INTRODUCTION
There is now an almost universal consensus that the prevalence of allergic diseases has increased considerably in the Western industrialised countries (1, 2), and several potential risk factors of allergenic sensitisation have been identified. Nevertheless, the reason for this increase remains obscure. The pathogenesis of allergy depends on the interaction between the time and amount of allergen exposure and the presence of these risk factors in genetically predisposed individuals. However, even if all factors that are known to affect the prevalence of allergic manifestations were to be combined, this would only partly explain their increase and the geographic differences in their prevalence (1-4).
Several studies of individuals belonging to the same ethnic group, but living under different conditions, clearly demonstrated that the prevalence of allergic disease is higher in industrialised countries than in rural areas or developing countries. Therefore, the relative risk of a positive skin prick test is 70 % higher among 11-year-old children living in a moderately polluted town in northern Sweden than among children living in the nearby countryside (3, 4). This finding has been linked, among other factors, to urban photochemical smog, which is characterised by high levels of nitrogen dioxide, ozone and particulate matter. In atopic subjects exposure to pollutants probably increases airway responsiveness to aeroallergens (5). Nevertheless, in regions such as China and Eastern Europe, with a high concentration of some air pollutants such as particulate matter and sulphur dioxide, generally had low rates of asthma prevalence and areas with a low degree of air pollution, such as those in New Zealand, had a high prevalence of asthma (1). The reported differences between close-by populations are not genetically determined. Instead, they seem to be explained by differences in living conditions. This findings lead us to think that even though it is plausible that urban air pollution plays a role in the increasing prevalence of allergic respiratory diseases, other causes should not be discarded.
Allergy to grass pollen is very common and recent surveys have shown that 35 % of young adults in a wide variety of countries have specific serum antibodies to grass pollen (6). The allergic symptoms caused by grass pollen are worse in urban districts than in rural areas. The pollen more prevalent as cause of pollen asthma in our area is Lolium perenne. In a recent epidemiological review of data corresponding to 16381 patients from our health area (rural and urban origin), we found a 7.3 % increase of grass pollen-induced asthma in the last decade (7), and the asthmatic patients were mainly from urban areas (OR: 1.77, 95 %CI 1.073 to 2.912, logistic regression).
A possible explanation for the above could be that grass from rural areas receives less damage than the same grass in urban regions, where these plants are exposed to a polluted environment, undergo repeated reaping and receive fumigated pesticides and other dusts. Continuous plant damage of the plant might cause an overexpression of stress proteins in the grass pollen. Since some of these proteins are major allergens, it seems reasonable to suggest that the same pollen can be more allergenic in contaminated urban areas than in rural ones. The aim of our study was to establish a possible difference in the allergenicity (in vivo and in vitro) and protein composition of different samples of Lolium perenne grass pollen, collected in two nearby rural and urban areas during two different pollination periods. If such difference exist, our study could have preventive and diagnosis implications. We also will try to speculate some possible causes to explain our findings. Obviously we cannot extrapolate the observations made in our area to other allergens in other areas, but a difference in allergenicity between rural and urban pollen in our zone might explain our previous epidemiological data (7).
METHODS
Collection of pollen samples
Spikes of Lolium perenne (around 1600 spikes) were gleaned during the peak pollination period of this plant in our area (May 1999). The spikes were shook and sieved and their pollen was collected, stored and kept refrigerated. This process was repeated the following pollen season (May 2000). About 400 mg of pollen was obtained in each case. We selected for the harvest in the two years the same ten contaminated areas of our city (Valladolid, Spain) and the same ten rural areas, 15 Km away from the city, without industries and other sources of atmospheric or pesticides contamination. Valladolid is a mild polluted city with 450.000 inhabitants, placed in a very important grass (cereal) crop area of our country with continental climate and with a high level of grass pollination during the month of May. The mean peak count concentration of Lolium perenne or rye grass pollen (the most prevalent grass in our area) in the last 6 years was 200 grains/m3 (data provided by Dr. Subiza, Aerobiology Comitee of the SEAIC) but with variability depending of preseasonal rainfall and average monthly humidity. In certain points of the city the pollution is high, due to traffic problems, with important levels of black smoke, SO2 and NO2 (data supplied by the Local Council Environment Department). These grasses are subjected to repeated reaping and pollution, and much of their spikes are cut before they flower. The rural areas selected were marked in a map in order to control the placement and sunshine average of the chosen plants in the two years. The urban and rural samples were labelled as A or B for 1999 and A' or B' for 2000. They were examined by a botanist in order to exclude the presence of other non-grass pollens. Contamination by mould spores was ruled out by microscopic observation and mould-culture of the samples. The samples from the first year were stored refrigerated in an airtight container until the second year in order to perform the "in vivo" and "in vitro" tests at the same time.
Pollen extraction
Lolium perenne extracts from the different pollen samples (A, A', B and B') were obtained after extracting 300 mg of pollen with 0.1 M NaHCO3 pH 8 at a 10 % w/v ratio for 90 minutes at 4 °C. After centrifugation (12,000 rpm for 30 minutes at 4 °C), the supernatant was dialysed against purified water and filtered through a 0.22 μm membrane. The protein concentration was determined by the Lowry method directly in the extract and in the protein pellet after precipitating the extracted proteins with 2.5 % phosphotungstic acid (PTA). All these extracts were 50 % glycerinated and stored at 20 °C until use.
"In vivo" quantitation of the biological activity of the extracts
The biological "in vivo" activity of the Lolium perenne extracts was measured in BU (Biological Units), according to previous studies (8). The different extracts used were standardised at 5 % (w/v). Skin prick tests (SPT) were carried out in 40 male patients suffering from grass pollen-induced rhinoconjunctivitis and wheal areas were measured by planimetry. We described "urban patient" as the patient coming from the city and "rural patient" as the individual coming from villages with less than 50,000 residents. We considered the social, environmental, genetic and biological variables that could act as confounders (age, sex, sensitisation to other allergens and change in place of living or working). Informed consents were obtained. Twenty patients were from rural areas and 20 from urban districts, with a mean age of 20 years (range 18-22), and a class 4 level of IgE antibodies to Lolium. Positive and negative controls were also tested (histamine 10 mg/mL and saline solution).
"In vitro" quantitation of the biological activity of the extracts
Paper disks were activated with BrCN and subsequently sensitised with a Lolium perenne pollen reference extract (ALK-Abelló, S.A. Madrid, Spain) according to Ceska et al (9). The disks were incubated with 50 mL of a pool of sera from grass pollen-allergic patients (1/4 dilution) and 50 μL of inhibiting extract (A, A', B, B' and the reference extract) at different protein concentrations, and then with 100,000 cpm of 125I-anti-IgE mAb HE-2, according to Sánchez-Madrid et al (10). The biological activity of extracts A, A', B and B', expressed in BU/mL, was determined by comparison of the inhibition curves obtained with the reference extract curve, which had been calibrated "in vivo" in BU/mL.
SDS-PAGE
Extracts A, A', B, B', the reference extract and the molecular weight markers (BioRad precision protein standards, Hercules, CA, USA) were separated by means of tricine- SDS-PAGE under reducing conditions (11). Precast 10-20 % polyacrylamide gels were used (Novex, San Diego, CA, USA). A volume of sample extract (5 % w/v) of 40 μl was applied except for extract B' in which 80 μl was applied.
Quantitation of the major allergen Lol p 5
The Lol p 5 content of extracts A, A', B and B' was determined by an ELISA technique based on specific monoclonal antibodies as indicated (12).
Statistical analysis
Data were analysed using the SPSS 7.5 programme. Paired t-tests were used to analyse the relationship between in vivo and in vitro parameters.
RESULTS
Morphological differences
Lolium perenne shrubs from rural and urban areas were similar during the year 1999, but more growth of the rural plant was observed in the year 2000. Nevertheless, microscopic alterations or differences among the various pollen samples collected were not observed.
Skin prick tests
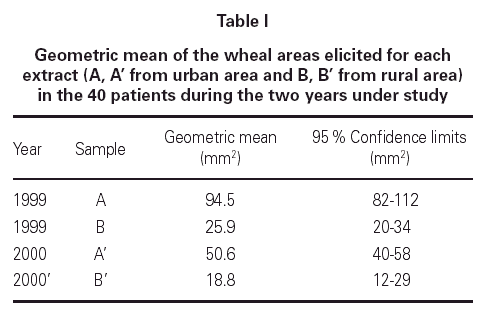
The geometric mean wheal areas elicited by the extracts from the years 1999 and 2000 can be seen in table I. Extracts A and A' correspond to Lolium pollen from urban areas and the extracts B and B' to the same species, but from a rural area. A significant difference in the skin reactivity to the extracts was demonstrated, with a greater response obtained to the pollen from the urban areas in the two years under study.
In vitro analysis
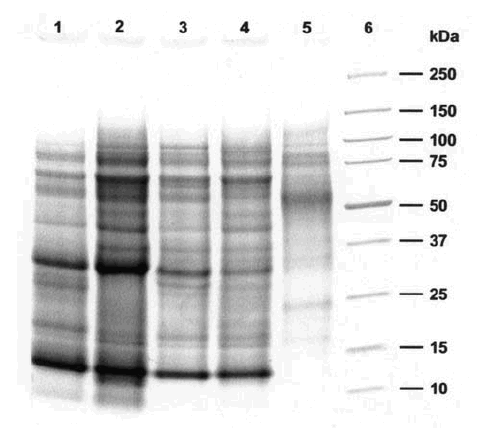
Figure 1 shows the SDS-PAGE analysis of the different pollen extracts. The samples of the year 1999 (A and B) showed minor differences compared between themselves and to the reference (lane 1). The major Lolium allergens (Lol p 1 and Lol p 5), with molecular weights between 30-35 kDa, correspond to bands located between the 25 and 37 markers. The SDS-PAGE analysis of the year 2000 samples showed that extract B' had a different profile compared with extract A' and with the year 1999 samples and reference. The protein bands of molecular weight lower than 50 kDa had a very low concentration (including the major allergens Lol p 1 and 5), and mainly higher molecular weight bands are observed, but their pattern is clearly different from the one observed with the rest of the extracts.
Figure 1.--Tricine-SDS-PAGE of extracts of the different pollen samples.
1:Lolium reference extract
2:Extract A, urban 1999
3:Extract B, rural 1999
4:Extract A', urban 2000
5:Extract B', rural 2000
6:Molecular weight markers
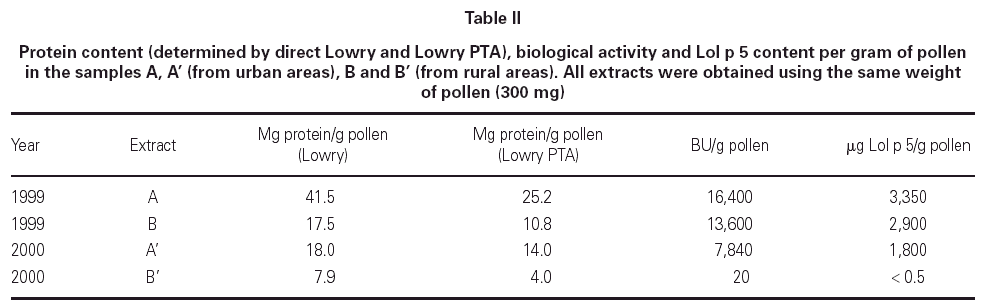
Table II shows the protein concentration, biological activity and Lol p 5 content of samples A, A', B and B'. The results are expressed per gram of pollen for comparison purposes. In the two years under study, samples A have approximately twice the protein content of samples B, as can be demonstrated by direct-Lowry and Lowry-PTA. In this last method, the extract protein is precipitated first with 2.5 % PTA (the same reagent used in the PNU determination), and then the pellet protein is solubilized and quantitated by Lowry. As a result, this method is more accurate because it gets rid of interfering substances, such as polyphenolic pigments present in pollen extracts. Regarding the biological activity, the differences observed in the year 1999 are not as important as in the year 2000, although sample A is 15 % more active than B. In contrast, samples from the year 2000 show that A' (urban) is considerably more active than B' (rural). The Lol p 5 content behaves in a similar way as the biological activity.
Statistical study
In the statistical study we compared the geometric mean areas of wheal obtained after SPT with the urban and rural extract in the two years under study (A: 94.5 and B: 25.9); (A': 50.6 with B': 18.8). The cutaneous response showed significant differences in the two years: p < 0.001, F: 3864.68 in 1999 and p < 0.005, F: 1189.69 in the year 2000. We also tried to found a significant correlation between changes in protein concentration and biological activity. A significant relationship was found between the protein concentration of each sample and the wheal area in skin-prick tests (0.959, p < 0.05), as well as between the protein concentration and BU/g pollen in the in vitro tests: 0.829, p < 0.05.
DISCUSSION
Allergic diseases are increasing both in prevalence and severity in most industrialised countries, and subjects living in urban areas are more likely to experience allergic respiratory symptoms, particularly those induced by pollen allergens, than subjects living in rural areas (13). Some studies suggest that pollen allergens can interact with components of air pollution. By adhering to the surface of allergenic pollen grains or paucitocromic allergen-carrying particles, air pollutants can act as adjuvants and enhance the immune response to pollen allergens (5). In addition, diesel exhaust emissions believed to stimulate IgE synthesis, thereby facilitating allergic sensitisation in predisposed subjects and the subsequent development of atopic respiratory diseases. Nevertheless, despite evidence of a correlation between respiratory pollen allergy and air pollution, the nature of this link is still a matter of speculation (13-17). Our study has demonstrated a higher "in vivo" allergenicity, as determined by skin-prick test, of the same species of pollen, collected in two different years, from an urban area with respect to that from a rural area. The "in vitro" studies point in the same direction. A lower protein content, allergenicity (as determined by RAST inhibition) and Lol p 5 content per gram of pollen was detected in the rural samples as compared with the urban ones. However, the effect is much more evident in the samples of the year 2000 than in those of 1999. According the results of our study the differences observed between wheal areas obtained in the year 1999 are higher and not related to comparable BU/g of pollen. In addition, these two extracts (1999 A and B) have comparable contents of Lol p 5. Nevertheless, during the year 2000, these differences in both parameters (wheal areas and BU/g of pollen and Lol p 5 content) are much more evident. During the year 1999 the difference in wheal areas may be justifiable by differences in protein concentration of the extracts, but in the year 2000 these differences are mostly accounted for the different biological activity and Lol p 5 content of each sample. In fact, the protein concentration of the urban sample of the year 2000 were approximately 1/2 of the concentration of the rural sample but the differences in BU/g of pollen and Lol p 5 content were of the order of 1/400. The pollution levels were similar during the years 1999 and 2000 in our city. This fact, together to the aforementioned results suggests that there was another uncontrolled variable that may have affected the protein concentration of pollens and suggest that exposure to pollutants does nor lead for itself to an increased allergen content. This difference might be explained by the climatic variation of these two years, with higher rainfall and wilder temperatures in the year 2000, which also led to the higher counts and peak concentration (240 grains/m3/24 h) of Lolium perenne in May-2000 compared with May 1999 (60 grains/m3/24 h). Another possible explanation is that the urban grasses might have been subjected to some damage in the year 2000 that not affected to rural wild grasses. This damage might have stimulated the expression of defense proteins. Different families of plant defense proteins (class I chitinases, LTPs, thaumatins and alpha-amylase inhibitors) have been recently described (18-20). These proteins are part to the plant's defense system, which includes molecules commonly located in the outer cell layer of the plant organs, and become overexpressed in response to attacks by pathogenic bacteria, fungi or pests. These defensive molecules have already been identified as important allergens, as demonstrated for pathogenesis related proteins expressed under stress conditions, which include Bet v 1, Cor a 1, Mal d 1, Api g 1 and others major allergens. An over expression of these defense proteins in pollen grains might explain the increase in the prevalence of allergic symptoms in urban areas.
In our previously reported work on the increase in the prevalence of grass pollen-induced asthma in the urban population (7), several confounding factors (indoor pollutants, sex, sensitisation to other allergens, diet) were taken into account, but the origin of our patients (urban/rural) seemed to act as an independent variable. In a recent survey, Nilsson et al. (17) support the existence of a period of increased susceptibility to allergic disease during the first years of life in urban children. Whether a rural lifestyle protects against allergy or whether urban pollutants are risk factors for the development of allergy is not clear from the above studies.
We suggest that pollen allergens, besides being potentated by air pollutants, could themselves trigger exacerbations of allergic respiratory diseases due to modifications in their allergenicity. The sensitisation power of certain food vegetables can change by over expression of defense proteins (21). Likewise, air pollution, repeated reaping and fumigation, or other unknown factors not controlled in our study, might modify the pollen from urban areas. In line with our suggestion, Thomas et al (22) studied the effect of a 4-h exposure to 50-200 p.p.b. nitrogen dioxide on the viability, germination and protein release from freshly collected birch, rye, alder and hazel pollen grains. They showed that exposure to 100 p.p.b. nitrogen dioxide modulated the protein content of pollen grains. Recent findings of D'Amato et al. (5), using RAST inhibition, have demonstrated that exhaust emission from non-catalytic cars increase the allergenic potency of Parietaria pollen. In addition, previous studies demonstrated that the major allergens Phl p Vb in timothy grass is a pollen RNase, considered as a stress protein (23), and increases of group 5 allergen content of Lolium perenne by effect of ozone have also found (24). In the same way, we found an increase in Lol p5 (25) that raises the possibility that an overexpression of these allergens could be the cause of the major sensitisation power of urban pollen. To prove that plant from urban areas express more stress proteins due to air pollution we should extend the investigated allergen spectrum to other major allergens and investigate the induction of these defense proteins at the mRNA level, studies that exceed the scope of our present work.
In summary, even though it is possible that urban air pollution plays a role in the onset and in the increasing prevalence of allergic respiratory diseases, other causes should not be discarded. Although the observed different allergenicity between the urban and rural pollen in our study may not be indicative of the general urban and rural situation in other countries, our work may be considered as a pilot study for a full research project in other areas and with other pollen species. Furthermore, the difference in protein concentration and biological activity of the same pollen in successive years might be kept in mind for preventive measures, diagnosis and specific immunotherapy.
ACKNOWLEDGEMENTS
We thank Dr. Javier Subiza for their advice and comments, and all members of Allergy Section of Rio Hortega Hospital for their help.
BU: Biological units
OR: Odds ratio
SDS-PAGE: Sodium dodecyl sulfate-polyacrylamide gel electrophoresis