Subclinical spirometric abnormalities may be detected in patients with rhinitis without asthma, proportional to the severity established by ARIA (Allergic Rhinitis and Its Impact on Asthma) guidelines. New criteria of rhinitis classification were recently validated according to the ARIA modified (m-ARIA), which allow the discrimination between moderate to severe grades. The impact of rhinitis on lung function according to frequency and severity is unknown.
ObjectivesTo investigate subclinical spirometric impairment in children and adolescents with allergic and non-allergic rhinitis without overt symptoms of asthma, according to the frequency and severity criteria of rhinitis classified by m-ARIA.
MethodsAn observational cross-sectional study, including children and adolescents aged 5–18 years with allergic and non-allergic rhinitis without asthma. We analysed the functional abnormalities and bronchodilator response with spirometry in relation to the grade of rhinitis established by m-ARIA using an adjusted logistic model. A value of p<0.05 was considered statistically significant.
ResultsWe studied 189 patients; 22.2% showed spirometric abnormalities. Patients with persistent rhinitis had greater impairment of lung function compared to intermittent grade (p=0.026). Lung functional impairment was more frequent in severe and moderate rhinitis than mild grade (p=0.005) and was independent of the atopic status to both frequency (p=0.157) and severity (p=0.538). There was no difference in bronchodilator reversibility between groups (p>0.05).
ConclusionsImpaired lung function was associated with persistence and severity of rhinitis and there was no significant difference between patients with moderate and severe rhinitis. The spirometric abnormality was demonstrated in patients with allergic and non-allergic rhinitis.
Rhinitis is a chronic inflammatory disease of the nasal mucosa, characterised by nasal congestion, rhinorrhea, sneezing, and nasal itching, which becomes relevant because of its high prevalence and the negative impact in the patients’ quality of life.1–3
The link between the upper and lower airways has been recognized since the beginning of the last century,4 but has been investigated in depth only in the last two decades, with the model of relationship between rhinitis and asthma.5,6 Epidemiological data indicate that over 80% of patients with asthma have rhinitis whereas asthma can affect up to 40% of patients with rhinitis; therefore, many authors have suggested the “one airway one disease” hypothesis as an expression of one indivisible anatomical and pathological entity.1,2,7,8 Based on this concept, the relationship between the upper and lower airway relies not only in their epidemiological interest but also in their pathophysiological and clinical interest; it also has direct therapeutic implications.6,9,10
The ARIA (Allergic Rhinitis and Its Impact on Asthma) guidelines,1,2 have proposed a clinical classification of rhinitis based on the frequency and severity of symptoms. Patients who develop symptoms for fewer than four days a week or fewer than four consecutive weeks, correspond to intermittent rhinitis whilst the presence of symptoms for more than four days a week and over four consecutive weeks, qualifies as persistent rhinitis.
The severity is determined by four items established in the ARIA guidelines: impairment in the school or work performance, daily activities, sleep disturbances and troublesome symptoms. It is considered mild when none of the items are affected and moderate to severe when one or more items are present (o-ARIA).
The PREDIAL multicentre study (Pediatric Allergic Rhinitis)11 has recently validated a new classification of rhinitis modified from the original ARIA guideline (m-ARIA), which allows to differentiate the moderate from the severe rhinitis. Thus, mild rhinitis has no affected items, moderate rhinitis compromises one to three items and the severe form includes patients with all of the parameters affected.12
New evidence has detected subclinical abnormalities in lung function in patients with allergic and non-allergic rhinitis with no symptoms suggestive of asthma,13–18 in a proportional way according to the original o-ARIA severity criteria16 and with increased responsiveness to a bronchodilator19,20; these findings could be the expression of a common disease that affects the entire respiratory tract.
The impact of rhinitis on lung function is still unknown according to the new classification of m-ARIA and considering frequency (intermittent-persistent) and severity (mild, moderate and severe).
The aim of this study was to examine spirometric abnormalities and their potential bronchodilator reversibility in children and adolescents with allergic and non-allergic rhinitis without asthma, and its relationship with the symptoms frequency and severity.
Materials and methodsStudy design: observational, cross-sectional analytical study.
Patients: inclusion and exclusion criteriaChildren and adolescents, between 5 and 18 years old referred to the Allergy and Immunology Division of the Clínica Universitaria Reina Fabiola, Universidad Católica de Córdoba, Argentina, were recruited from December 30, 2011 to May 31, 2013. The main inclusion criterion was a clinical diagnosis of rhinitis based on the presence of two or more nasal symptoms (rhinorrhea, blocked nose, itching and/or sneezing). Patients with allergic and non-allergic rhinitis were consecutively included, according to the presence or absence of aeroallergens sensitivity (determined by skin prick tests) and classified according to the symptoms duration and severity.11,12
The exclusion criteria were as follows:
- a.
Prior history of asthma or equivalent symptoms (cough, dyspnoea and/or wheezing and shortness of breathing).
- b.
Acute or chronic upper and lower airways infection.
- c.
Anatomic nasal disorders, nasal polyposis, septum deviation, etc.
- d.
Previous or current use of allergen-specific immunotherapy (subcutaneous or sublingual).
- e.
Use of intranasal or systemic steroids, antihistamines, leukotriene antagonists and alpha-adrenergic (nasal or systemic) during the previous four weeks.
- f.
Active smokers and/or exposed to cigarette smoke at home.
Was established according to the m-ARIA criteria.11,12 According to duration of the symptoms, patients with fewer than four days a week or fewer than four consecutive weeks were classified as suffering from intermittent rhinitis; the presence of symptoms for more than four days a week and over four consecutive weeks was considered as persistent rhinitis. The rhinitis severity was determined according to the number of affected items (limitations in school performance and daily activities, sleep disturbance or the existence of troublesome symptoms) as mild (no affected items), moderate (one to three quality of life items compromised) and severe (four affected items).
Despite the fact that ARIA guidelines are developed for allergic rhinitis, the Global Allergy and Asthma European Network suggested to classify non-allergic rhinitis with similar criteria to allergic rhinitis.21 Therefore, we classified patients with non-allergic rhinitis according to the same definitions used for the allergic ones in terms of duration and severity of symptoms.21,22
Studied variablesAge, gender, body mass index (BMI) and duration of rhinitis were considered. Spirometry and allergen skin prick test were performed.
The duration of rhinitis was established by the difference between the age of onset of the symptoms and the child's age at the moment of diagnosis. The percentage of life affected was the result of the following equation: [diagnosis age−age of onset/diagnoses age]×100.
Skin prick testFor the skin prick tests a standardised panel was used with the following allergens: house dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis), fungi (Alternaria sp., Aspergillus sp., Cladosporium, Mucor, Rhizopus, Penicillium), dog, cat and pollens from trees and grasses mix, Compositae mix (Alergo-Pharma®, Buenos Aires, Argentina). Histamine hydrochloride (10mg/dl) and sterile saline solution 0.9% were used as positive and negative controls. All tests were performed in the anterior forearm, using a Pricker type lancet (Diater Laboratories®, Buenos Aires, Argentina) and read after fifteen minutes using a millimetre rule. A wheal diameter ≥3mm was considered as a positive reaction.23 The existence of one or more positive skin tests to allergens was associated with allergic rhinitis; absence was compatible with a non-allergic rhinitis phenotype.
Lung function test and bronchodilator responseA flow volume loop was performed using a Vitalograph® 2120 UK spirometer, according to international standards of the American Thoracic Society/European Respiratory Society (ATS/ERS).24 Forced Vital Capacity (FVC), Forced Expiratory Volume in the first second of the FVC (FEV1), coefficient FEV1/FVC and Forced Expiratory Flow between 25% and 75% of FVC (FEF 25–75%) were recorded; the values were calculated by the software program included in the device, according to the predicted values of Knudson.25 Abnormal values were considered as those lower to 80% for the first three parameters and 65% for FEF 25–75% in relation to the normal predictive values.26
A bronchodilator response was measured after the administration of 200μg salbutamol (Ventolin®, GlaxoSmithKline) and expressed as positive when an improvement in FEV1≥12% compared to baseline pre-bronchodilator values were obtained.26
The best of three baseline and three post-bronchodilator measurements was chosen, meeting the criteria for acceptability and reproducibility according to ATS/ERS.24
Skin tests with allergens and spirometry were performed by the same operator without knowledge of any of the studied variables. To avoid circadian variations, all studies were conducted between 9 and 12a.m.
Statistical analysisAnalysis of the occurrence of impaired lung function (whatever its origin), the involvement in each one of the respiratory parameters and the positive bronchodilator response in relation to the severity of rhinitis, was performed by adjusting a logistic model. The model included severity and duration of rhinitis as variables, and sex, age, weight, height, BMI, atopy, family history of allergy, rhinitis duration and percentage of life affected as co-variables. The purpose of including these co-variables was to eliminate possible confounding effects to the severity of rhinitis. A significance level of 5% was considered to establish statistical significance. To adjust for these models, the function glmer from lme4 library27 of R was used,28 implemented under InfoStat software interface.29
ResultsOne hundred and eighty-nine children and adolescents, aged 5–18 years old, were included (106 males; 56%); the demographic and general characteristics distributed according to frequency and severity of rhinitis are shown in Tables 1 and 2.
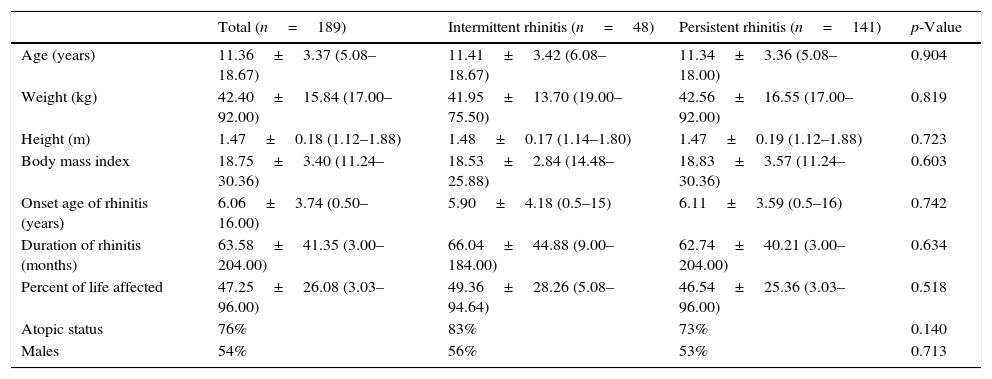
Demographic characteristics of patients with rhinitis according to the frequency of symptoms classified by the mARIA* criteria.
| Total (n=189) | Intermittent rhinitis (n=48) | Persistent rhinitis (n=141) | p-Value | |
|---|---|---|---|---|
| Age (years) | 11.36±3.37 (5.08–18.67) | 11.41±3.42 (6.08–18.67) | 11.34±3.36 (5.08–18.00) | 0.904 |
| Weight (kg) | 42.40±15.84 (17.00–92.00) | 41.95±13.70 (19.00–75.50) | 42.56±16.55 (17.00–92.00) | 0.819 |
| Height (m) | 1.47±0.18 (1.12–1.88) | 1.48±0.17 (1.14–1.80) | 1.47±0.19 (1.12–1.88) | 0.723 |
| Body mass index | 18.75±3.40 (11.24–30.36) | 18.53±2.84 (14.48–25.88) | 18.83±3.57 (11.24–30.36) | 0.603 |
| Onset age of rhinitis (years) | 6.06±3.74 (0.50–16.00) | 5.90±4.18 (0.5–15) | 6.11±3.59 (0.5–16) | 0.742 |
| Duration of rhinitis (months) | 63.58±41.35 (3.00–204.00) | 66.04±44.88 (9.00–184.00) | 62.74±40.21 (3.00–204.00) | 0.634 |
| Percent of life affected | 47.25±26.08 (3.03–96.00) | 49.36±28.26 (5.08–94.64) | 46.54±25.36 (3.03–96.00) | 0.518 |
| Atopic status | 76% | 83% | 73% | 0.140 |
| Males | 54% | 56% | 53% | 0.713 |
Data are expressed as mean±SD, in parenthesis: range.
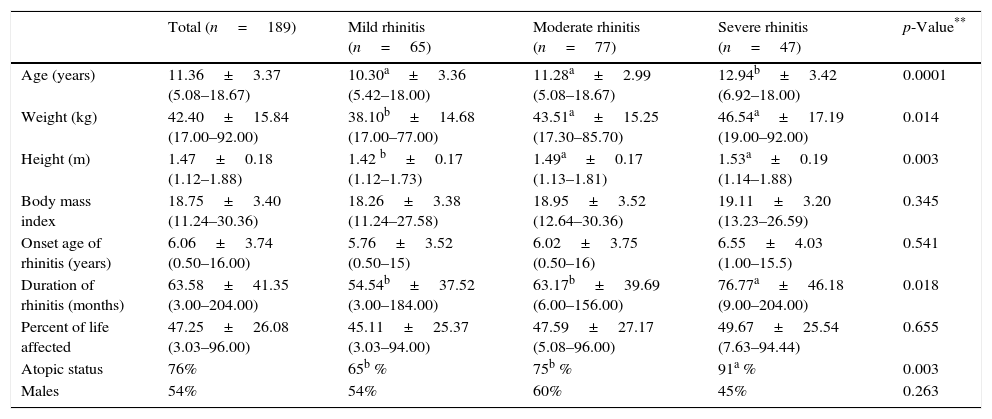
Demographic characteristics of patients with rhinitis according to the severity of symptoms classified by the mARIA* criteria.
| Total (n=189) | Mild rhinitis (n=65) | Moderate rhinitis (n=77) | Severe rhinitis (n=47) | p-Value** | |
|---|---|---|---|---|---|
| Age (years) | 11.36±3.37 (5.08–18.67) | 10.30a±3.36 (5.42–18.00) | 11.28a±2.99 (5.08–18.67) | 12.94b±3.42 (6.92–18.00) | 0.0001 |
| Weight (kg) | 42.40±15.84 (17.00–92.00) | 38.10b±14.68 (17.00–77.00) | 43.51a±15.25 (17.30–85.70) | 46.54a±17.19 (19.00–92.00) | 0.014 |
| Height (m) | 1.47±0.18 (1.12–1.88) | 1.42 b±0.17 (1.12–1.73) | 1.49a±0.17 (1.13–1.81) | 1.53a±0.19 (1.14–1.88) | 0.003 |
| Body mass index | 18.75±3.40 (11.24–30.36) | 18.26±3.38 (11.24–27.58) | 18.95±3.52 (12.64–30.36) | 19.11±3.20 (13.23–26.59) | 0.345 |
| Onset age of rhinitis (years) | 6.06±3.74 (0.50–16.00) | 5.76±3.52 (0.50–15) | 6.02±3.75 (0.50–16) | 6.55±4.03 (1.00–15.5) | 0.541 |
| Duration of rhinitis (months) | 63.58±41.35 (3.00–204.00) | 54.54b±37.52 (3.00–184.00) | 63.17b±39.69 (6.00–156.00) | 76.77a±46.18 (9.00–204.00) | 0.018 |
| Percent of life affected | 47.25±26.08 (3.03–96.00) | 45.11±25.37 (3.03–94.00) | 47.59±27.17 (5.08–96.00) | 49.67±25.54 (7.63–94.44) | 0.655 |
| Atopic status | 76% | 65b % | 75b % | 91a % | 0.003 |
| Males | 54% | 54% | 60% | 45% | 0.263 |
Data are expressed as mean±SD, in parenthesis: range.
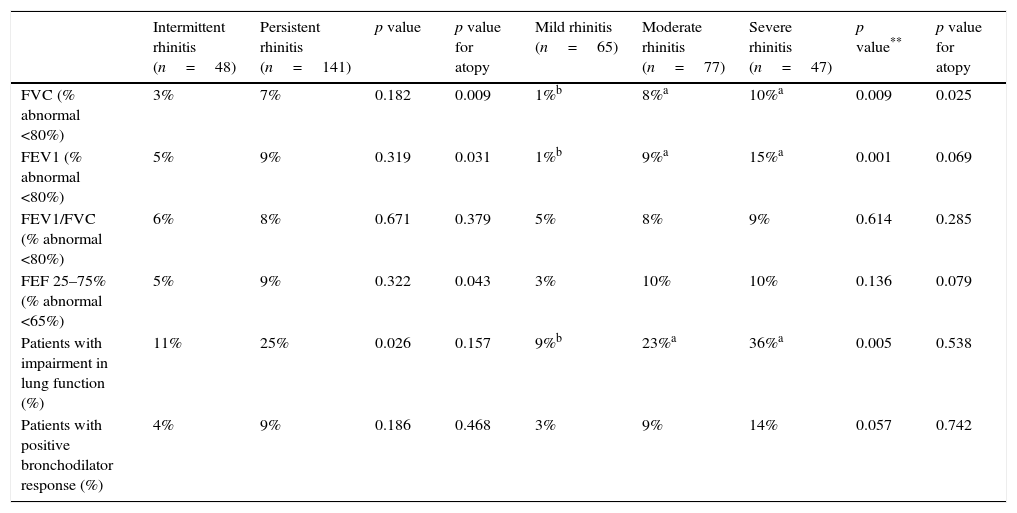
In 42 of 189 patients (22.2%) at least one impaired lung function parameter was detected. FEV1 and FEV1/FVC index were most frequently affected in 20 patients (10.6%), either alone or combined with another spirometric parameter, whilst FEF 25–75% was altered in 18 patients (9.5%).
The analysis also demonstrated that patients with persistent rhinitis had a greater impairment of lung function than those patients with intermittent rhinitis (p=0.026) (Table 3).
Distribution of patients with impairment in lung function, abnormalities in different spirometric parameters and bronchodilator response according to frequency and severity of rhinitis classified by the mARIA* criteria.
| Intermittent rhinitis (n=48) | Persistent rhinitis (n=141) | p value | p value for atopy | Mild rhinitis (n=65) | Moderate rhinitis (n=77) | Severe rhinitis (n=47) | p value** | p value for atopy | |
|---|---|---|---|---|---|---|---|---|---|
| FVC (% abnormal <80%) | 3% | 7% | 0.182 | 0.009 | 1%b | 8%a | 10%a | 0.009 | 0.025 |
| FEV1 (% abnormal <80%) | 5% | 9% | 0.319 | 0.031 | 1%b | 9%a | 15%a | 0.001 | 0.069 |
| FEV1/FVC (% abnormal <80%) | 6% | 8% | 0.671 | 0.379 | 5% | 8% | 9% | 0.614 | 0.285 |
| FEF 25–75% (% abnormal <65%) | 5% | 9% | 0.322 | 0.043 | 3% | 10% | 10% | 0.136 | 0.079 |
| Patients with impairment in lung function (%) | 11% | 25% | 0.026 | 0.157 | 9%b | 23%a | 36%a | 0.005 | 0.538 |
| Patients with positive bronchodilator response (%) | 4% | 9% | 0.186 | 0.468 | 3% | 9% | 14% | 0.057 | 0.742 |
Patients with moderate and severe rhinitis have more functional lung impairment compared to patients with rhinitis in mild grade (p=0.005); however, no significant differences were detected among the first two (Table 3). The spirometric impairment was independent of atopic status for both frequency (p=0.157) and severity (p=0.538), and no differences were found in the bronchodilator response between groups (Table 3).
DiscussionIn the present study, differences in pulmonary function impairment were studied, considering the duration (intermittent-persistent) and severity (mild, moderate and severe) of rhinitis according to the m-ARIA criteria.11,12
Some research has detected subclinical abnormalities in lung function in patients with rhinitis with no evidence of clinical asthma13–17; in a previous study,17 we demonstrated that at least one altered respiratory function parameter was found in one quarter of patients with allergic rhinitis and that the FEV1/FVC ratio was the most commonly affected parameter, either alone or combined with other functional measures. Our present findings confirm previous evidence that a high percentage of children and adolescents with rhinitis show some impairment in spirometric parameters, because 22.2% of the patients with allergic and non-allergic rhinitis, but without asthma, showed this alteration. Patients with persistent rhinitis had a higher functional respiratory impairment than those with intermittent rhinitis. Likewise, those rated as moderate and severe presented a greater lung impairment than those classified as mild, although no significant differences were detected between them.
A single study assessed lung function in relation to the severity of rhinitis in patients without asthma. Mohammad et al.16 demonstrated in a group of young adult patients with allergic rhinitis, an impairment of the lung function that was proportional to the degree of rhinitis classified by the original ARIA guide. The moderate-severe persistent degree had a significantly greater bronchial involvement than the intermittent mild and moderate-severe and mild persistent.16 Other studies showed changes in lung function and bronchial hyper-reactivity (BHR) in different patient populations with persistent rhinitis showing moderate and severe symptoms.18,30 BHR was more frequent in patients with allergic rhinitis than in those with non-allergic rhinitis, and also that persistency of rhinitis was a significant predictor of BHR.30 These findings highlight the concept that a bronchial involvement is frequent in rhinitis, also in the absence of overt asthmatic symptoms and is consistent with the hypothesis that the greater the severity of rhinitis, the greater the impact on lung function.
This ventilatory abnormality observed in children and adults with rhinitis in the absence of asthmatic symptoms, is potentially reversible to the effects of a bronchodilator.19,20 In adults with allergic rhinitis 8.4% of patients presented abnormal FEV1, 24.7% impaired FEF 25–75% and 66.1% showed reversibility to bronchodilator31 suggesting that these alterations may indicate a “pre-asthmatic” status which can gradually evolve to clinically demonstrated asthma with the progression of the “atopic march”. This could have therapeutic implications; however, in our study and considering the dissociated conditions frequency and severity, we could not reproduce similar findings because the reversibility obtained between groups was not significant. This is probably because the bronchodilator response was rated using not only patients presenting obstructive defects but also those with alterations in FVC only.
The abnormality in lung function was independent of the atopy condition: whilst a higher prevalence of allergic sensitisation was observed in patients with severe rhinitis (Table 2), both phenotypes of rhinitis (allergic and non-allergic), showed similar functional involvement. Chawes et al.15 found similar impairment of the specific airway resistance (sRaw), measured by whole body plethysmography in young children with allergic and non-allergic rhinitis, although the former ones had significantly higher values of exhaled nitric oxide (FeNO). These findings suggest that the impact of rhinitis on lung function, in absence of asthma, would be proper to the condition of rhinitis and would not be associated with its aetiology.
Rhinitis in pediatric patients has a sub-clinical bronchial disease process that is proportional to the magnitude of the disease, but the prognosis value of these findings has not yet been determined.
It has been shown that rhinitis is an independent risk factor for developing asthma32–35 and that the use of intranasal steroids can improve the functional defect in children with allergic rhinitis without asthma,36 but no prospective research has established that patients with rhinitis and impaired lung function have a higher risk of developing asthma. Possibly this depends on common genetic factors for a possible progression of rhinitis to asthma,37,38 which could have an initial sub-clinical expression in intra-thoracic airway obstruction.
These potential diagnostic, prognostic and therapeutic implications are sufficient reasons to suggest that patients with rhinitis alone should be evaluated for asthma, based on their clinical history, a careful chest examination and lung function assessment just as proposed by the ARIA document.1,2 Our findings support the indication of spirometry only in patients with persistent, moderate and severe degree of rhinitis, making unnecessary the routine determination in patients with rhinitis classified as mild and intermittent. This management strategy could contribute to financial savings for public health, a suggestion which should be confirmed with studies involving a larger sample of patients and reproducible results with other centres.
Our study has the strength to confirm the asymptomatic impairment of lung function in children and adolescents with rhinitis, also observed by other authors13–18,31 and that data analysis excluded potentially confounding variables. We recognise the limitations imposed by the clinical classifying degrees of rhinitis, because these can manifest variability over time and be influenced by subjectivity between operators.
In conclusion, this study highlights that impaired lung function appears in a substantial percentage of patients with allergic and non-allergic rhinitis. The involvement was more common in persistent, moderate to severe rhinitis, with no significant differences between these last two degrees of severity. The bronchial involvement was independent of the atopic status suggesting a link between the upper and lower airway diseases beyond the inflammation associated to the allergy status.
Our results also allow us to suggest that subjects with rhinitis and without asthma should be evaluated for their lung function, because this somehow can influence the course of the disease.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.
FundingThis study was carried out using funds from the Secretaría de Investigación y Vinculación Tecnológica of the Universidad Católica de Córdoba.
The authors would like to acknowledge the fellows in training Yanina Berardi and Ana Gabriela Sosa for their collaboration in the patients’ recruitment and to Eugenia Concari for her technical assistance.