Parthenolide is the active constituent of the plant ‘Tanacetum parthenium’ (Feverfew) which has been used for centuries as a folk remedy for inflammatory conditions.
Aim of the studyIn this study we aimed to investigate the effects of parthenolide in a murine model of chronic asthma.
Materials and methodsThirty-five BALB/c mice were divided into five groups; I (control), II (placebo), III (dexamethasone), IV (parthenolide) and V (dexamethasone and parthenolide combination). Lung histology was evaluated after treatment with the study drugs. Levels of interleukin (IL)-4 and IL-5 were determined by ELISA.
ResultsHistologic parameters except the number of mast and goblet cells improved in the parthenolide group when compared with placebo. All parameters except basal membrane thickness and number of mast cells were improved significantly better in the group receiving dexamethasone when compared with the parthenolide group. Improvement of most of the histologic parameters was similar in Groups III and V. Interleukin-4 levels were significantly reduced in the parthenolide group when compared to the placebo group.
ConclusionWe demonstrated that parthenolide administration alleviated some of the pathological changes in asthma. But parthenolide alone is not efficient as dexamethasone therapy and the parthenolide and dexamethasone combination also did not add any beneficial effect to the dexamethasone treatment.
Asthma is a chronic inflammatory disease of the airways characterised by airway hyperresponsiveness, airflow obstruction and remodelling. Airway inflammation is the principal factor in the pathogenesis of asthma in which numerous cell types and mediators released from these cells have been demonstrated to play significant roles. The transcription factor, nuclear factor κB (NF-κB) is known to be a critical modulator of inflammation in the pathogenesis of asthma. It is expressed in many cell types, which plays a key role in the expression of many pro-inflammatory genes, leading to synthesis of cytokines, adhesion molecules, chemokines, growth factors and enzymes.1 Nuclear factor κB pathway is known to be active in asthma. In previous studies it has been demonstrated that levels of NF-κB p65 and NF-κB p50 were increased in asthmatic patients, while in mice lacking NF-κB p50, diminished eosinophilic infiltration was observed in response to an aerolised allergen in a model of allergic asthma.2–5
Current strategies for the management of asthma focus mainly on suppressing airway inflammation, and steroids are the most effective anti-inflammatory drugs in asthma.6 Inhaled steroids are currently the mainstay of asthma therapy but they also have several systemic and local side effects when used at high doses for a long time. Research for the use of alternative and complementary treatments that reverse histopathological changes and have fewer side effects is continuously increasing.
Sesquiterpene lactones are the active constituents of medicinal plants from the Asteraceae family which have been used for centuries as a folk remedy in inflammatory conditions such as migraine, inflammation and arthritis.7,8 Parthenolide is one of the important sesquiterpene lactones found in plant Tanacetum Parthenium (feverfew) and the active constituent of this plant is responsible for its inflammatory effects.7,8 Previous studies suggest that anti-inflammatory effects of parthenolide are related to inhibition of NF-κB pathway. As this pathway is also known to be active in asthma pathogenesis, it may be speculated that parthenolide may be beneficial in the treatment of asthma. To our knowledge there is no study in the medical literature evaluating the efficacy of parthenolide on chronic airway changes of asthma. The aim of the present study was to investigate the effects of parthenolide on chronic changes of lung in a murine model of asthma for the first time.
Materials and methodsAnimalsConventionally raised, 6- to 8-week-old, 35 female BALB/c mice weighing 20–25g were used in the experiment. The animals were fed a commercial diet ad libitum and housed in an air-conditioned facility on a 12-h light/12-h dark cycle. The studies adhered to the National Institutes of Health guidelines for the experimental use of animals. The Local Animal Care and Use Committee granted approval for the study.
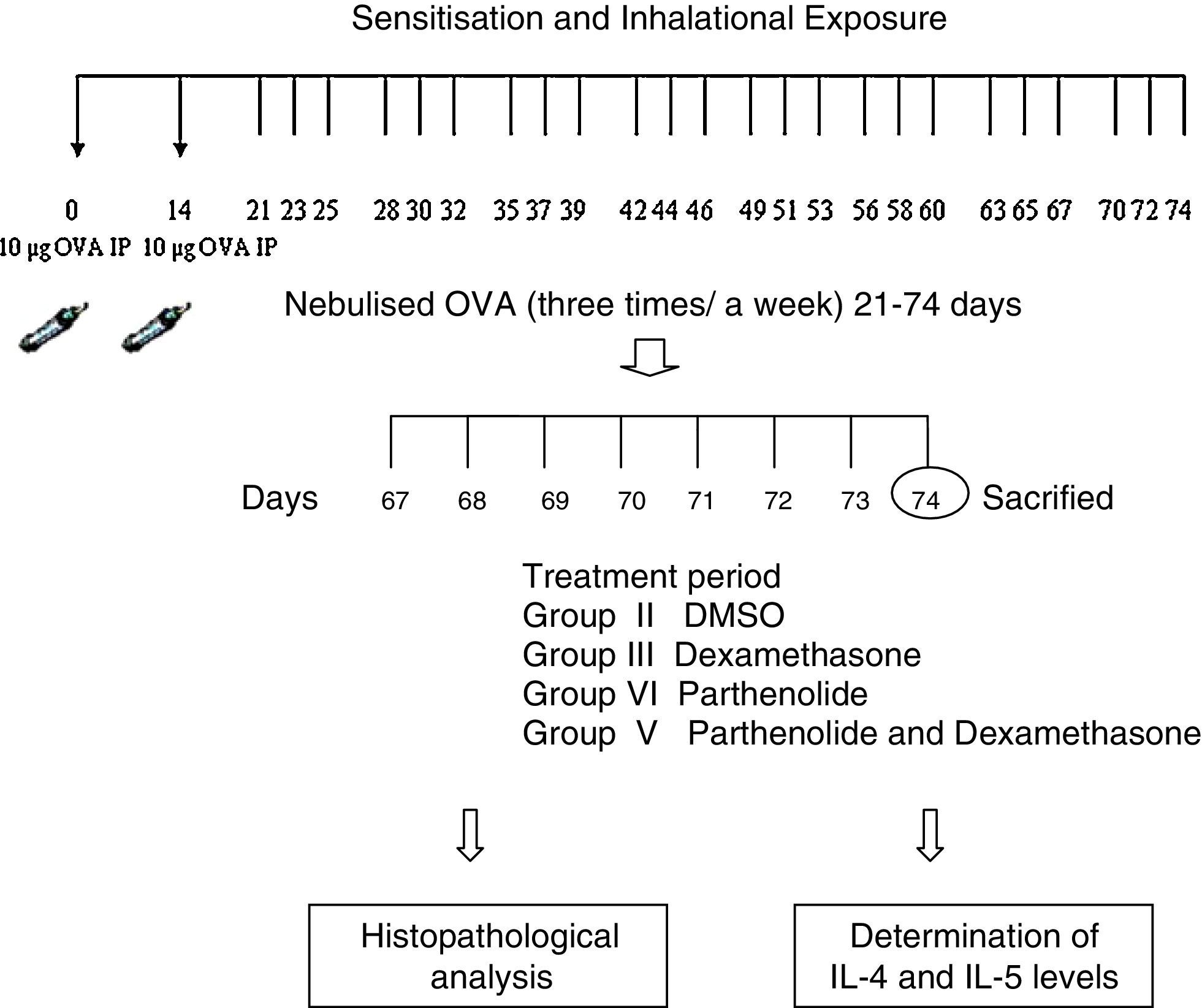
Experimental protocolMice were sensitised by an intraperitoneal (ip) injection of ovalbumin (10μg/0.1ml, two weeks apart, i.e. on day 0 and day 14) consisting of a chicken egg albumin (ovalbumin, grade V, ≥98 pure, Sigma, St. Louis, MO, USA) with alum adjuvant as described by Temelkovski et al.9. Mice in study groups II, III, IV, V and VI were then challenged with an aerosol of 5ml 2.5% ovalbumin (OVA) in saline for 30min per day on three days of the week for eight weeks beginning from the 21st day of the study. The mice in the control group (group I) received normal saline with alum intraperitoneally on days 0 and 14 of the experiment and aerosolised saline without alum for 30min per day on three days of the week for eight weeks beginning from the 21st day of the study. Exposures were carried out in a whole body inhalation exposure system in a plexiglass chamber with 40×60×120 diameters designed for placement of cages. Temperature and relative humidity were maintained at 20–25°C and 40–60%, respectively. A solution of 2.5% ovalbumin in normal saline was aerosolised by delivery of compressed air to a sidestream jet nebuliser with a flow rate of 6L/min (Medicair, UK) and injected into a chamber. The aerosol generated by this nebuliser comprised >80% particles with a diameter of <4μm. Particle concentration was maintained in the range of 10–20mg/mm3 in the chamber.10
Study drugsMice in group III received dexamethasone 1mg/kg;11 group IV received parthenolide 3μg/g;12 group V received both dexamethasone 1mg/kg and parthenolide 3μg/g; and group II received DMSO (solvent of parthenolide) 50μL13 once a day in the last seven days of the challenge period. Parthenolide was obtained from Calbiochem (Merck Millipore, Darmstadt, Germany). Animals were sacrificed by an overdose of ketamin 24h after the last drug administration. Experimental protocol and study drugs are presented in Table 1.
Preparations of lung homogenatesAnimals were sacrificed by an overdose of ketamin 24h after the last drug administration. Lungs were removed and washed with cold PBS three times in order to remove blood. Lung tissues were taken into 2ml microcentrifuge tubes and stored at −80°C until analysis. On the study day, frozen lungs were thawed, weighed (60–80mg), transferred into different tubes on ice containing 5ml of stainless beads, 0.1% SDS, protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA) and 0.1mg/ml phenylmethanesulfonyl fluoride (PMSF) in PBS. Microcentrifuge tubes were transferred to precooled Tissuelyser LT racks and placed into TissueLyser (Qiagen, Germany) homogenisator. Frequency and time were set to 50 and 5min, respectively. The homogenates were then centrifugated at 15,000×g for 1h at 4°C. The supernatants were stored at −80°C.
Measurement of cytokinesLevels of IL-4 and IL-5 were quantified in the supernatants of the lung tissue by standard ELISA protocols using commercial mouse IL-4 and IL-5 (Bender MedSystems, MedSystems Diagnostics GmbH, Vienna, Australia). Detection levels were 2.0pg/ml for IL-4 and 3.3pg/ml for IL-5.
Histologic evaluationExaminations were conducted blinded by two of the investigators. Tissue specimens were obtained from the mid zone of the left lung of mice. Samples were fixed in 10% formalin for light microscopic evaluation. Some tissue samples of 1–2mm3 taken from adjacent regions were stocked in 2% gluteraldehyde for electron microscopic evaluation. After fixation, samples were embedded in paraffin for light microscopic evaluation. Paraffin blocks were placed in Leica RM2125 rotary microtome (Germany) and serial sections of 5μm thickness were obtained. After choosing the first section randomly, 10 sections in each mouse were selected by skipping over 10 sections and proceeded to the staining process. For light microscopic evaluation, four different staining processes were used. The first 10 samples were stained with haematoxylin and eosin (H&E). In these samples general tissue features were examined and thicknesses of epithelium and subepithelial smooth muscle layers of the medium and small airways were measured. In order to evaluate the thicknesses of epithelium and subepithelial smooth muscle layers, measurements were performed from four points of each airway at levels of 3, 6, 9, and 12 O’clock. Considering that each section contained two to three airways, nearly 20 or more airways were evaluated in each mouse.
Photomicrographs were taken by Olympus DP25 (Japan) camera, which was adapted on Olympus CX-41 model microscope (Olympus Optical, Tokyo, Japan). The histological analysis was carried out with a computer-assisted image analyser system (DP2-BSW).
The consecutive 10 sections were stained with Toluidine Blue and the other 10 sections with periodic acid–Shiff (PAS). The last sections were stained with Masson Trichrome stain for demonstration of collagen deposition. Photomicrographs were taken randomly from five fields of each section which were stained with Toluidine Blue. For mast cell enumeration, a standard transparent counting frame representing an area of 20,000μm2 was used manually and 10 fields in each photograph were examined for each mouse. Goblet cells stained with PAS were enumerated in 10 sections of each mouse. In each section, three to five randomly selected airways were photographed. Circumferences of all airways were measured and goblet cell numbers in these areas were recorded. For standardisation, goblet cell numbers in 100μm were analysed by division of total goblet cell number to the total length of airway circumferences and multiplying the result by 100.
For ultrastructural investigations, the tissues were postfixed with osmium tetroxide (OsO4), dehydrated in a graded series of alcohol, and then embedded in Araldite® CY212. The thin (60–90nm) sections were obtained with ultra-microtome (Leica) and stained with uranyl acetate and lead citrate, examined on a transmission electron microscope (Carl Zeiss Libra 120 EFTEM, Germany), and digitally photographed. In each mouse, five to seven ultrathin sections were taken from each two blocks and epithelium of the airway, the surrounding structures, and the intercellular connections were evaluated. Thicknesses of the basement membrane of the respiratory epithelium were measured from 20 points that were at equal distances to each other and the data were recorded.
Statistical analysisSPSS 15.0 package programme was used in the statistical analysis. Data were presented as mean±standard deviation (SD) in each group. All data were analysed by Kruskal–Wallis and then Mann–Whitney test for dual comparisons. p<0.05 was considered statistically significant.
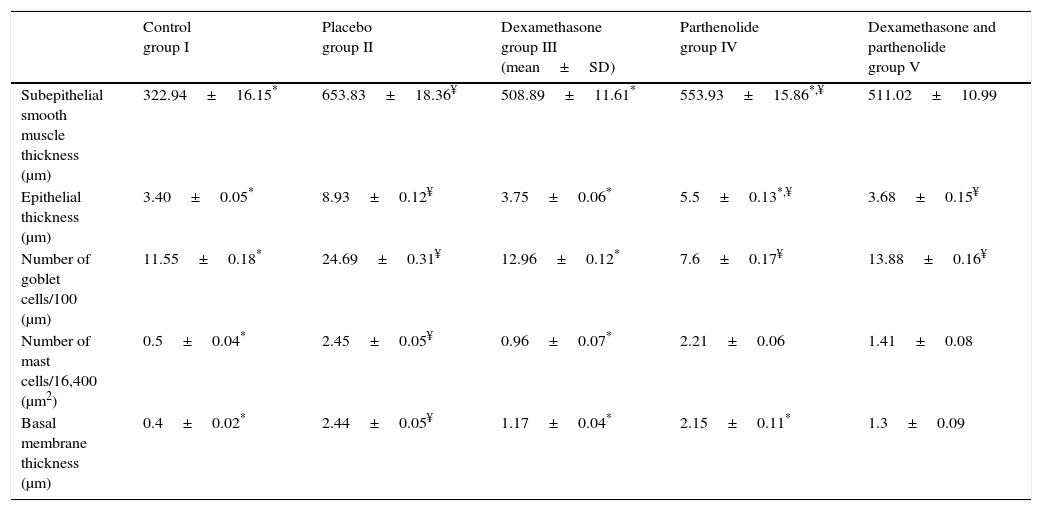
ResultsEach group consisted of seven mice at the beginning of the experiment and all of the mice completed the study. We compared the control group with the placebo in order to show that the model of asthma was established. In the placebo group, all histologic parameters, namely thickness of basal membrane, epithelium and subepithelial smooth muscle layer and the number of mast cells and goblet cells were significantly higher than the control group (Table 2 and Fig. 1). These results showed that the model of chronic asthma was established.
Histological comparison of the study groups.
| Control group I | Placebo group II | Dexamethasone group III (mean±SD) | Parthenolide group IV | Dexamethasone and parthenolide group V | |
|---|---|---|---|---|---|
| Subepithelial smooth muscle thickness (μm) | 322.94±16.15* | 653.83±18.36¥ | 508.89±11.61* | 553.93±15.86*,¥ | 511.02±10.99 |
| Epithelial thickness (μm) | 3.40±0.05* | 8.93±0.12¥ | 3.75±0.06* | 5.5±0.13*,¥ | 3.68±0.15¥ |
| Number of goblet cells/100 (μm) | 11.55±0.18* | 24.69±0.31¥ | 12.96±0.12* | 7.6±0.17¥ | 13.88±0.16¥ |
| Number of mast cells/16,400 (μm2) | 0.5±0.04* | 2.45±0.05¥ | 0.96±0.07* | 2.21±0.06 | 1.41±0.08 |
| Basal membrane thickness (μm) | 0.4±0.02* | 2.44±0.05¥ | 1.17±0.04* | 2.15±0.11* | 1.3±0.09 |
n:7 in each group.
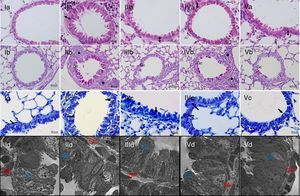
Light and electron microscopic findings of study groups. I; control (n:7), II; placebo (n:7), III; dexamethasone (n:7), IV; parthenolide (n:7), V; parthenolide and dexamethasone (n:7), A; H&E, B; PAS, C; Toluidine staining. (A) Arrow with two heads; sub-epithelial smooth muscle thickening, * peribronchial mononuclear cell infiltration. (B) arrows; goblet cells (C) arrows; mast cells. (D) Electron microscopic views. Increased goblet cells in placebo group (Gc), smooth muscle thickening (Sm).
In Group III (Dexamethasone) all histologic parameters were improved when compared with placebo (p<0.05, Table 2). In Group IV (Parthenolide), basal membrane thickness, subepithelial smooth muscle thickness and epithelial thickness were significantly lower when compared to placebo group (p<0.05) while the number of mast and goblet cells were similar with the placebo group (p<0.05). When histological parameters of Group IV were compared with Group III, dexamethasone seems to be superior in lowering subepithelial smooth muscle thickness, epithelial thickness and the number of goblet cells (p<0.05) but the effect of two drugs was similar in basal membrane thickness and the number of mast cells (Table 2).
In Group V in which the study drugs were parthenolide and dexamethasone combination, all histologic parameters were significantly better compared to placebo and improvement was similar to dexamethasone in all parameters except epithelial thickness and the number of goblet cells (Table 1 and Fig. 1). This showed that this combination did not add any improvement on administration of dexamethasone alone.
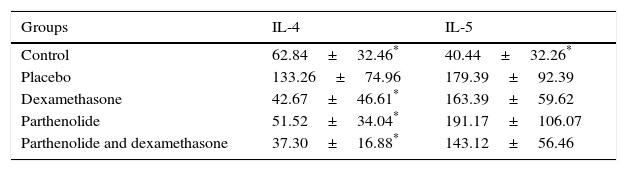
Interleukin 4 and 5 levels were significantly higher in placebo group compared to control group, as expected. Only significant decreases of IL-4 levels were detected in study groups when compared with placebo. Interleukin-5 levels of the groups III, IV and V were all similar with the placebo group (Table 3).
DiscussionParthenolide, a naturally occurring molecule derived from feverfew (T. Parthenium) exhibits its anti-inflammatory properties by inhibition of NF-κB and resulting decreased signal induction of pro-inflammatory cytokines.14–16 Anti-inflammatory effects of parthenolide are also mediated by inhibition of prostoglandin synthesis.17–19 Although parthenolide has been used for asthma therapy for many centuries, there is no study investigating the role of parthenolide in asthma therapy. We firstly demonstrated that parthenolide was partially effective in reversing abnormal histological findings of asthma in a murine model of asthma.
Nuclear factor-κB which is the target of parthenolide, has a critical role in the pathogenesis of lung diseases.20 Nuclear factor-κB activation has been shown to be robust and prolonged in airway epithelium of asthmatics.21 Activation of NF-κB in airways of allergen sensitised mice also resulted in enhancement of the inflammation and airway hyperesponsiveness22. On the contrary, it has been shown that mucus secretion, Th2 cytokines and cellular infiltration were diminished when NF-κB activation was inhibited in a murine model of asthma.23 In another study with OVA-sensitised mice having Clara cell specific IKK-β deletions, peribronchial fibrosis, airway eosinophilia, eotaxin and TARC chemokine were diminished when compared with mice having functional IKK-β.24 All these studies highlighted the importance of NF-κB pathway in asthma and paved the way for studies about therapeutics inhibiting NF-κB pathway and effects of existing therapeutics on NF-κB pathway.25 Decoy oligonucleotides and small molecules inhibiting NF-κB were studied in animal models of asthma and it is concluded that inhibition of NF-κB resulted in a decrease in inflammatory cytokines, mucus secretion and airway hyperresponsiveness.26,27
Parthenolide, a naturally occurring inhibitor of NF-κB, is used in only a few studies involving respiratory cell lines. A study investigating the role of parthenolide in cultured human respiratory epithelial cells showed that parthenolide inhibited NF-κB activity by IL-8 gene expression in these human respiratory epithelial cell lines.28 In a study using defective cystic fibrosis transmembrane conductance regulator (CFTR) cell lines and CFTR- knockout mice, it is also shown that parthenolide inhibited IL-8 secretion and prevented NF-κB activation, IκBα degradation and IκB kinase activity. Inhibiting NF-κB translocation resulted in attenuation of subsequent inflammatory response.29
In our study, parthenolide has been shown to improve most of the histopathological parameters in a murine model of asthma when compared with placebo. Histological parameters except the number of goblet cells and mast cells significantly improved in the group receiving parthenolide while in the group receiving dexamethasone, all histological parameters improved significantly compared to placebo. Dexamethasone was superior to parthenolide in most of the parameters except basal membrane thickness and number of mast cells. Combination of parthenolide and dexamethasone also had no additional benefit over dexamethasone therapy. We only detected decrease of IL-4 levels in the study groups (receiving either dexamethasone or parthenolide) suggesting that parthenolide may exert its anti-inflammatory properties by IL-4 similar to dexamethasone. This seems different from a previous study investigating the consequences of NF-κB inhibition by decoy oligonucleotides in experimental asthma.26 Nuclear factor-κB decoys inhibited IL-5, IL-13 and eotaxin but did not change IL-4 production in the airways of OVA-challenged mice.26 Although it is known that typical Th2 cytokines are often coordinately expressed, selective production of IL-4 or IL-5 has been reported in several situations and this may be attributed to differential transcription factor activation or inhibition.30,31
In conclusion, administration of parthenolide partially alleviated airway inflammation and remodelling in a murine model of asthma and combination therapy with dexamethasone did not have additive effects. Parthenolide as a naturally occurring NF-κB inhibitor exhibiting anti-inflammatory properties is still a prominent candidate for further studies and drug development. But specific mechanisms regarding its effects in asthma should be elucidated in oncoming studies of parthenolide.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Authors’ contributionsZAA and NU contributed in design of the study, OY and MK participated in animal study of the work, SO carried out conventional histological work, AB carried out electron microscopy, OK participated in design and coordination of the work. ZAA wrote the manuscript. All authors read and approved the manuscript.
Conflict of interestThe authors have no conflict of interest to declare.