INTRODUCTION
At present no single diagnostic test exists for bronchial asthma. The evaluation of asthma severity relies on multiple observations including lung symptoms and function measurement. Other measures of disease activity may be of great value but are more time-consuming and cannot be performed in routine clinical practice at the moment, especially in young children. Such measurements include enumeration of sputum eosinophils, inhalation challenge, bronchoscopy with bronchoalveolar lavage, and bronchial biopsies. Knowledge about the pathophysiological processes in asthma gives the possibility to analyze and quantitate cells or products involved in the pathology and follow changes as a reflection of bronchial disease process.
During recent years, the importance of eosinophil inflammation in the pathogenesis of asthma has been clearly established (1, 2). Activated eosinophils release granule proteins: eosinophil protein X (EPX) and the eosinophil cationic protein (ECP), which is cytotoxic to the airway epithelium (2). Serum ECP and EPX has been measured to monitor disease activity in bronchopulmonary obstruction (3-7), and recently urinary eosinophil protein X (U-EPX) has been suggested as a marker of inflammation in children with asthma (8-14). EPX is the only basic eosinophil granule protein that can be accurately measured in the urine (15), and U-EPX has been shown to correlate both with blood and bronchoalveolar eosinophil cells counts (16). Urine sampling is sample, non-invasive, and pain-less, and measurements of U-EPX may therefore be of particular value for supporting the diagnosis and monitoring disease activity in children.
The aim of this work is to evaluate the importance of eosinophil inflammation in asthmatic children and to study the value of the U-EPX in the prediction of the severity and the activity of asthma in children.
SUBJECTS AND METHODS
Data collection
96 non-atopic asthmatic children with an acute exacerbation requiring hospital admission were recruited from the outpatient and the emergency department between January 1996 and July 1998. The diagnosis of asthma was based on a history of recurrent episodes of wheezing and on the physical examination. Patients who has received drugs that might affect the U-EPX concentration (e. g. systemic or inhaled corticosteroids, cromolyn sodium or any oral antihistaminic drugs) during the 2 weeks prior to enrolment in the study were excluded (17, 18). 80 patients completed the study, 6 were discharged at their request before the complete recovery of the acute attack and 10 were lost to follow-up.
Control group
Twenty-five healthy children of matching age and sex referred to our out-patient clinic and who had no history of wheezing or any other recurrent or chronic airway disease were recruited as a control group. These children had neither a parenteral history of atopy or asthma nor a history of atopic dermatitis, food allergy or allergic rhinoconjunctivitis. Informed consent to participate in the study was obtained from the parent or guardian of each child.
Clinical assessment
A detailed medical history was recorded and complete clinical examination was conducted for all the patients. Before any therapeutic intervention was given, the asthmatic patients were classified as having mild (21 cases), moderate (35 cases) or severe (24 cases) attack of asthma according to an asthma score (19, 20). The asthma score used included both clinical parameters (respiratory rate, alertness, dyspnea, accessory muscle use, skin color, auscultatory findings) and physiological parameters (peak expiratory flow rate, oxygen saturation) (19-21). The asthmatic patients had plain X-ray of the chest done to exclude other potential causes of wheezing. Oxygen saturation was determined using a pulse oximeter and peak expiratory flow rate (PEFR) was measured using a Wright flowmeter and was expressed as the percentage of the predicted normal value for the sex and the height of the patient using a previously reported standard (22).
Management and follow-up
All the asthmatic children were managed according to the asthma protocol used in the hospital, i.e. 2 cc of nebulized salbutamol solution (0.01 %) for mild cases and nebulized salbutamol plus systemic corticosteroids (Solumedrol 2 mg/kg/dose every 8 hours) for moderate and severe cases. Patients were discharged from the hospital when they fulfilled the following improvement criteria: no respiratory distress, no tachypnea, oxygen saturation greater than 95 % and PEFR greater than 80 % of expected.
We hypothesized that for a test to be clinically useful, it must distinguish between symptomatic and asymptomatic asthma and must normalize as the patient's condition comes under control. Accordingly, we repeated the investigations 2 weeks after the patient's acute attack resolved. At follow-up, none of the patients had any asthma symptoms, their oxygen saturation and PEFR as a percentage of predicted were comparable to normal. No patient, either before or after enrolment in the study, was receiving maintenance therapy.
U-EPX DETERMINATION
Urine from the children with asthma was collected within 4h of admission and stored in a refrigerator until aliquoting and freezing (20 °C) within 10 h of sampling; controls had a urine sample taken in the morning, which was frozen within 8 h of sampling (14). U-EPX was analyzed by a specific radioimmunoassay (Pharmacia, Uppsala, Sweden).The detection limit of the assay was < 3μg/l, the within-assay coefficient of variation was < 5 %, and the between-assay coefficient 250 system (Ortho Clinical Diagnostics, Rochester, USA). All measurements were carried out in duplicate. Considering the children's renal function, the EPX concentrations were referred to the urine creatinine concentration and expressed as μg/mmol creatinine.
Counting of blood Eosinophils
Blood samples from every child were carefully obtained (vacutainer system) before any therapeutic intervention. The number of eosinophils in blood samples with EDTA was determined with (Max M Coulter STKS; Coulter Corp., Miami, USA) hematology analyzer.
Statistical analysis
Statistical significance was determined using the student t-test, F-test (ANOVA), Chi-squared test (χ2) and correlation coefficient (r). Statistical significance was attained when the p-value was less than 0.05.
RESULTS
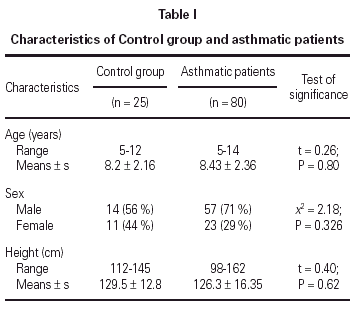
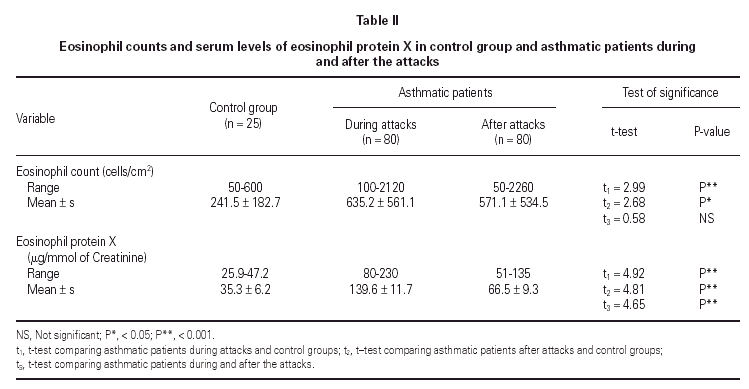
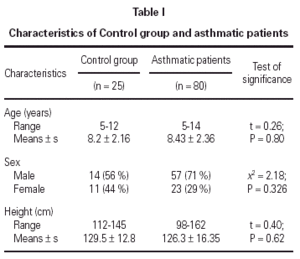
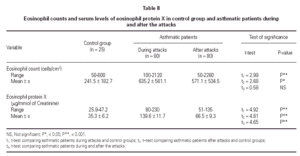
No statistically significant difference was found between the asthmatic patients and the control group with regard to age, sex, and height (table I). The mean eosinophil counts were significantly higher for the asthmatic patients, both during and 2 weeks after their attack, when compared to the control children. However, no statistically significant difference was found within the asthmatic patients group between the counts during and after the attacks (table II).
The mean U-EPX concentration was significantly higher in asthmatic patients, both during and after their attacks when compared with the control group. Moreover, the mean EPX concentration in asthmatic patients was significantly higher during the asthma attacks than after (table II).
Asthmatic subgroups during asthma attacks
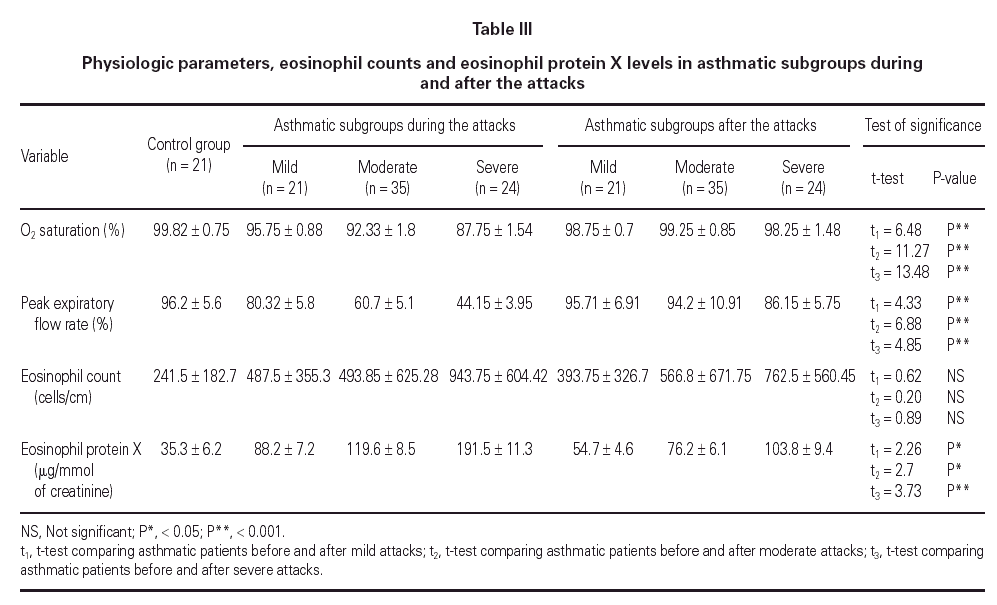
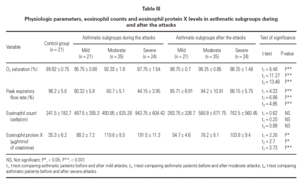
Mean oxygen saturation and PEFR were significantly different between the asthmatic children and the control group, as well as within the asthmatic group itself (F = 194.74, P < 0.001; F = 224.78, P < 0.001). As expected, the lowest values were recorded in the severe attack subgroup and were normal in the control group.
The mean eosinophil count was significantly higher only in patients having severe attacks of asthma as compared with the control group (P < 0.05). No statistically significant difference was found between the eosinophil counts of patients with mild or moderate attack when compared with the control group.
The mean U-EPX concentration was significantly higher in each of the asthmatic subgroups when compared with the control group. Furthermore, the level was significantly higher in asthmatic patients with severe asthma attacks when compared with those with mild and moderate attacks (P < 0.01). However, no significant difference was found between mean U-EPX concentrations in patients with mild attacks when compared with those with moderate attacks.
Asthmatic subgroups after asthma attacks
Oxygen saturation and PEFR increased significantly for all asthmatic subgroups 2 weeks after resolution of the attack. No statistically significant difference was found between patients after resolution of the asthma attack and the control group with regard to oxygen saturation and PEFR (table III). At follow-up, the mean eosinophil count was significantly higher in asthmatic patients who had a severe attack when compared with the control group (P < 0.01) but no statistically significant difference was found for the other 2 subgroups. Furthermore, the eosinophil counts for each subgroup of patients did not differ during the attack and at the follow-up visit.
The U-EPX concentrations showed no statistically significant difference between the control group and the asthmatic patients after resolution of the mild or moderate attack. There was also a significant difference in levels in the patients with the severe attack at the follow-up when compared both to normal patients (p < 0.001) and to the patients who had mild (P < 0.001) and moderate (P < 0.05) attacks. The EPX concentrations decreased significantly in all asthmatic subgroups after resolution of the attack when compared with the levels during the attack (table III).
Correlation between U-EPX concentrations and other parameters
No statistically significant correlation could be found between U-EPX concentrations, oxygen saturation (r = 0.311; P = 0.094), PEFR (r = 0.390; P = 0.09), eosinophil count (r = 0.065, P = 0.790) or age (r = 0.198; P = 0.365). In addition, no statistically significant differences were found between U-EPX concentrations in males when compared to females, both in the control group (t = 0.7, P = 0.6) and in the asthmatic group (t = 0.21; P = 0.78).
DISCUSSION
Bronchial asthma is a chronic inflammatory disease of the airway, and eosinophils are the main cell responsible for causing this inflammation (23-25). Eosinophil airway inflammation is one of the hallmarks of asthma (26), and markers of eosinophil activation have therefore been studied as possible predictors of asthma in children (27, 28). EPX is easily measured in the urine (15), and the concentration of U-EPX is thought to reflect eosinophil activation in the airways of asthmatic children (9, 10, 29).
The present study demonstrated higher levels of U-EPX in children with nonatopic asthma than in controls, consistent with other studies (9, 29). In a recent study, U-EPX concentrations are higher in atopic than in nonatopic asthmatic children (29). High concentrations of U-EPX have also been demonstrated in children with atopic dermatitis (30, 31).However, in the present study the children with asthma had no clinical signs of atopic dermatitis or any other allergic disease at the time of inclusion, suggesting that high concentrations of U-EPX reflect eosinophil activation in the airways of these children.
Our study showed that the U-EPX concentration was significantly elevated for all the asthmatic children, both during and 2 weeks after the acute attack. Moreover, the U-EPX concentration for all the asthmatic children during the acute exacerbation was significantly higher than after the resolution of the attack 2 weeks later, as reported in previous studies (12, 32).
These results suggest that in severe asthma cases, despite clinical improvement, the inflammatory process is ongoing, supporting the current recommendations for prolonged, intensive anti-inflammatory treatment of severe asthmatics (33). Furthermore, other investigators have reported a decrease in U-EPX after treatment of asthmatic children with inhaled corticosteroids suggesting that the concentration of U-EPX at least partly reflects bronchial airway inflammation (10, 33).Therefore, U-EPX concentrations would not only help determines the severity of the ongoing inflammation but could also allow the monitoring of therapy adequacy (10).
In accordance with other studies, our results showed no age or sex-related differences in U-EPX concentrations (9, 34). No significant correlation was found between the U-EPX concentration and the physiological parameters used in our assessment of asthma severity, namely oxygen saturation and PEFR.
Contradictory results have been reported regarding the relationship between pulmonary function tests, such as FEVI and PEFR, and U-EPX concentrations. A negative correlation between U-EPX concentrations and pulmonary function has been reported in asthmatic children by Reichenberg (34). Our results, however, agree with data from Niggemann et al (35) and Oymar and Bjerknes (29) who could not find a significant correlation between U-EPX and either airway hypersensitivity or pulmonary function. The lack of a correlation may be explained by the fact that the kinetics of changes in lung function may differ from those of changes in inflammatory parameters.
Blood eosinophil counts have been viewed for several decades as a valuable tool for indicating disease severity, possibly because they reflect the degree and extent of inflammation in the asthmatic lung (23). Studies have reported a correlation between the number of blood eosinophils and the severity of asthma (36-38). In the clinical management of patients with asthma, elevated blood eosinophil counts was considered a risk factor, indicating exacerbation of the disease (36, 39-41).
In our study, the mean eosinophil count was significantly higher for asthmatic patients both during and after the acute attacks when compared to a control group. Nevertheless, when the asthmatic subgroups were analyzed, there was no difference between the eosinophil counts of patients with mild or moderate attacks when compared to the control group. The difference reached statistical significance only when the attack was severe. Furthermore, no significant difference was found between counts during the attack and at the follow-up visit. The eosinophil count thus cannot be used as a blood marker of disease activity in childhood bronchial asthma.
A more refined way of measuring the disease activity is thus not measuring the absolute number of cells participating in the inflammatory process, but the degree of activation of these cells by using the U-EPX concentrations (39).
CONCLUSION
This study showed high concentration of U-EPX in asthmatic children, especially during acute exacerbation. In addition, our data suggests that the measurement of U-EPX concentration may be useful in quantifying bronchial inflammation. This result can further be used as a marker of severity of the disease exacerbation and it would not only facilitate early diagnosis and staging of inflammatory and allergic disorders but it would also allow the monitoring of therapy and intervention.