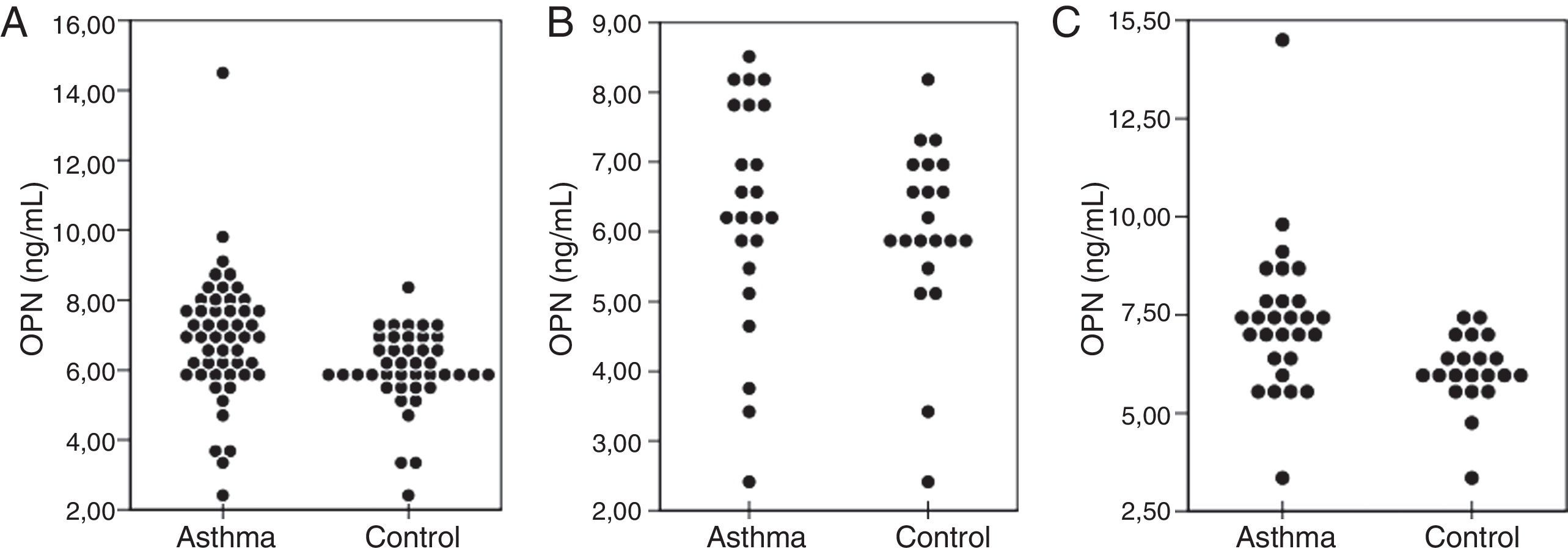
When Asthma Group 2 and Control Group 2 were compared; eosinophil count along with the ECP and IgE levels were higher in the asthma group (p=0.001, p=0.016, and p=0.003, respectively). The neutrophil count, interleukin levels (IL-6, IL-10, IL-13, and IL-17), TGF-β and hsCRP levels of both groups were found to be similar (p>0.025). When compared with the control groups, no difference was observed with respect to OPN levels in ≤5-years asthmatic patients, while the levels in >5-years age asthmatic patients were shown to be statistically higher (p=0.539 and p=0.001, respectively) (Table 3) (Fig. 1).
Laboratory characteristics of study population according to age groups.
| ≤5 Years | >5 Years | |||||
| Asthma Group 1 (n=23) | Control Group 1 (n=21) | p* | Asthma Group 2 (n=28) | Control Group 2 (n=21) | p* | |
| Eosinophilia (count/mm3) | 141(12–553) | 91(32–152) | 0.328 | 231(10–1297) | 110(5–270) | 0.001 |
| IgE (IU/ml) | 55.0(2.6–294.0) | 51.0(8.3–99.0) | 0.851 | 120.0(6.0–2000.0) | 50.0(18.0–98.0) | 0.016 |
| ECP (ng/ml) | 29.0(5.0–110.0) | 12.5(4.6–128.0) | 0.005 | 34.0(8.9–124.0) | 13.4(4.6–128.0) | 0.003 |
| Neutrophiliaa (count/mm3) | 10 100 (4 000–16 300) | 8 100 (4 300–12 800) | 0.009 | 8 100 (5 000–14 100) | 7 300 (5 500–12 800) | 0.77 |
| IL-6 (pg/ml) | 9.6(0.2–303.0) | 4(0.01–55.7) | 0.079 | 3.5(0.10–19.0) | 4.6(0.01–37.4) | 0.315 |
| IL-10 (pg/ml) | 2.8(0.1–21.6) | 2.8(0.5–69.2) | 0.884 | 3.5(0.5–18.6) | 1.4(0.3–10.4) | 0.291 |
| IL-13 (pg/ml) | 5.7(0.08–32.8) | 3.8(0.06–20.5) | 0.824 | 3.8(0.02–20.1) | 2.3(0.01–13.1) | 0.530 |
| IL-17 (pg/ml) | 4.5(0.03–28.9) | 0.09(0.03–52.8) | 0.01 | 0.06(0.04–13.9) | 0.08(0.04–72.2) | 0.487 |
| TGF-β (ng/ml) | 1.6(0.03–3.7) | 0.9(0.0–2.1) | 0.004 | 1.1(0.01–3.7) | 1.0(0.0–3.3) | 0.602 |
| hsCRP (mg/dl) | 1.4(0.1–5.2) | 0.3(0.1–8.7) | 0.101 | 0.55(0.10–6.50) | 0.30(0.10–4.30) | 0.445 |
| OPN (ng/ml) | 6.2(2.4–8.5) | 6.0(2.3–8.2) | 0.539 | 7.2(3.4–14.5) | 6.0(3.2–7.3) | 0.001 |
ECP: eosinophil cationic protein; TGF-β: transforming growth factor beta; hsCRP: high sensitive C reactive protein; OPN: osteopontin.
Comparison of serum OPN levels in the asthma and control groups. Overall, OPN levels in the asthma group were higher than that of the control group (p=0.004) (A). OPN levels of the children with asthma ≤5-years age were similar to the levels in the control group (p>0.025) (B). OPN levels of the children with asthma >5-years age were higher than those of the control group (p=0.001) (C). OPN=osteopontin.
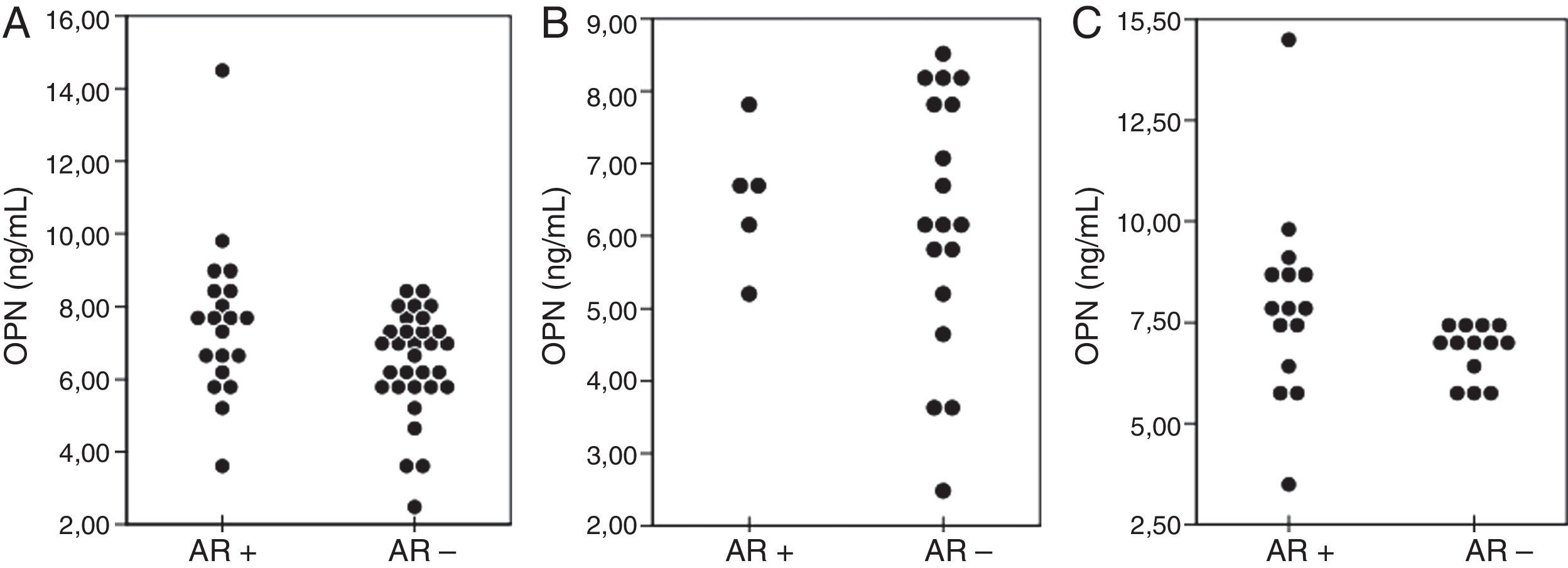
The asthmatic group was divided into two subgroups according to the presence of allergic rhinitis. OPN levels (ng/ml) of the cases who had allergic rhinitis (n=20) were higher than those of the patients without (n=31) allergic rhinitis (7.6±2.2; 6.4±1.4, respectively, p>0.025). When compared in terms of age, OPN levels in the ≤5-years age asthmatic group were similar in patients with (n=5) and without (n=18) allergic rhinitis (p>0.025). However, in the >5-years age group OPN levels (ng/ml) of the patients with allergic rhinitis (n=15) were higher than those of the patients (n=13) without allergic rhinitis (6.7±0.6; 7.9±2.4, respectively, p=0.021) (Fig. 2).
The comparison of OPN levels between the age groups according to the presence (AR+) or absence (AR−) of allergic rhinitis. Overall, AR+ asthmatic children had higher levels of OPN when compared with AR− children (p=0.04) (A). OPN levels of the children with asthma ≤5-years age who were AR+ were similar to the AR- group (p>0.025) (B). Children with asthma >5-years age who were AR+ had higher levels of OPN than those of the AR− group (p=0.021). OPN=osteopontin; AR=allergic rhinitis.
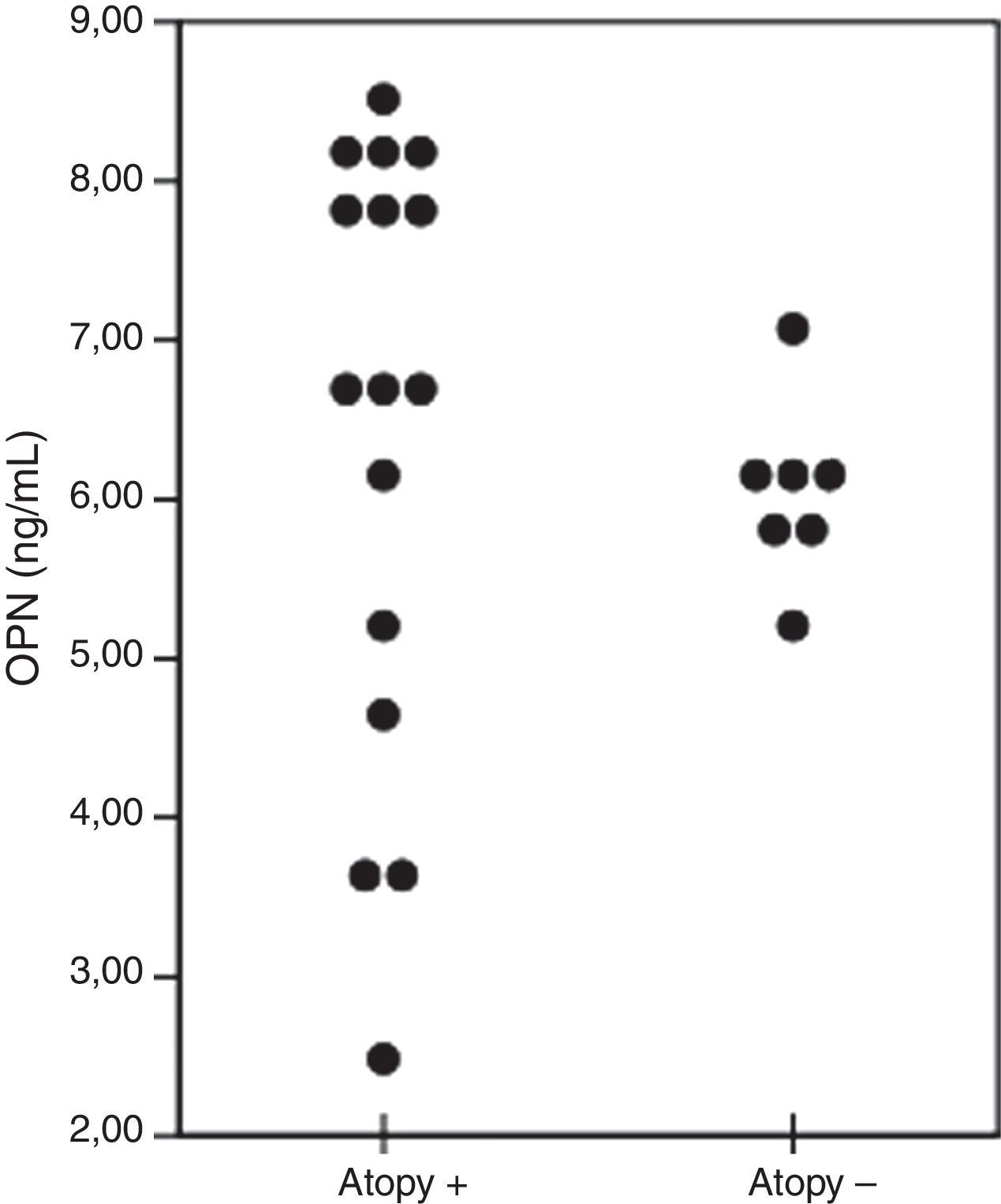
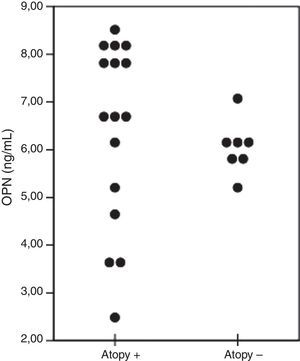
≤5-Years age asthmatic children were divided into two groups with regard to the presence of atopy. OPN levels (ng/ml) of the patients were 6.8 (2.4–8.5) and 5.9 (5.3–6.2) in the atopy positive (n=16) and atopy negative (n=7) groups, respectively (p=0.149) (Fig. 3).
>5-Years age asthmatic children were divided into two subgroups as ≤10 and >10 years of age, to investigate the effect of puberty on OPN levels. OPN levels (ng/ml) of the patients were 7.0 (5.7–9.8) and 7.3 (3.4–14.5) in the ≤10 years of age (n=10) and >10 years of age (n=18) groups, respectively (p=0.695). In addition, OPN levels were similar in both Control Group 1 and Control Group 2 (p>0.025).
No statistically significant correlation was observed between the OPN levels and IL-6, IL-10, IL-13, IL-17, TGF-β, serum IgE, ECP, and eosinophil count in both asthma groups. In addition, no statistically significant correlation was exhibited between asthma severity, exposure to second-hand smoke and the OPN level (data not shown).
DiscussionOPN is a protein expressed during the inflammatory processes related to Th2-mediated diseases including asthma. Xanthou et al.6 were the first to show the role of OPN in asthma by demonstrating the expression of OPN in the asthmatic lung and revealing the decreased levels of Th2 response due to recombinant OPN in a rat model. Studies have revealed that OPN is induced by the Th1 cytokine IFN-gamma, and that it is suppressed by IL-4 and IL-13, which are released by monocyte and monocyte-derived DCs.12,13 Kurokawa et al.14 showed that the ovalbumin (OVA)-induced specific IgE levels are higher in OPN-deficient mice when compared to those of the control group. In addition, OVA-specific IgE production decreased after recombinant intraperitoneal OPN application. All these studies support the hypothesis that OPN might be protective in asthma by decreasing the Th2 response.
On the contrary, recent studies show that the increased levels of OPN could increase the inflammatory reaction and play a role in remodelling in asthmatic patients. An asthmatic rat model revealed that decreased levels of OPN might prevent airway hyperresponsiveness and remodelling.15 Samitas et al.4 have demonstrated that the serum and bronchoalveolar lavage fluid (BALF) levels of OPN in adult asthma patients in the steady state were higher in comparison to the control group, however, during an asthma attack, the OPN levels decreased. In addition, OPN was found to be expressed in bronchial tissue cells (i.e. epithelium, smooth muscle cells, myofibroblast and T lymphocyte) and this expression is in correlation with the reticular basal membrane thickness. More recently, Liu et al.16 have demonstrated an increase in serum OPN levels in positive correlation with total nasal symptom score, total eosinophil count, serum ECP and IL-5 in patients with allergic rhinitis. OPN levels in patients with a comorbidity of asthma and allergic rhinitis were significantly higher than in patients with allergic rhinitis without asthma in the same study. In several other studies conducted in adult patients with asthma, OPN levels were found to be increased in serum, saliva and BALF.3–5,7,17 Studies conducted on asthmatic patients have shown that the OPN levels in saliva and BALF were high and in correlation with eosinophilia. In addition, it has been shown that OPN increases eosinophil chemotaxis and plays role in the migration of lymphocytes, monocytes and neutrophils to the inflammation region.5,18 Similarly, OPN levels in the lung tissue and BALF were found to be high in murine models with induced allergic inflammation.5,18,19 In fact, it was demonstrated that OPN levels are suppressed with corticosteroid treatment.20 In summary, OPN is related with eosinophilia, remodelling and basal membrane thickening of the lung tissue in severe asthmatic patients. OPN also has positive correlation with disease severity in asthmatic patients.3,4,16,17
Our study is the first in the literature that focuses on the relationship between childhood asthma and OPN. The results indicated that the OPN levels of children >5-years age who were diagnosed with asthma were higher than those of the control group. This finding correlates with similar studies carried out on adults.4,7,17 However, when a subgroup analysis was performed; OPN levels of patients aged ≤5 years were similar to those of the control group. When it is considered that OPN levels increase in Th2 mediated inflammatory diseases, the absence of this increase in patients aged ≤5 years can be explained by the presence of transient wheezing in this group.
Serum total IgE and eosinophil levels of the preschool-age group were not found to be elevated when compared with the control group owing to the presence of viral wheezing in preschool-age children. This finding is supported by the elevated levels of neutrophils in asthmatic children compared with the control group. OPN levels of the >5-years age asthmatic children who had allergic rhinitis were found to be higher than that of the children without allergic rhinitis and this finding was consistent with the previous report by Liu et al.16 Inconsistent with the findings of Hillas et al.3 there was no difference in terms of OPN levels between children who had exposure to second-hand smoke and children who did not have such exposure. This might be due to less exposure of children to passive smoke and milder chronic pulmonary changes at this period of life when compared with adult smokers. Levels of neutrophils, IL-17 and TGF-β could be found to be elevated, since the majority of children ≤5-years age had viral infection induced wheezing. Nevertheless, we think that elevated levels of ECP, IgE, OPN, and total eosinophil count are related with allergic inflammation in asthmatic children >5-years age. In addition, similar levels of serum interleukins in both the school age asthmatic and control groups can be explained with the relatively small number of cases in the subgroups and variability in the serum levels of interleukins. Although OPN levels were high in the atopic ≤5-years asthmatic children, this increase was not statistically significant. This would have been significant with more patients in the group. Since levels of OPN in Th2-mediated inflammation has a tendency to increase in ≤5-years age atopy negative asthmatic patients, asthmatic patients who had positive atopy and aged >5-years, might be significant in showing Th2-mediated inflammation in childhood asthma. In addition, when >5 years asthmatic patients were divided into groups as <10 years and ≥10 years, OPN levels were similar between the groups, which suggests that puberty does not affect OPN levels. Moreover, OPN levels were similar in both control groups, which suggests that OPN levels are not affected by age. Although previous studies revealed a correlation between OPN levels and clinical severity of asthma, we have not demonstrated such a correlation. The low numbers of patients in the subgroups could cause this when >5-years age asthmatic patients were divided into groups with regard to clinical asthma severity.
The main restrictions of our study were the low number of cases and that longer follow-up of the ≤5-years age group could not be carried out. Another limitation of this study was the investigation of interleukin and OPN levels only in serum, excluding BALF. OPN has been shown to increase in positive correlation with IL-5, IL-13, ECP and eosinophil count in previous studies.4,16 However, we did not find such correlation in asthmatic children which might be due to two reasons; firstly serum cytokine levels are variable and easily affected by many factors and secondly this might be due to the limited number of patients within this subgroup. We believe that it would better reflect the inflammatory response if the test was performed in BALF or saliva.
The studies have demonstrated that OPN may be regarded as an inflammatory marker, which increases in Th2-mediated inflammatory reactions. The elevated levels of OPN in children >5-years age in our study, are consistent with these findings. The similar results of OPN levels between the preschool-age asthmatic patients and the control group can be attributed to the transient wheezing episodes in the latter group. There is no available marker to predict if wheezing occurring in early childhood will persist in late childhood. In this context, OPN may be regarded as a predictor of persisting wheezing in preschool-aged wheezing children. To clarify this, the relationship between OPN and the development of persistent wheezing should be investigated in long-term follow-up studies.
ConclusionThis study reveals that OPN plays a role in Th2-mediated inflammation, however its role in childhood asthma is not yet completely known. This is the first study that demonstrates the role of OPN in childhood asthma. While an increase in OPN levels in preschool-age asthmatic children with heterogeneous wheezing has not been determined, an increase has been observed in school-age asthmatic children similar to that seen in asthmatic adults. We believe that OPN could be used as an important therapeutic target in school-age asthmatic children. Studies carried out on larger groups with long-term follow up are required in order to be able to determine the role of OPN in childhood asthma, to use it as a precursor of persistent wheezing in pre-school age children and to develop OPN guided treatment strategies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank Dr. Bulent Bozkurt, Dr. Ferit Kulali, and our senior nurse practitioner Asiye Tasci for their contributions.