Food allergy (FA) prevalence is well documented in developed countries and appears to be increasing, but remains unknown in most Latin American countries. We aimed to evaluate on a population basis the parent-reported prevalence of FA and its clinical characteristics in Mexican schoolchildren.
MethodsA validated Spanish version of a structured written questionnaire was administered to parents of schoolchildren aged 5–13 years old from Culiacan, Mexico.
ResultsA total of 1049 parents responded to the survey (response rate, 84%). The estimated prevalence rates (95% CI) were: adverse food reactions 10.0% (8.3–11.9), “perceived FA, ever” 5.5% (4.3–7.0), “physician-diagnosed FA, ever” 4.9% (3.7–6.3), “immediate-type FA, ever” 4.4% (3.3–5.8), “immediate-type FA, current” 3.5% (2.6–4.8), and anaphylaxis 1.2% (0.72–2.1). Immediate hypersensitivity reactions were mainly triggered by the consumption of shrimp (1.3%), other shellfish (0.7%), strawberry (0.6%), chocolate (0.5%), and egg (0.4%). Schoolchildren with “immediate-type FA, current” had more atopic dermatitis and allergic rhinitis (p<0.05), but not asthma or drug allergy (p>0.05) than children without FA. All cases of anaphylaxis sought medical attention, but only one child had physician-diagnosed anaphylaxis and was advised to acquire an epinephrine autoinjector.
ConclusionsThe prevalence of “immediate-type FA, current” to any food is 3.5% in Mexican schoolchildren. The poor recognition of anaphylaxis and the low frequency of prescription of epinephrine autoinjectors suggest that acute food-induced allergic reactions are not optimally managed in Mexico.
Food allergy (FA) is a prevalent and potentially severe condition that affects children and adults worldwide. This immune disorder appears to be increasing and has become an important health concern in developing and developed countries.1 It has been estimated that the condition affects more than 1–2% but less than 10% of the general population.2 However, the epidemiology of FA remains unknown in most Latin American countries1,3 with only three population-based studies published to date.4–6 Notably, only one of these studies applied strict criteria for defining FA5 and this has high sensitivity for positive specific food IgE in affected patients.7–9 To our knowledge, no population-based studies of FA have been carried out in Mexico, a country inhabited by more than 120 million people. Thus, the aim of this study was to evaluate the parent-reported prevalence of FA and the clinical characteristics of this condition in a Mexican population of schoolchildren.
Materials and methodsPopulation surveyWe conducted a population-based cross-sectional survey in Culiacan, Sinaloa, Mexico. All data were collected during the period from September 2014 to August 2015. The sampling was made by convenience in ten elementary schools (private and public schools) that geographically cover five areas of the city of Culiacan, Mexico (two schools in each of the following areas; North, South, East, Southeast, and downtown area). At least 20 schoolchildren per grade (120 per school, six grades) were included in the study except for two private schools that reported a reduced number of students (<100), but agreed to participate in the study. The questionnaires and informed consents were handed out to the teachers who in turn attached them to the children's homework notebooks. This process was carried out only once. If the questionnaire and signed informed consent were not returned back after three working days, this was considered as non-response by the parents.
QuestionnaireA validated Spanish version of a structured questionnaire designed to estimate the parent-reported prevalence of food allergy in schoolchildren5 was slightly adapted to be used in this study. The adjustments were intended to enable the self-administration of the questionnaire by Mexican parents, but the parameters to measure the variables of interest were not modified. This instrument is composed of some questions that were taken from a validated Spanish questionnaire,4 which was later customised for screening purposes,5 and others from an in-depth questionnaire, which was validated in English9 and Spanish.5 To identify those children that at the time of the survey still had allergic reactions to the suspected foods, we included a key question in the instrument final version (is your child now able to eat the suspected food without any reactions), as previously described.9
Respondents first answered questions related to basic demographic and clinical information about the child. All respondents with a positive response to perceived food-related recurrent symptoms completed the second part of the questionnaire. This section incorporated standardised questions about symptoms suggestive of IgE-mediated FA; time of appearance of the symptoms after food ingestion; the foods involved in the allergic/adverse food reaction; and treatments prescribed during allergic reactions among others.
An Ethics Review Board of the Universidad Autónoma de Sinaloa approved the study protocol (ethic approval number CE-UACNYG-2014-AGO-001).
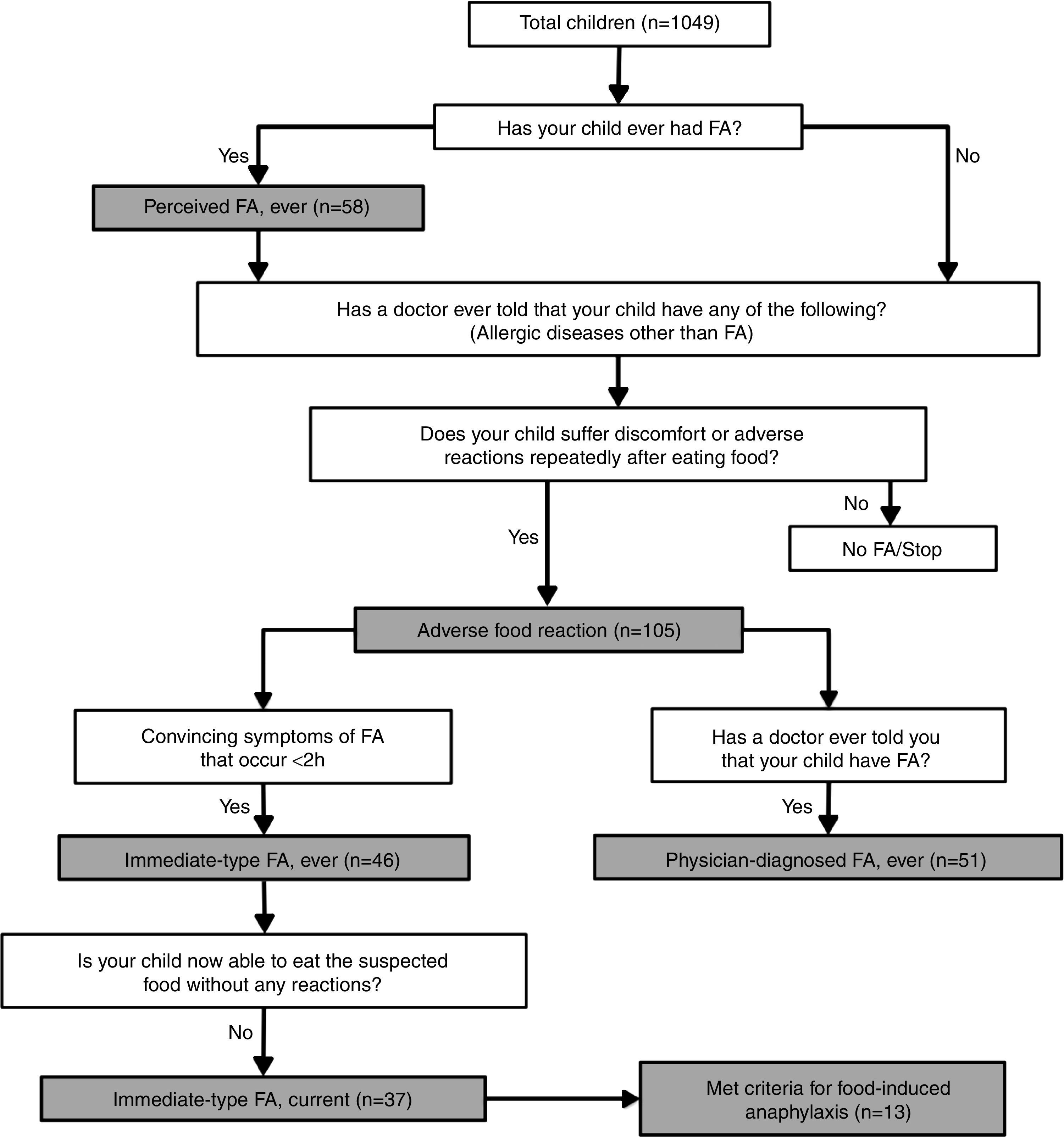
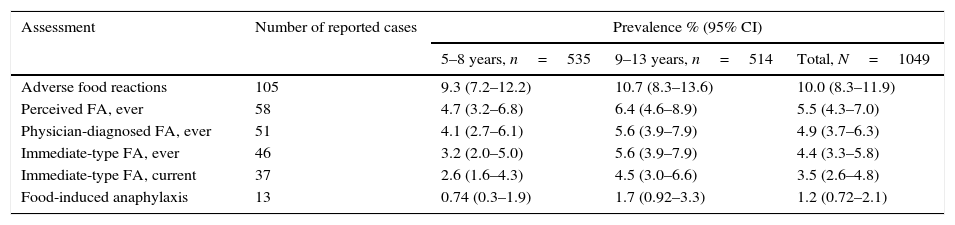
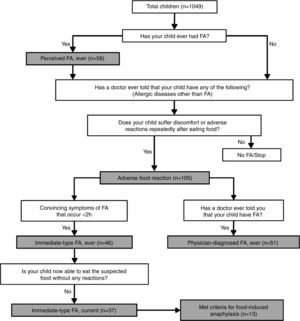
DefinitionsAdverse food reactions and FAs were defined according to the algorithm shown in Fig. 1. Briefly, a child was regarded as having “perceived FA, ever” if the parents stated that their child had had allergic reactions to food.10 An adverse food reaction was defined as any symptomatic recurrent adverse reaction to a specific food potentially mediated or not by immune mechanisms.11 “Immediate-type FA, ever” was defined as having symptomatic recurrent adverse food reactions that were “convincing” of immediate hypersensitivity allergic reactions. This included skin with hives, angio-oedema, trouble breathing, wheezing or throat tightness, vomiting and diarrhoea, among other symptoms typical of immediate hypersensitivity reactions that occurred within 2h after food ingestion; as previously described.5,7–9 “Immediate-type FA, current” was defined as those cases that met criteria for “immediate-type FA, ever”, but answered negatively to the question “is your child now able to eat the suspected food(s) without any reactions”.9 In addition, “physician-diagnosed FA, ever” was defined as those cases that met criteria for adverse food reactions and answered positively to the question, “Has a doctor ever told you that your child has FA?”
Food-depending anaphylaxis was defined as those cases that met criteria for “immediate-type FA, current” and according to the three following criteria: (1) acute onset of an illness with involvement of the skin, mucosal tissue or both and respiratory compromise or reduced blood pressure; (2) two or more of the following that occur rapidly after food ingestion: (a) involvement of the skin-mucosal tissue, (b) respiratory compromise, (c) reduced blood pressure, (d) persistent gastrointestinal symptoms; and (3) reduced blood pressure after exposure to a food allergen.12
Statistical analysesStatistical analysis was carried out using PASW statistics version 18.0 (SPSS Inc., IL, USA). Categorical variables were summarised by descriptive statistics including total numbers and percentages, and associations of FA with other atopic diseases, age, and season of birth were analysed by two-tailed Fisher exact test. Continuous variables were summarised by mean and range with differences between two groups calculated using the Student t-test. A p-value <0.05 was considered statistically significant. Prevalence rates were calculated using OpenEpi software version 3.03a (www.OpenEpi.com, updated 04/05/2015, and accessed 28/08/2015). Rates were reported as rate (95% confidence intervals) per 100 inhabitants. Based on the prevalence of expected FA in schoolchildren (5.5%), a 95% confidence level, and 2% accuracy, the total of questionnaires collected was representative of the 160,038 children that attend elementary school in Culiacan, Mexico.
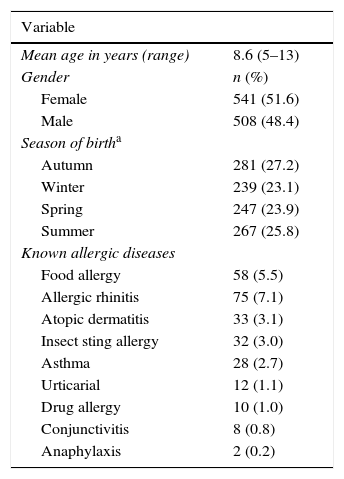
ResultsStudy participants and demographic characteristicsThe questionnaire was sent to 1248 subjects. Of these, 1049 were retrieved with valid responses (valid response rate, 84.0%) and 199 could not be retrieved or had invalid data. The demographic and clinical characteristics of the subjects surveyed are summarised in Table 1. The proportions of girls and boys that participated in the study as well as seasons of birth did not differ significantly (p>0.05). Allergic diseases were reported by 17.1% of the participants and 4.6% reported more than one allergic disease.
Demographic and clinical characteristics of the study population.
| Variable | |
|---|---|
| Mean age in years (range) | 8.6 (5–13) |
| Gender | n (%) |
| Female | 541 (51.6) |
| Male | 508 (48.4) |
| Season of birtha | |
| Autumn | 281 (27.2) |
| Winter | 239 (23.1) |
| Spring | 247 (23.9) |
| Summer | 267 (25.8) |
| Known allergic diseases | |
| Food allergy | 58 (5.5) |
| Allergic rhinitis | 75 (7.1) |
| Atopic dermatitis | 33 (3.1) |
| Insect sting allergy | 32 (3.0) |
| Asthma | 28 (2.7) |
| Urticarial | 12 (1.1) |
| Drug allergy | 10 (1.0) |
| Conjunctivitis | 8 (0.8) |
| Anaphylaxis | 2 (0.2) |
Prevalence estimations of adverse food reactions and FA are given in Table 2. Overall, more than 40% of the adverse food reactions were perceived as allergic reactions. In the group of “immediate-type FA, current”, 54.0% (n=20) of the cases were female (p>0.05 compared to male) and 75.7% (n=28) had “physician-diagnosed FA, ever”. Stratified by age groups (5–8 years, 9–13 years), prevalence estimations were higher in the 9–13 years group, but these age-related differences were not statistically significant (p>0.05) (Table 2). Foods causing anaphylaxis were shrimp (n=4), milk (n=2), chocolate (n=2), strawberry (n=2), egg (1), chili (n=1), and tree nut (n=1). One child that reported shrimp-induced anaphylaxis also reported anaphylaxis upon the consumption of other shellfish. However, an epinephrine autoinjector was prescribed in only 1 out of 13 cases of anaphylaxis, and in another case of “immediate-type FA, ever”. Seventeen subjects reported convincing FA symptoms but delayed onset (over two hours) of which six reported that they still had allergic reactions upon food exposure.
Prevalence estimations.
| Assessment | Number of reported cases | Prevalence % (95% CI) | ||
|---|---|---|---|---|
| 5–8 years, n=535 | 9–13 years, n=514 | Total, N=1049 | ||
| Adverse food reactions | 105 | 9.3 (7.2–12.2) | 10.7 (8.3–13.6) | 10.0 (8.3–11.9) |
| Perceived FA, ever | 58 | 4.7 (3.2–6.8) | 6.4 (4.6–8.9) | 5.5 (4.3–7.0) |
| Physician-diagnosed FA, ever | 51 | 4.1 (2.7–6.1) | 5.6 (3.9–7.9) | 4.9 (3.7–6.3) |
| Immediate-type FA, ever | 46 | 3.2 (2.0–5.0) | 5.6 (3.9–7.9) | 4.4 (3.3–5.8) |
| Immediate-type FA, current | 37 | 2.6 (1.6–4.3) | 4.5 (3.0–6.6) | 3.5 (2.6–4.8) |
| Food-induced anaphylaxis | 13 | 0.74 (0.3–1.9) | 1.7 (0.92–3.3) | 1.2 (0.72–2.1) |
Twenty-seven out of 46 children (58.7%) that met criteria for “immediate-type FA, ever” and 122 out of 986 (12.4%) without convincing FA symptoms had history of allergic diseases other than FA (p<0.05). Similar analysis between those with “immediate-type FA, current” and those without reported convincing FA symptoms was also statistically significant (p<0.05). Children with immediate-type FA, either “ever” or “current”, were more frequently reported to have allergic rhinitis, atopic dermatitis, insect sting allergy, and urticarial (p<0.05), but not asthma, drug allergy or allergic conjunctivitis (p>0.05) than children without convincing FA symptoms (n=986). Previous studies have shown that children with FA are born more frequently in autumn/winter than spring/summer months.13,14 In our study a higher rate of children with “immediate-type FA, current” were born in autumn/winter (57.8%) than spring/summer months (42.2%), but this was not statistically significant (p>0.05).
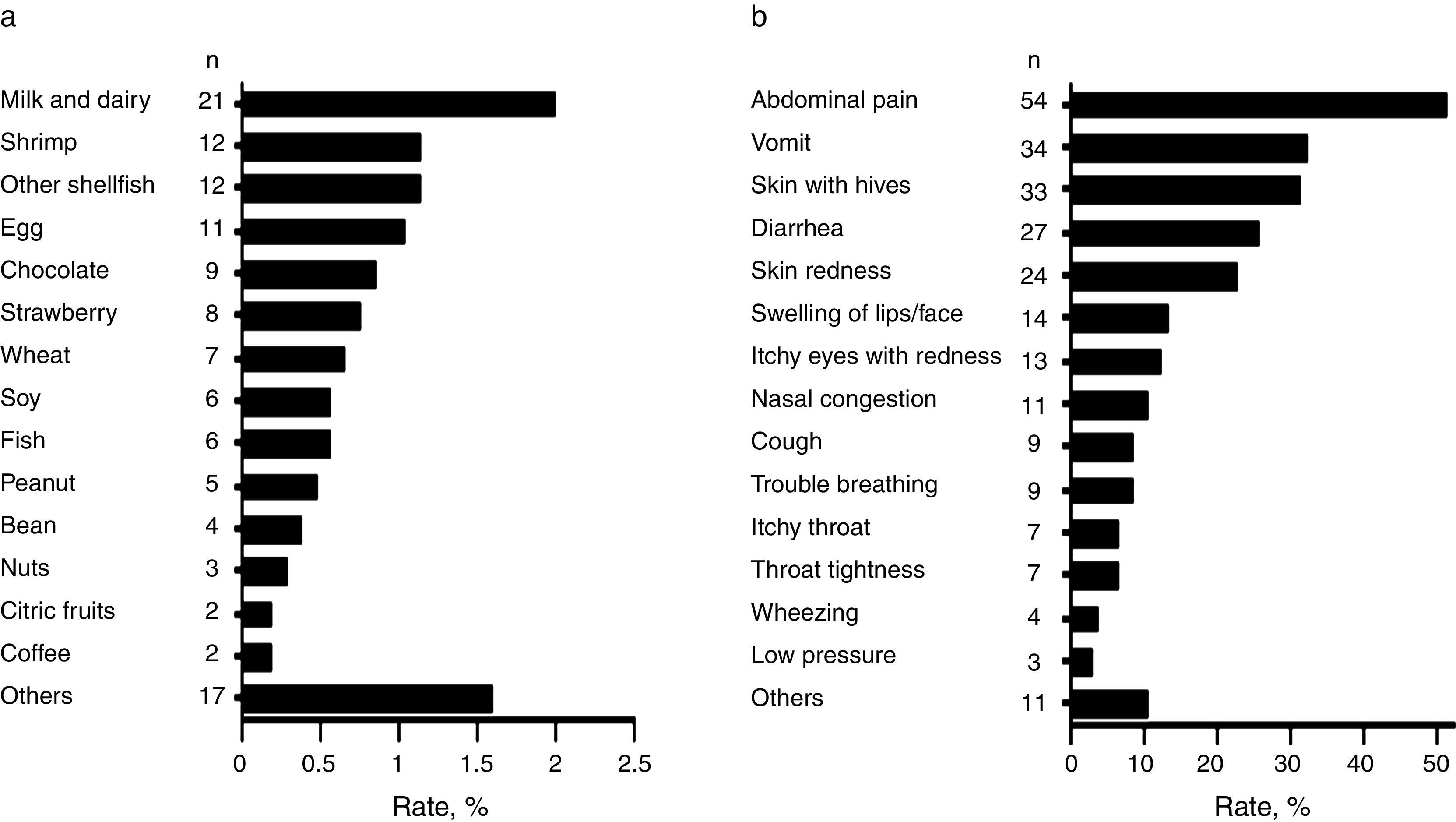
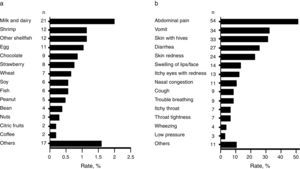
Foods causing symptomatic adverse reactionsThe most commonly implicated foods causing recurrent adverse reactions and the specific symptoms are shown in Fig. 2a and b respectively. Among those that reported adverse food reactions (n=105), 67.6% (n=71) sought medical attention and 59.0% (n=62) removed the causative food from the diet. Of those on restriction diets (n=62), 38.7% (n=24) had no “physician-diagnosed FA, ever” and 17.7% (n=11) neither had “physician-diagnosed FA, ever” nor met criteria for immediate-type FA, either “ever” or “current”. These results suggest that some schoolchildren were on restriction diets due to other adverse food reactions different from immediate-type FA or they could unnecessary be on restriction diets.
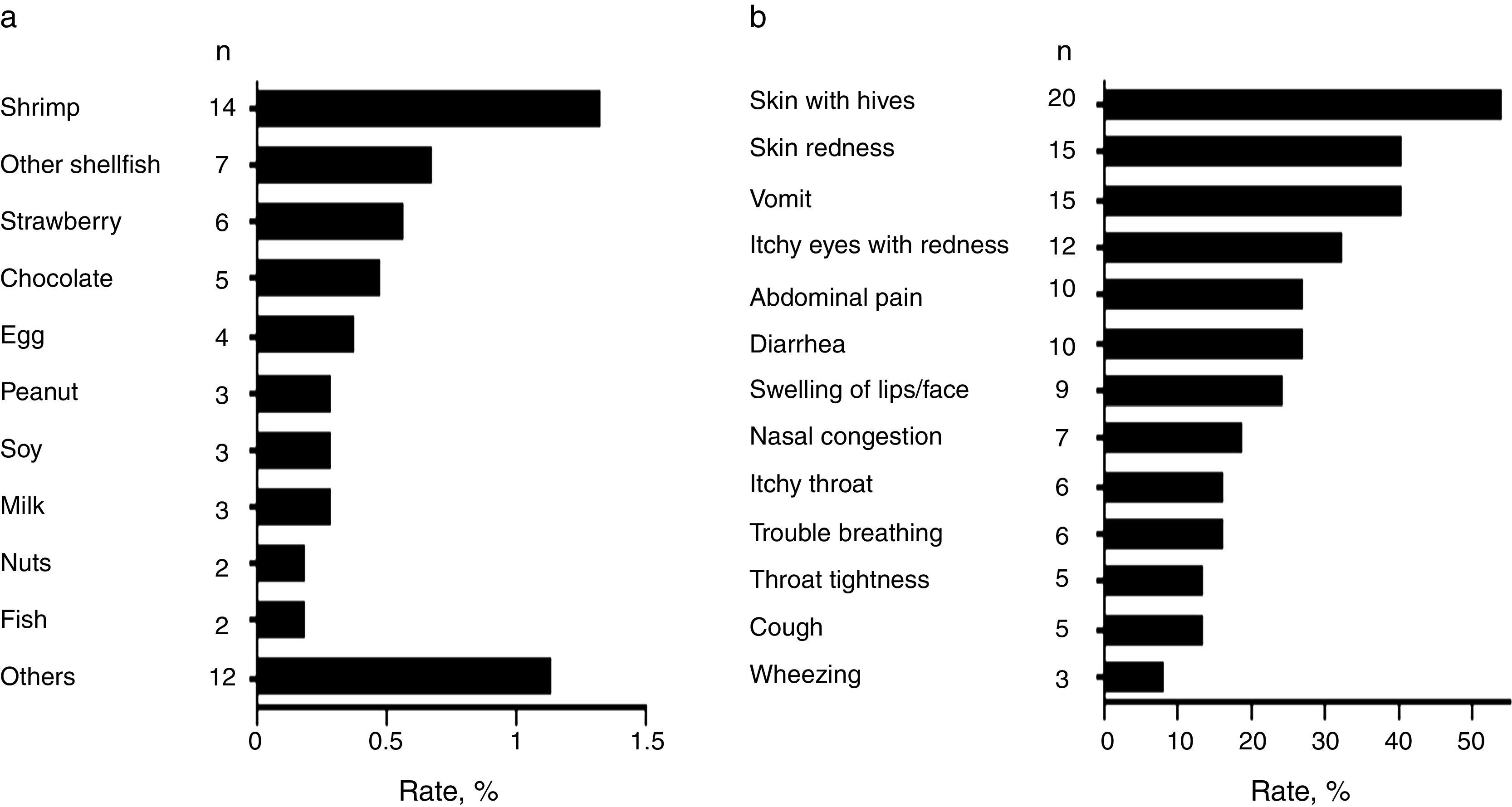
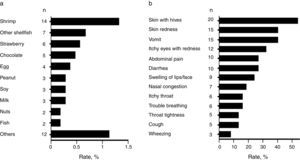
Common food allergens and clinical characteristics of FANext, we analysed children with a convincing history of FA. Most frequently reported food allergens in the studied population were shrimp (1.3%, 95% CI: 0.8–2.2), other shellfish (0.7%, 95% CI: 0.32–2.3), and strawberry (0.6%, 95% CI: 0.26–1.1) followed by chocolate (0.5%, 95% CI: 0.2–1.1) and egg (0.4%, 95% CI: 0.14–0.97) (Fig. 3a). Other allergens were peanut, soy, and milk (0.29% each, 95% CI: 0.09–0.83), as well as nuts and fish (0.19% each, 95% CI: 0.05–0.69) (Fig. 3a). Overall, shellfish allergy including shrimp accounted for 43.2% (n=16; due to five cases of allergy to both shrimp and other shellfish) of the “immediate-type FA, current” cases with a prevalence rate of 1.5% (95% CI: 0.94–2.4).
The FA-related adverse reactions in children with “immediate-type FA, current” mainly included skin (62.2%), gastrointestinal (48.6%), and respiratory (45.9%) symptoms. The most frequently reported specific symptoms are shown in Fig. 3b. Adverse food reactions were perceived at the age of 1.6 years (range 0.5–4) and 2.3 years (range 0.5–4) in those with “immediate-type FA, current” and in those with non-convincing FA symptoms respectively (p<0.05), showing that immediate hypersensitivity reactions to foods are more likely to appear or to be perceived earlier in life than other adverse food reactions.
DiscussionThis population-based study highlights that both perceived adverse food reactions and immediate hypersensitivity reactions to food are common in Mexican schoolchildren. The prevalence of adverse food reactions was 10.0% and the triggers most frequently reported were milk and dairy and shrimp. Similarly, a recent survey that only included an adult Mexican population reported these foods as the main triggers of adverse food reactions, but the prevalence of the reactions was 16.7%.15 Oral allergy syndrome was the main clinical manifestation. On the contrary, abdominal pain and vomit were the leading symptoms related to adverse food reactions in our cohort of Mexican schoolchildren. Age differences between populations and the increased prevalence rate of adverse reactions to fruits and vegetables in the adult Mexican population (6.12%),15 compared to what we found in Mexican schoolchildren (1.04%), could explain the different clinical manifestations.
Our study also highlights that the cumulative prevalence of shrimp and other shellfish allergy is relatively high in Mexican schoolchildren (1.5%). This is more than twofold the prevalence of shellfish allergy reported in Chilean schoolchildren (0.7%) using a similar approach,5 but these data are in line with studies carried out in Asian countries where seafood is abundant and shellfish is usually reported as the most common food allergen.9,16 The results also suggested that the prevalence of other common food allergies such as peanut (0.29%) and tree nut (0.19%) allergy in this part of Mexico is relatively low compared to the other countries in North America (>1%).8,17 Even in other countries such as the United Kingdom, Australia and Chile the reported prevalence rates of peanut allergy exceed 1% in children.5,18,19 These results support the notion that there is marked heterogeneity in the prevalence of FA between populations20 making of interest the evaluation of FA prevalence to specific foods in unexplored regions.
We observed a significant association of FA with atopic dermatitis and allergic rhinitis, but not asthma, which is commonly associated to FA. This lack of association can be explained by the low prevalence rate of self-reported asthma in our study (2.7%), which is far lower than the prevalence rates reported in other regions of Mexico (rates between 5.8–12%) using the International Study of Asthma and Allergies in Childhood (ISAAC) methodology.21,22
In this study, the prevalence of food-induced anaphylaxis was 1.2%. This is twofold lower than that reported in Chilean schoolchildren using the same definitions of anaphylaxis,5 but it is almost equal to that reported in adult Mexican population (1.3%).15 Although the preferred mean for emergency treatment of anaphylaxis is the use of epinephrine autoinjectors,23 our data showed a low frequency of prescription of this emergency treatment. Out of 46 children that met criteria for immediate-type FA, only two were advised to acquire an epinephrine autoinjector. One of these two cases reported a physician-diagnosed food-induced anaphylaxis, but epinephrine autoinjectors were not prescribed in another 12 cases of anaphylaxis, even though all of them sought medical attention. This data indicates that food-induced anaphylaxis induced by food is not optimally managed in Mexico and highlights the need to educate healthcare personnel regarding the risks of FA and treatment of acute food-induced allergic reactions. This could be expected as epinephrine autoinjectors were and are still not available in the mainstream drugstores of the city of Culiacan, Mexico (data not shown), and this is a cause of concern. In line with this, similar surveys carried out in Asian countries and Chile reported that epinephrine autoinjectors were not available or not prescribed by physicians.5,9
The major strengths of our study are its population-based design, the relatively high participation rate (84.0%) in a sample representative of the schoolchildren living in the city studied, and the criteria used to estimate the prevalence rates of immediate-type FA. It has been shown that most subjects fulfilling these criteria (93%) had IgE antibody to the implicated food.7 However, it should be acknowledged that our study has some limitations. First, the use of self-reporting to estimate prevalence rates has been found to overestimate the real prevalence rates,20 and our data were not confirmed by more specific diagnostic studies such as skin prick tests, specific IgEs, or oral food challenges. Secondly, our study had a limitation in assessing the effects of family history of atopic disease on FA in children. A family history of allergic disease has been associated with higher risk of FA in this population.24 In addition, it is possible that some children that have outgrown FA could still be on elimination diets due to uncertainty about their condition or because they disliked the taste of the avoided food,25 and this could influence the estimated prevalence rate of “immediate-type FA, current”. Despite these limitations, the present study provides useful epidemiological data regarding FA in Mexican schoolchildren and serves as groundwork for further epidemiological and clinical studies based on objective diagnostic criteria.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
The authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingThis work was funded by Universidad Autónoma de Sinaloa (grant PROFAPI 2013/026). The authorities responsible for financial support did not participate in any stage of the research conducted or publication process.
Author contributionsN. O.: study concept and design, manuscript preparation; E.E. V.-M.: Responsible for substantial acquisition of the data, statistical analysis, manuscript preparation; M.J. V.-G.: Study design, acquisition of the data, and analysis of the data; V.A. C.-R.: Acquisition of the data, manuscript preparation, analysis and interpretation of the data; A. B.: Analysis and interpretation of the data, statistical analysis, reviewed the manuscript for important intellectual content; F. C.-C.: Study concept and design, manuscript preparation. All authors have read and approved the final version of this manuscript.
Conflicts of interestNone declared.
The authors are grateful to Giovanni I. Ramírez-Torres, Ivan R Chiquete-Elizalde, Jesús A. Ibarra-Diarte, Jesús G. Arámburo-Galvez, and Jesús A. López-Gallardo for assistance in data collection. Thanks to PROFAPI 2013/026.