To analyse specific immune response to the 23-valent pneumococcal polysaccharide vaccine by measuring pneumococcal antibodies in children with asthma and with respiratory recurrent infection (RRI) as compared to healthy children.
MethodsThe study included 60 children, divided into three groups: 20 with asthma, 20 with RRI, and 20 healthy controls. Post-vaccination specific IgG antibodies against 10 pneumococcal serotypes (S1, S3, S4, S5, S6B, S9V, S14, S18C, S19F, and S23F) contained in the 23-valent pneumococcal polysaccharide vaccine (PPV) were measured. A specific IgG concentration ≥1.3μg/mL was considered a protective response to the vaccine. For statistical analysis, levels of specific IgG antibodies against each of the 10 pneumococcal serotypes were compared across the three groups of children using the x2 test.
ResultsAll of the children showed antipneumococcal antibody levels >1.3μg/mL for over 70% of the serotypes, considered within the normal range of response. Average IgG antibody levels and percentages of children protected were statistically comparable among the three groups studied.
ConclusionThe asthmatic children without RRI had pneumococcal antibody levels and percentages of serotype-specific protection to PPV comparable to those of healthy children. Asthmatic children with recurrent infections should be evaluated for specific antibody deficiency (SAD). Because asthma patients are at high risk for invasive pneumococcal infections, it would be worthwhile to explore systematic administration of PPV in children over the age of two years who have not received a pneumococcal conjugate vaccine, considering the positive response to PPV reported here.
In subjects with recurrent respiratory infections older than two years and with normal serum immunoglobulins, response to the pneumococcal polysaccharide vaccine (PPV) is used as diagnostic test for selective antibody deficiency (SAD).1 In a prior study, we found a strong association between asthma and this primary immunodeficiency (PID).2
Certain disorders involve elevated risk for invasive pneumococcal disease, due to deficient immunological response to encapsulated agents. Groups at greatest risk include children under the age of two years, sickle cell anaemia carriers, patients with functional or surgical asplenia, and patients with primary or secondary immunodeficiencies. Other high-risk groups are patients with diabetes, with nephropathy, with chronic heart disease, with chronic pulmonary damage, and others over the age of two years who are recommended to receive the 23-valent PPV.3 Children with asthma are also at high risk for invasive pneumococcal infections.4 Asthma is one of the most common chronic diseases in children, affecting from 1% to 25% of various populations studied, and its prevalence seems to be rising.5 In Chile, studies have reported a prevalence of 7% in a population of children aged 6–7 years and 23% in children aged 13–14 years.6
It has been found that patients with asthma have a 2.4-fold greater risk of developing an invasive pneumococcal infection than their healthy counterparts.4 Even those with mild asthma have a risk 1.7 times higher than that of the general population, while those with severe asthma have a risk 2.6 times higher.4,7
Children with asthma are at particularly elevated risk for invasive pneumococcal infections when the clinical presentation of the asthma is severe or requires corticosteroid treatment.4 Studies of patients with severe pneumococcal infections confirm that asthma patients are at higher risk for pneumonia and positive Streptococcus pneumoniae sputum culture.8 Children with asthma develop pneumonia at a rate higher than that of the general population. According to a U.S. study of over 500 children under the age of 18 years, asthma patients acquire pneumonia more frequently than children without known risk factors.9 Asthmatic children treated with inhaled corticosteroids also have higher rates of oropharyngeal colonisation with S. pneumoniae.10
In many reports, approximately 50% of primary immunodeficiencies are antibody deficiencies,11 and more than 10% of children with recurrent respiratory infections have selective antibody deficiency (SAD).12,13 The frequency of this condition in asthmatic children has not been established, but it is noteworthy that a large proportion of children with recurrent respiratory infections are asthma patients. Associations between asthma and allergic illnesses with other types of primary immunodeficiencies have been reported.14
The objective of this study was to assess the specific immune response measured through pneumococcal antibody levels, to administration of 23-valent polysaccharide pneumococcal vaccine (PPV) in asthmatic children older than two years without recurrent respiratory infections and normal immunoglobulin concentrations, and to compare the response to PPV in children with respiratory recurrent infections without immunodeficiency, and in healthy children.
Patients and methodsProspective, controlled clinical study of 60 children divided in three groups from an urban area (Santiago, Chile):
Group A: Twenty patients between 2 and 15 years old, random included, with a diagnosis of GINA-defined mild-to-moderate, well-controlled asthma, without history of recurrent respiratory infections, selected from a primary care office.
Group B: Twenty patients between 2 and 15 years old, with a history of recurrent respiratory infections, selected from the Immunology Unit of Dr. Exequiel Gonzalez Cortes Hospital (Santiago, Chile), according to at least one of the following criteria: six or more episodes per year of respiratory infection; one or more episodes per month of respiratory infection during the winter period; or three or more lower respiratory tract infections.15 Children were excluded if there was another cause of recurrent respiratory infection, such as immunodeficiency, local anatomical damage resulting in localised pneumonia, or concomitant neutropenia.
Group C: Control group of 20 children between 2 and 15 years old, with no history of pathology, selected randomly from the same primary care office as Group A.
Parents of all participating children provided written informed consent, and the study was approved by the Ethics Committee of the University of Chile.
Patients were excluded for previously-diagnosed primary or secondary immunodeficiency; local anatomical defect (cause of recurrent localised pneumonia); concomitant neutropenia; pathology such as cystic fibrosis, nephropathy, cardiac disease, chronic respiratory disease, sickle-cell anaemia, functional or surgical asplenia, or diabetes; or inability to obtain the informed consent of parents.
Baseline immunological studies were performed for serum IgG, IgM, and IgA, complement C3, and IgG subclasses, as well as haemogram with absolute lymphocyte and neutrophil count. All children included had normal immunoglobulin concentrations and normal immunological laboratory parameters in order to excluding hypogammaglobulinaemia and other primary and secondary immunodeficiencies.
All children received the 23-valent PPV (Pneumo 23®, Sanofi Pasteur), containing 25μg of each of the 23 polysaccharides included in the formulation. The IgG-class anti-polysaccharide pneumococcal antibody levels for 10 serotypes (S1, S3, S4, S5, S6B, S9V, S14, S18C, S19F, and S23F) were measured using third-generation ELISA techniques as standardised by the Centers for Disease Control (CDC),16 at study enrolment (T1) and 45–60 days post-vaccination (T2). Antibody concentration equal to or greater than 1.3μg/mL of specific IgG for each serotype was considered a normal protective response.
For statistical analysis, serological response was measured as the concentration of specific IgG antibody against each of the 10 pneumococcal serotypes in the three groups (children with asthma, children with recurrent respiratory infections, and healthy children), using x2 for the geometric mean titres expressed in micrograms per ml of blood in each group, for each serotype, at two times: at study enrolment (T1) and at 45–60 days post-vaccination (T2).
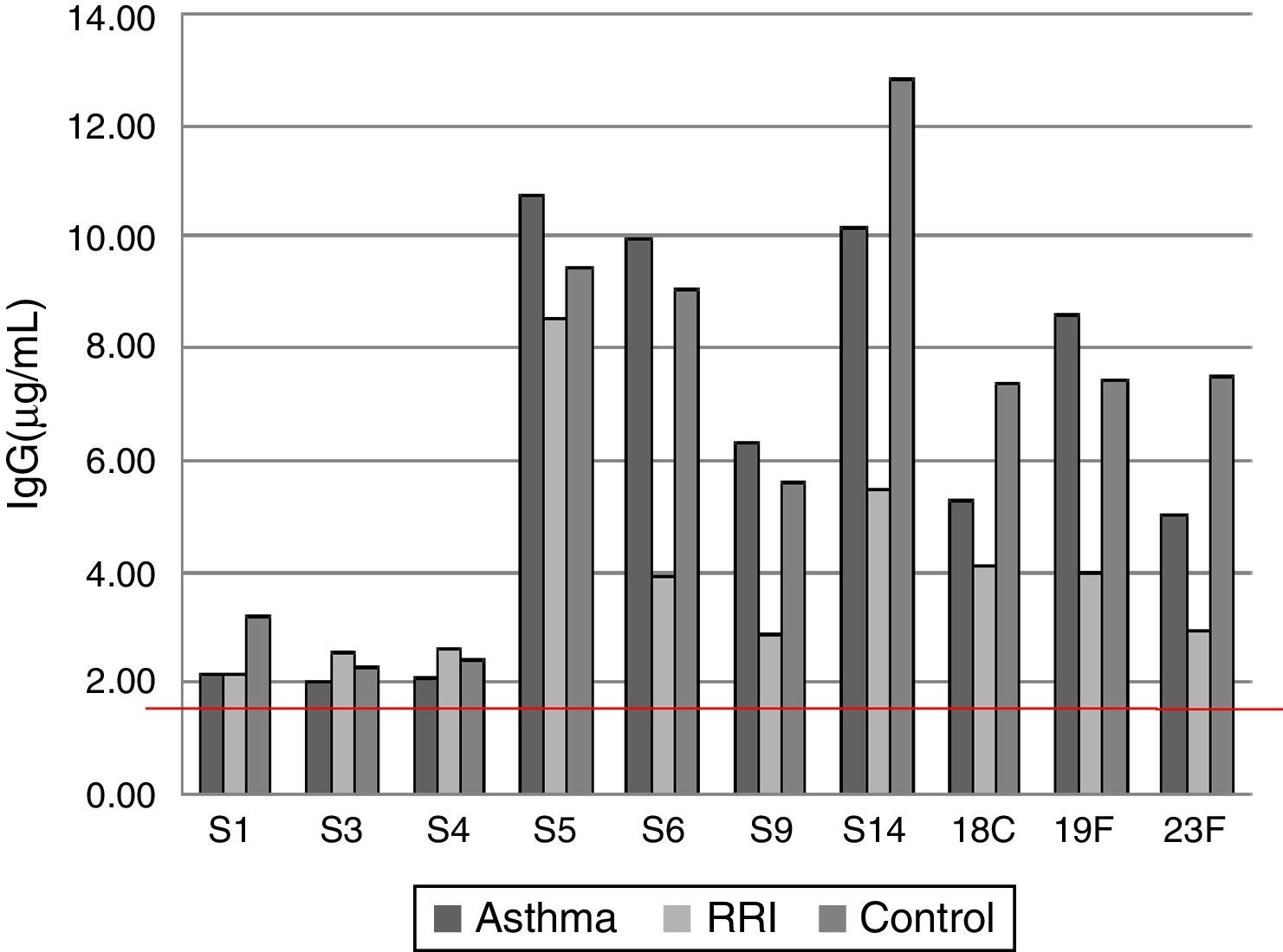
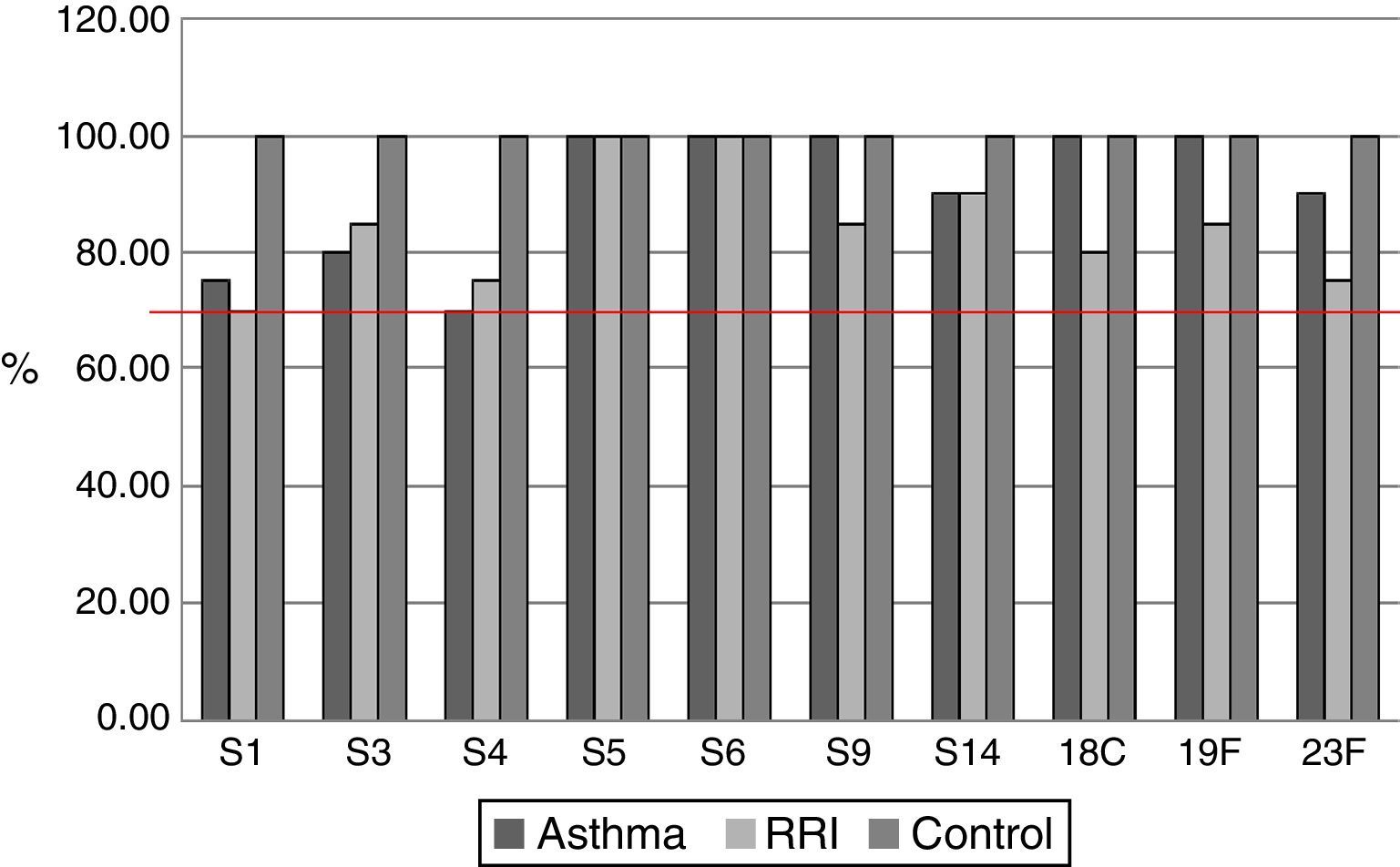
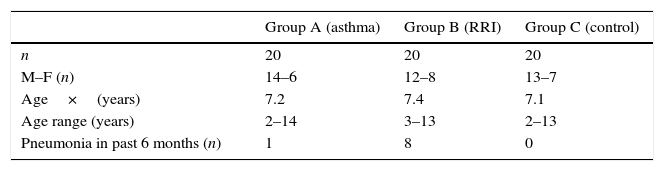
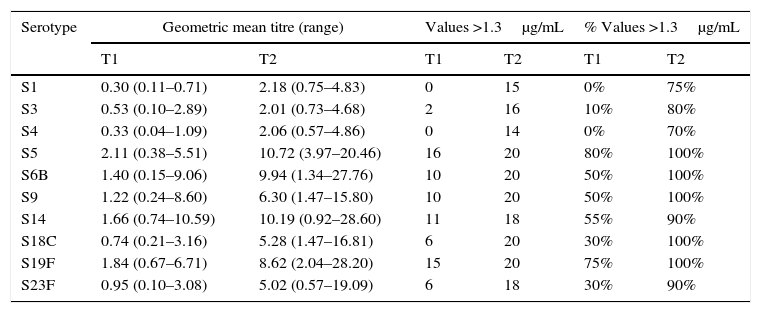
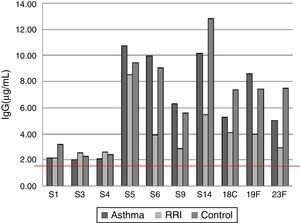
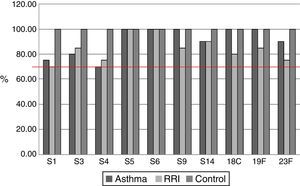
ResultsOf the 20 children with asthma (group A), 14 were male, and the age range was 2–14 years. One patient had an episode of pneumonia during the six months prior to vaccination (Table 1). In this group of children, 15/20 children responded with levels above 1.3μg/mL for the 10 serotypes tested, and the average values for four serotypes, S5, S6B, S14, and S19F, were greater than 1.3μg/mL before vaccination, with levels rising five- to seven-fold after vaccination (Table 2). All serotypes reached geometric mean titres above 1.3μg/mL by day 45–60 post-vaccination, with levels ranging from 2.01 (S3) to 10.72μg/mL (S5); an adequate protective response was observed in 70% to 100% of subjects (Figs. 1 and 2). The lowest individual value observed pre-vaccination was 0.04μg/mL, for S4, and the highest was 10.59μg/mL, for S14. After vaccination, individual levels varied from 0.57μg/mL, for S4 and S23F, to 28.6μg/mL, for S14 (Table 2).
Specific IgG antibody response against 10 pneumococcal serotypes in children with asthma.
| Serotype | Geometric mean titre (range) | Values >1.3μg/mL | % Values >1.3μg/mL | |||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| S1 | 0.30 (0.11–0.71) | 2.18 (0.75–4.83) | 0 | 15 | 0% | 75% |
| S3 | 0.53 (0.10–2.89) | 2.01 (0.73–4.68) | 2 | 16 | 10% | 80% |
| S4 | 0.33 (0.04–1.09) | 2.06 (0.57–4.86) | 0 | 14 | 0% | 70% |
| S5 | 2.11 (0.38–5.51) | 10.72 (3.97–20.46) | 16 | 20 | 80% | 100% |
| S6B | 1.40 (0.15–9.06) | 9.94 (1.34–27.76) | 10 | 20 | 50% | 100% |
| S9 | 1.22 (0.24–8.60) | 6.30 (1.47–15.80) | 10 | 20 | 50% | 100% |
| S14 | 1.66 (0.74–10.59) | 10.19 (0.92–28.60) | 11 | 18 | 55% | 90% |
| S18C | 0.74 (0.21–3.16) | 5.28 (1.47–16.81) | 6 | 20 | 30% | 100% |
| S19F | 1.84 (0.67–6.71) | 8.62 (2.04–28.20) | 15 | 20 | 75% | 100% |
| S23F | 0.95 (0.10–3.08) | 5.02 (0.57–19.09) | 6 | 18 | 30% | 90% |
T1 – prevaccination; T2 – postvaccination.
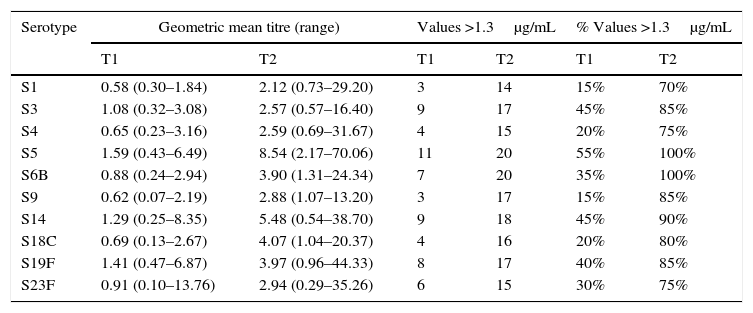
Of the 20 children with RRI (group B), 12 were male, and the age range was 3–13 years. Eight patients had episodes of pneumonia during the six months prior to vaccination, three of whom had two episodes (Table 1). All of the children in this group showed a normal response at 45–60 days post-vaccination, and 14/20 children reached levels above 1.3μg/mL for the 10 serotypes measured. At 45–60 days, the geometric mean titres of the IgG antibodies against all 10 serotypes studied were above 1.3μg/mL, ranging from 2.12 (S1) to 8.54 (S5)μg/mL. An adequate protective response was observed for 70–100% of serotypes (Table 3).
Specific IgG antibody response against 10 pneumococcal serotypes in children with recurrent respiratory infections.
| Serotype | Geometric mean titre (range) | Values >1.3μg/mL | % Values >1.3μg/mL | |||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| S1 | 0.58 (0.30–1.84) | 2.12 (0.73–29.20) | 3 | 14 | 15% | 70% |
| S3 | 1.08 (0.32–3.08) | 2.57 (0.57–16.40) | 9 | 17 | 45% | 85% |
| S4 | 0.65 (0.23–3.16) | 2.59 (0.69–31.67) | 4 | 15 | 20% | 75% |
| S5 | 1.59 (0.43–6.49) | 8.54 (2.17–70.06) | 11 | 20 | 55% | 100% |
| S6B | 0.88 (0.24–2.94) | 3.90 (1.31–24.34) | 7 | 20 | 35% | 100% |
| S9 | 0.62 (0.07–2.19) | 2.88 (1.07–13.20) | 3 | 17 | 15% | 85% |
| S14 | 1.29 (0.25–8.35) | 5.48 (0.54–38.70) | 9 | 18 | 45% | 90% |
| S18C | 0.69 (0.13–2.67) | 4.07 (1.04–20.37) | 4 | 16 | 20% | 80% |
| S19F | 1.41 (0.47–6.87) | 3.97 (0.96–44.33) | 8 | 17 | 40% | 85% |
| S23F | 0.91 (0.10–13.76) | 2.94 (0.29–35.26) | 6 | 15 | 30% | 75% |
T1 – prevaccination; T2 – postvaccination.
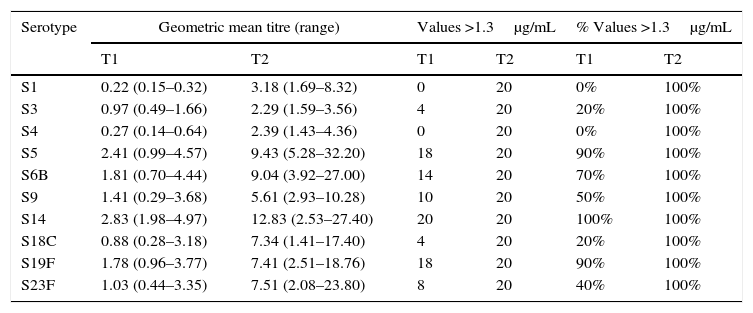
Of the 20 healthy children (group C), 13 were male, and the age range was 2–13 years. No child in this group had a pneumonia episode during the six months prior to vaccination (Table 1). Serotypes with pre-vaccine levels above 1.3μg/mL were S5, S6B, S9, S14, and S19F, with levels rising four-fold after vaccination. All children showed an adequate protective response to the vaccine, with 100% showing values above 1.3μg/mL for all 10 serotypes. Geometric mean titres ranged from 2.29 for S3 to 12.83μg/mL for S14 (Table 4).
Specific IgG antibody response against 10 pneumococcal serotypes in healthy children.
| Serotype | Geometric mean titre (range) | Values >1.3μg/mL | % Values >1.3μg/mL | |||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| S1 | 0.22 (0.15–0.32) | 3.18 (1.69–8.32) | 0 | 20 | 0% | 100% |
| S3 | 0.97 (0.49–1.66) | 2.29 (1.59–3.56) | 4 | 20 | 20% | 100% |
| S4 | 0.27 (0.14–0.64) | 2.39 (1.43–4.36) | 0 | 20 | 0% | 100% |
| S5 | 2.41 (0.99–4.57) | 9.43 (5.28–32.20) | 18 | 20 | 90% | 100% |
| S6B | 1.81 (0.70–4.44) | 9.04 (3.92–27.00) | 14 | 20 | 70% | 100% |
| S9 | 1.41 (0.29–3.68) | 5.61 (2.93–10.28) | 10 | 20 | 50% | 100% |
| S14 | 2.83 (1.98–4.97) | 12.83 (2.53–27.40) | 20 | 20 | 100% | 100% |
| S18C | 0.88 (0.28–3.18) | 7.34 (1.41–17.40) | 4 | 20 | 20% | 100% |
| S19F | 1.78 (0.96–3.77) | 7.41 (2.51–18.76) | 18 | 20 | 90% | 100% |
| S23F | 1.03 (0.44–3.35) | 7.51 (2.08–23.80) | 8 | 20 | 40% | 100% |
T1 – prevaccination; T2 – postvaccination.
The geometric mean titres of IgG antibodies against serotypes S6B, S9, S14, S18C, S19F, and S23F were lower in children with RRI versus controls, but there were no statistically significant differences between groups in the percentage of children who reached protective levels above 1.3μg/mL.
DiscussionResponse to the pneumococcal polysaccharide vaccine, expressed as the geometric mean titres of the IgG antibodies and the percentage of children who showed an adequate protective response, was similar across the three groups studied. Given the high percentages of children in all three groups with antibody levels over 1.3μg/mL after vaccination for the various serotypes analysed, with no significant differences between the groups of children with asthma and those with recurrent infections, it can be inferred that the immunological response is independent of the baseline pathologies studied in this series of patients.
It is interesting that the pre-vaccine titres for serotypes 5 and 14 were above 1.3μg/mL and then showed the robust increases after vaccination. It is possible that the patients had been exposed to these serotypes, as carriers or during a prior infection. Epidemiological studies in developing South American countries have shown that the serotypes responsible for the most prevalent infections in children are S14, S5, S6A/B, and S1.17
We recently published a report in which we found a relationship between asthma and selective antibody deficiency2; however, in the present study, we excluded children with primary or secondary abnormalities in immune antibody response using the available methodology. It would be interesting to perform a follow-up study with repeated measurements of antibody levels, to evaluate the duration of protection conferred by the vaccine.
In terms of responses to the different serotypes contained in the pneumococcal vaccine, studies have reported variations in asthmatic children. For instance, after the pneumococcal 7-valent conjugate vaccine, protection (defined as a geometric mean titre of specific IgG antibodies above 1μg/mL) varied from 40.9%, for serotype 6B, to 90.9%, for serotype 19F. After the polysaccharide vaccine, protective responses ranged from 31.6%, for serotypes 6B and 23F, to 81.6%, for serotype 19F.18
In children of asthmatic mothers, higher rates of nasopharyngeal colonisation with S. pneumoniae have been found beginning in the new-born period,19 and children with other atopic conditions such as dermatitis show a deficient antibody response to S. pneumoniae after the PPV.20
Preschool-aged children with persistent wheezing in the absence of acute infectious symptoms show elevated bacterial counts in bronchoalveolar lavage fluid and high rates of positive culture for bacterial agents such as S. pneumoniae; after antibiotic treatment, obstructive symptoms improve, suggesting that chronic bacterial infection may underlie the persistent wheezing.21
Patients with asthma are not presently included among the risk groups recommended to receive the pneumonia vaccine, unless they are immunosuppressed or their symptoms are severe enough to warrant chronic or frequent treatment with systemic corticosteroids.22
A systematic Cochrane review evaluating use of the PPV in children with asthma updated in 2015 concluded that there was limited evidence supporting routine use of the vaccine, and that randomised trials were needed to confirm its efficacy in this group.23
Asthma is not currently included on the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention list of indications for receiving the pneumococcal vaccine. A recent recommendation proposes that children over the age of six years who have not received the conjugate pneumococcal vaccine and who are at high risk for invasive pneumococcal infection should be given a dose of the 13-valent conjugate vaccine followed by the 23-valent polysaccharide vaccine after eight weeks, and then revaccinated with the PPV five years later.24
Studies on response to the polysaccharide vaccine in children with recurrent infections have provided dissimilar results. In general, immunogenicity seems to improve with age.16 It is has been impossible to establish definitive conclusions, due to the use of different vaccination schedules, for example, the combination of the PPV with the conjugate vaccine versus separate administration, and the use of heterogeneous patient groups, for example, the inclusion of individuals with and without immunosuppressive conditions.25 In the present study, we excluded for immunodeficiencies in the group with recurrent infections, and the children studied had not previously received either the conjugate or the polysaccharide vaccine.
Different parameters have been used as the cut-off for a protective response to the polysaccharide vaccine.26 In this study, we used specific IgG serum concentration above 1.3μg/mL for each serotype in order to rule out children with recurrent infections and specific antibody deficiency.16
Immune response to the PPV depends on various factors such as age, baseline disease, and immunogenicity of the various serotypes; however, the advantages of the vaccine are greater than the potential risks.25 Various studies in healthy children on the immunogenicity of the PPV have noted more robust response in poorest low incomes areas and countries27; differences in response according to age for the various serotypes; and poor specific IgG antibody response for S14, S6B, and S19F before 24 months of age.28 One study reported a significant, four-fold increase in specific IgG levels at two weeks post-vaccination for 18 of the 23 serotypes in children who received the vaccine at 12 months of age, with poorest response for S14, S19F, and S23F.29
In conclusion, asthmatic children without RRI responded with pneumococcal antibodies and percentages of serotype-specific protection comparable to those of healthy children. The children with RRI without PID also responded normally to the PPV, and although they had lower titres, the differences were not statistically significant. Asthmatic children with recurrent infections and normal immunoglobulin levels should be evaluated for SAD. Because children with asthma are at high risk for invasive pneumococcal infection, it would be worthwhile to evaluate the systematic use of the PPV in children over the age of two years who have not received the conjugate vaccine, considering the positive response to this vaccine reported here.
Conflicts of interestThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.