Mites are the main factor involved in respiratory disorder. Acarus siro is the most allergenic species of mite detected in the samples collected from flour mills.
ObjectiveThis work aimed to ameliorate the A. siro faeces allergenic disorder by garlic extract.
MethodsAlbino experimental rats were classified into three groups (native, inhaled and treated). Mites extract, ELISA and leukocytes differential counts techniques were used.
ResultsThe data obtained showed that the highest densities of A. siro in the samples collected from flour mills in El-Minia governorate during the period of February 2009 to January 2010 were recorded during the spring and autumn seasons. In addition, significantly higher serum levels of INF-γ and IgE were found in rats treated with faeces than the other groups, especially the garlic-treated group. In contrast, IL-4 was lower in faeces-treated rats than the others; however, the native group had the highest level of IL-4. The leukocytes differential count showed that eosinophil and basophil percentages in faeces-inhaled group are higher than both the native group and the garlic-treated group. Statistical analysis of data showed significant difference between garlic-treated group and either control or faeces-treated group (P<0.05).
ConclusionsThe population of A. siro mites peaked in spring and autumn. The immunological disorder caused by repeated exposure to A. siro faeces might be modulated by garlic.
Garlic (Allium sativum, Linn), the member of Liliaceae family, is probably one of the earliest known medicinal plants.1 Its bulbs had been used as a cure-all in ancient Egypt and are mentioned in the Ebers Papyrus, one of the earliest treatises on medicinal plants.2 Garlic-derived compounds, extracts and other type of preparations have benefits for disease prevention and treatment.3 This prompted us to proper garlic as an asthmatic modulatory medicinal plant.
Understanding the nature of environmental triggers is fundamental in attempts to prevent/reduce allergic diseases. As previously noted, exposure to common aeroallergens, especially perennial inhalable allergens such as mite, is associated with a significantly increased risk for asthma.4
Mite infestation is associated with negative effects on man and his resources. Storage mites consume stored grain and oil seeds, transfer toxicogenic microorganisms and produce allergens, thereby causing occupational allergies and endangering food safety.5
The flour mite Acarus siro is known as one of the commonest and most cosmopolitan of the stored product mite species.6 It has often been reported on various kinds of grain, processed cereal products, cheese, and litter in poultry houses and on beehive debris. The physical limitations (temperature and humidity) for A. siro have been studied by various acarologists.7
Several studies demonstrated that storage mites caused symptoms of bronchial asthma, particularly in rural occupational environments. The asthma symptoms are caused by inhalation of live mites and fragments of dead mites, or their excretory pellets.8
Bronchial asthma is a chronic inflammatory disease of the airway.9 Chronic exposure to allergens triggers a distinct array of immunobiological and biochemical reactions that directly stimulate and induce abnormalities of airway structure resulting in the development of clinical symptoms.10 Allergic disorder symptoms are associated with high level of serum allergens-specific IgE and eosinophilia.11 In addition, both allergen exposure protocols result in immune-mediated airway inflammation defined by elevated levels of IgE, the T-helper cell 2 (TH2) cytokines IL-4 and eosinophils.12
The pro-inflammatory cytokine, IFN-γ, promotes T-helper type-1 (Th1) responses, which downregulate the Th2-like immune responses that are hallmarks of allergic diseases, including asthma.13
The objective of the present investigation was to study the composition of the allergic mite species occurring in El-Minia Governorate flour mills, seasonal population of the most dominant allergenic species and their immunological disorders in albino rats. Modulation of these disorders was tested by a garlic extract.
Materials and methodsAllergenic flour mills mite populationOne hundred dusty flour samples were collected from flour mills in El-Minia Governorate. Each sample was place into a plastic bag, labelled with the origin of its collection and the type of product stored there. For the mite population, samples were collected in February 2009–January 2010. They were transferred to the laboratory and examined as soon as possible.
The Berlese-Tullgren method was used to extract mites from the samples. Ten grams from each sample were used in this method. Mites were sorted out, preserved and mounted on microscope slides for species identification.6,14A. siro figures were captured by light microscopy at ×400 and ×600 magnifications.
Extract preparationPreparation of Acarus siro crude faeces extractThe dominant allergenic A. siro mites used for experiments were cultured and grown in small Petri dishes with paper tissues under their covers. Petri dishes were kept in a desiccator containing about 50ml of saturated KCl resulting in 75% relative humidity. Cultures were kept at 25°C, and fed with dry flour. When the population density was sufficiently high, A. siro crude faeces were isolated by the Berlese-Tullgren method. Five grams from these faeces pellets were used for each inhalation treatment.
Preparation of crude garlic extractGarlic cloves were purchased from the local market in El-Minia, Egypt. They were then cleared of any adhering dried material. Fifty grams of the edible portion was homogenised in 100ml of distilled water and then filtered by passage through a 25mm pore-size filter (Millipore, St. Quentin, France) to give a crude aqueous extract of 500mg of garlic/ml. This was collected in a sterile vial and stored at 4°C until used.15 Dose 10mg garlic extract per kg albino rat body weight (b.wt.) was used.16
Experimental animalsMale albino rats, Rattus norvegicus (6–8 weeks old, weight 100–120g) were purchased from the Biological Supply Center, Theodore Bilharz Research Institute, TBRI, Cairo, Egypt and housed under specific pathogen-free conditions and maintained on a 12-h light–dark cycle, with food and water ad libitum. Animals were classified into three groups (10 animals each). The first group (control) was untreated. The second group was intranasal inhaled with A. siro faeces extract daily for 10 consecutive days. The third group was intra-peritoneal injected daily with garlic extract at a dose of 10mg/kg b.wt. and intranasal inhaled with A. siro faeces extract daily for 10 consecutive days.
Cytokine and immunoglobulin in serumSeventy-two hours after the last treating, ten animals from each group were sacrificed under chloroform anaesthesia. Blood samples were taken and centrifuged at 3000rpm for 30min. Sera were removed and kept at −20°C for the estimation of the cytokines (IFN-γ and IL-4) and IgE antibody levels by ELISA using kits purchased from R&D Systems (Minneapolis, MN, USA).
Leukocyte differential countFreshly collected blood samples of about 20μl were spread on clean slides into a thin film using another smooth-edged glass slide. Each blood smear was left to air dry before being fixed with methanol for 2–3min and then labelled by the number of the animal. Blood smears were stained with 10% Giemsa's stain (Aldrich) in buffered distilled water containing 0.021M Na2HPO4/0.015M KH2PO4. pH 7–7.2 for 30min far from sun light. After that, the stain was removed by gentle washing with distilled water and the slides were air-dried at room temperature.17 Using light microscopy at ×400 magnification, different types of blood leukocytes were recorded. At least double smears for each blood samples were counted.
Statistical analysisData were analysed using SPSS program version 13.0. Statistical analysis of the obtained data was performed using one way analysis of variance (ANOVA) test followed by least square differences (LSD) analysis for comparison between means. Results were expressed as mean±standard error (SE). Values of P<0.05 were considered statistically significant, while value of p>0.05 were considered statistically non-significant.
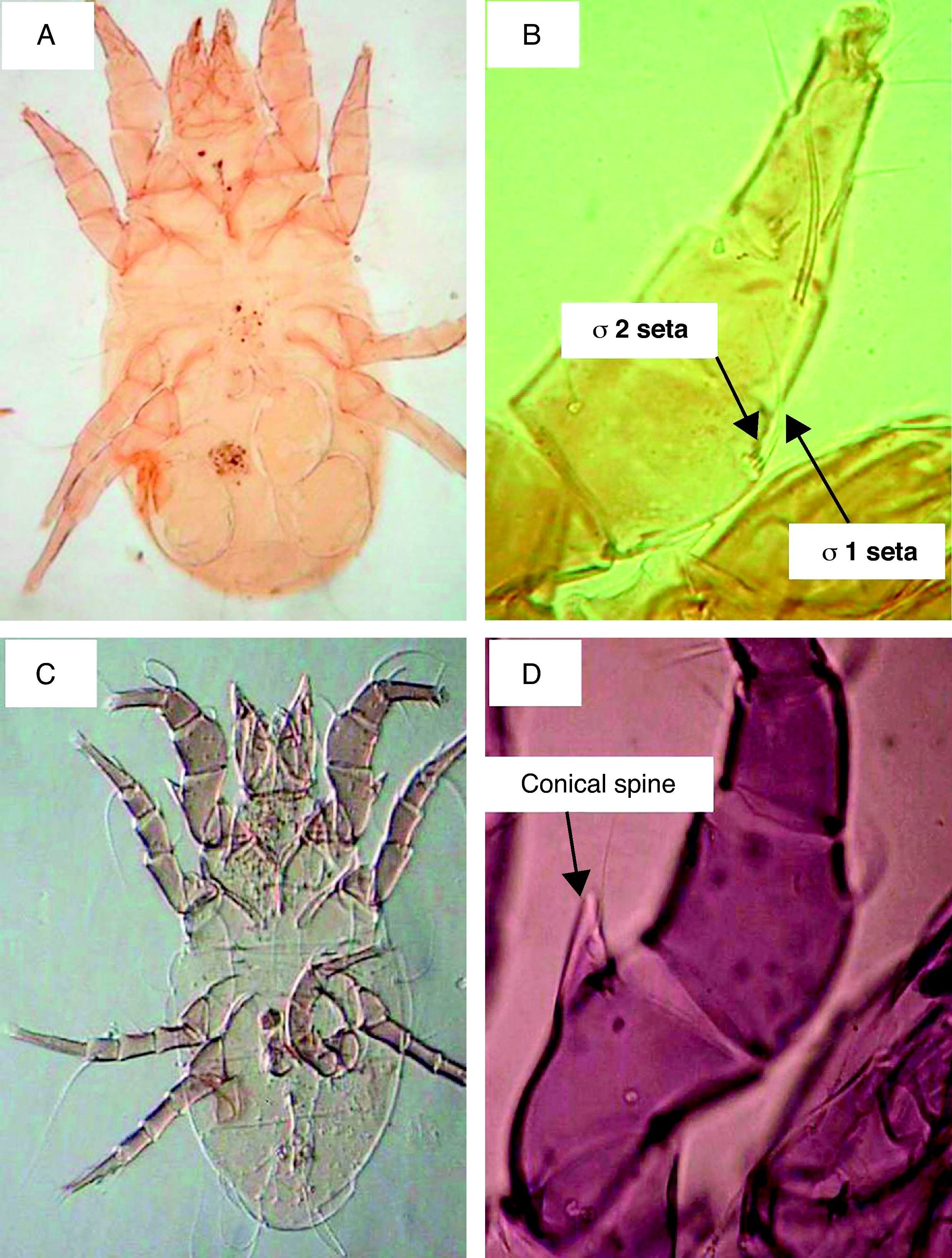
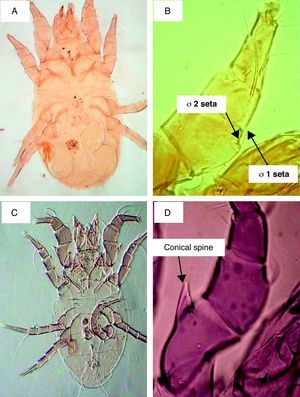
ResultsSeasonal population of flour mills mitesResults obtained on the acarofauna of mites in samples collected from visited flour mills revealed that A. siro is the most abundant species recorded in the present study. It comprised about 90% of the total acarofauna. This species belongs to the family Pyroglyphidae. Its Genu I has different setae. The seta sigma 1 (σ1) is more than three times longer than the seta sigma 2 (σ2). In addition, the male forelegs femur is enlarged and bearing ventrally a conical process (Fig. 1).
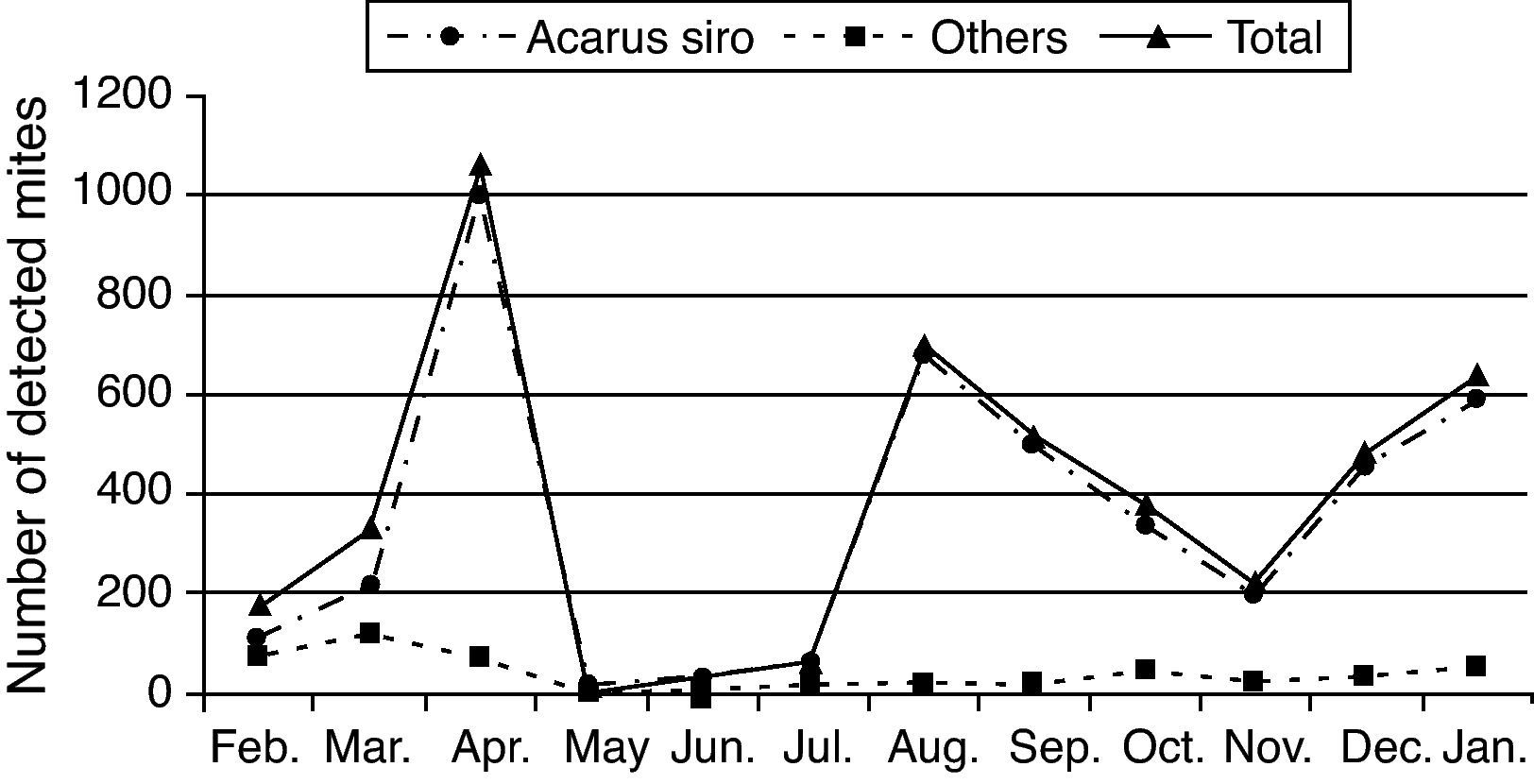
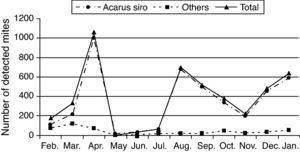
One-year collected data from February 2009 to January 2010 showed that flour mills A. siro mite population reached its peak in spring and autumn seasons, other mite species were also detected but in small numbers (Table 1 and Fig. 2).
Acarofauna composition found in five visited flour mills samples collected from El-Minia governorate through one year (from February 2009 to January 2010).
| Mite species | February | March | April | May | June | July | August | September | October | November | December | January |
| Acarus siro | 111.00±1.528 | 212.67±3.712 | 993.00±3.512a | 13.00±0.578 | 28.00±1.155 | 48.67±0.882 | 676.33±3.179a | 496.67±1.764 | 336.00±3055 | 196.00±3.055 | 453.00±3.512 | 586.67±8.819 |
| Others | 70.33±0.882 | 116.00±2.082 | 73.00±3.512 | 2.67±0.333 | 5.67±0.882 | 10.33±0.882 | 18.00±1.155 | 22.00±1.155 | 43.33±1.764 | 20.67±1.764 | 32.00±1.155 | 51.67±1.856 |
| Total | 181.33±2.404 | 328.67±5.783 | 1066.00±7.024 | 15.6±0.882 | 33.67±2.028 | 59.00±1.732 | 694.33±4.256 | 518.67±2.906 | 379.33±4.807 | 216.67±4.667 | 485.00±4.583 | 638.33±10.551 |
Values expressed are mean±SE of ten samples.
Statistic analysis of the obtained data showed a significant difference (P<0.05) in mite population recorded in spring and autumn compared to those recorded during the summer and winter seasons.
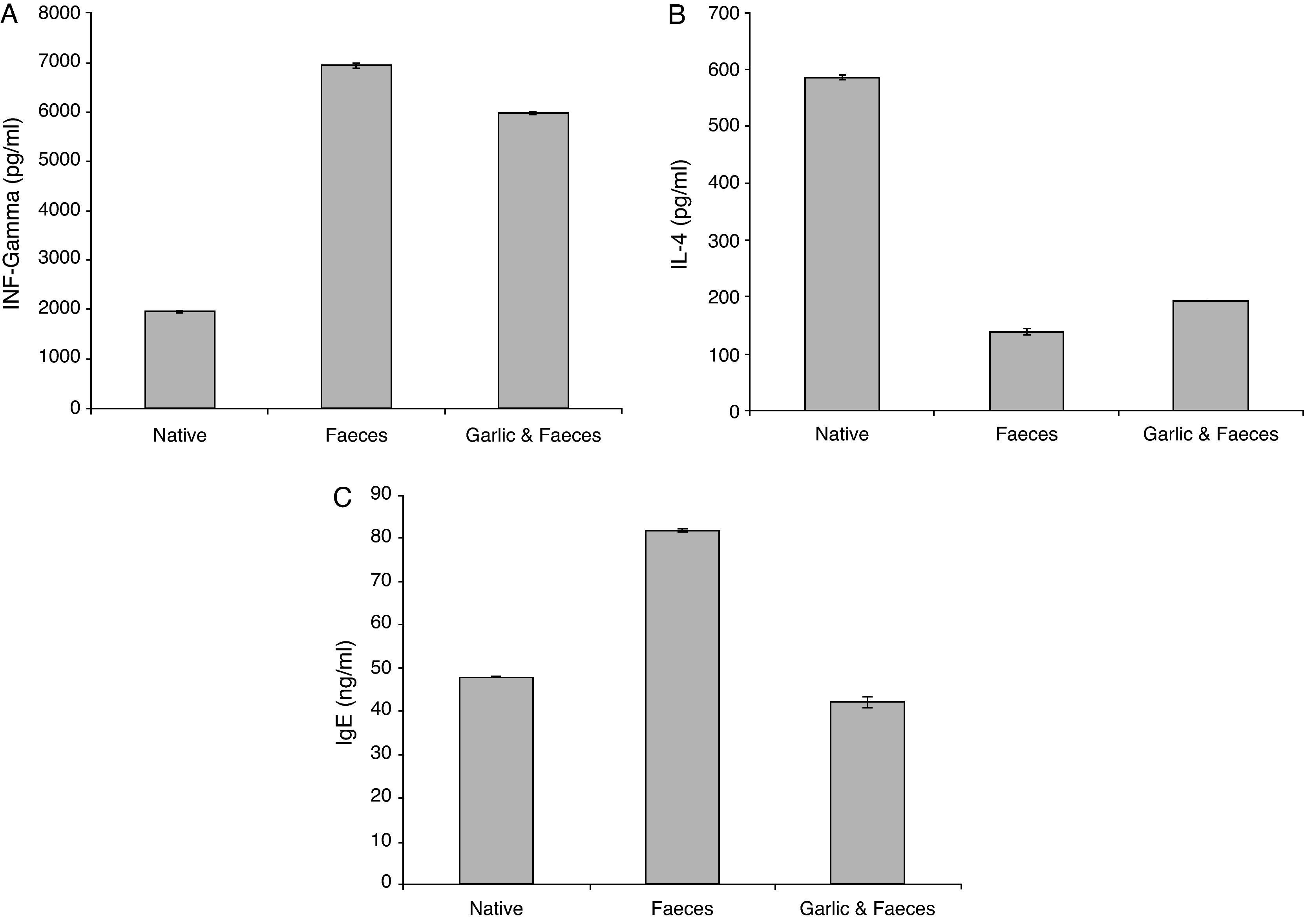
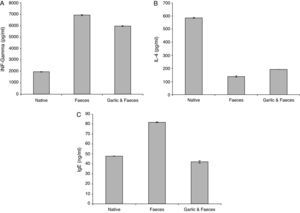
Estimation of INF-γResults of ELISA in Table 2 and Fig. 3A showed that INF-γ recorded 1965.90±21.798pg/ml in native group. Inhaled group with A. siro faeces counted high level of INF-γ (6932.46±17.752pg/ml). In contrast, the group treated with garlic had low level of INF-γ (5974.24±07.571pg/ml). Statistical analysis showed a significant difference in INF-γ between garlic-treated group and either control or faeces-treated group, P<0.05.
Changes in INF-γ, IL-4 and IgE levels in male albino rats inhaled with Acarus siro mite faeces and treated with garlic.
| Groups | INF-γ (pg/ml) | IL-4 (pg/ml) | IgE (ng/ml) |
| Native | 1965.90±21.798 | 585.14±4.553 | 47.87±0.307 |
| Faeces | 6932.46±17.752a | 138.50±0.894a | 81.87±1.367a |
| Garlic and faeces | 5974.24±07.571a | 191.32±2.990a | 42.09±0.153a |
Values expressed are mean±SE of ten samples.
The IL-4 ELISA value demonstrated that the garlic-treated group (191.32±2.990pg/ml) is higher than the faeces-treated group (138.50±0.894pg/ml) and lower than the native group, which recorded 585.14±4.553pg/ml (Table 2 and Fig. 3B). This study showed also that there is a significant difference in IL-4 level between garlic-treated group and either native or faeces-treated group (P<0.05).
Estimation of IgEIn contrast to IL-4, ELISA tests of IgE showed that the faeces-treated group (81.87±1.367ng/ml) is higher than both the native group (47.87±0.307ng/ml) and the garlic-treated group (42.09±0.153ng/ml). These differences can be seen in Table 2 and Fig. 3C. Statistical analysis showed that there is a significant difference between the garlic-treated group and either the control or faeces-treated group (P<0.05).
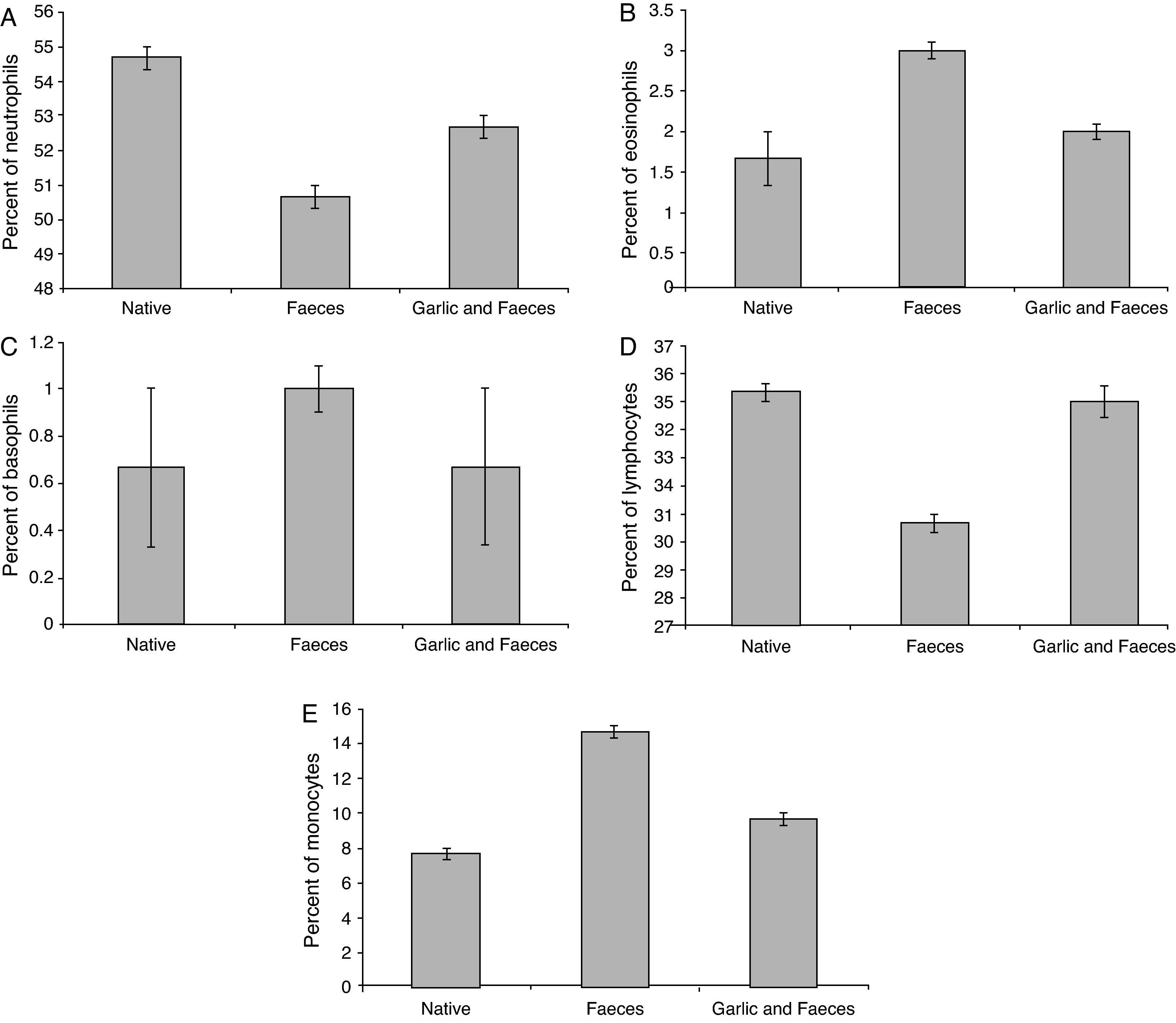
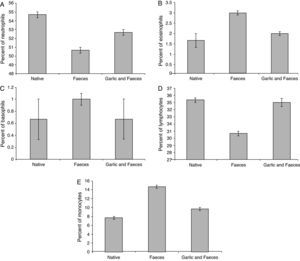
Leukocyte differential countTable 3 and Fig. 4A showed that the percentage of neutrophils in faeces-inhaled group (50.67±0.333) is lower than both the native group (54.67±0.333) and the garlic-treated group (52.67±0.333). On the other hand, eosinophils and basophils percentages in faeces-inhaled group (3.00±0.000 and 1.00±0.000) are higher than both the native group (1.67±0.333 and 0.67±0.333) and the garlic and faeces-treated groups that count 2.00±0.000 and 0.67±0.333, respectively (Table 3 and Fig. 4A and C).
Changes in differential leukocyte counts in male albino rats inhaled with Acarus siro mite faeces and treated with garlic.
| Group | Neutrophils | Eosinophils | Basophils | Lymphocytes | Monocytes |
| Native | 54.67±0.333 | 1.67±0.333 | 0.67±0.333 | 35.33±0.333 | 7.67±0.333 |
| Faeces | 50.67±0.333a | 3.00±0.100a | 1.00±0.100a | 30.67±0.333a | 14.67±0.333a |
| Garlic and faeces | 52.67±0.333a | 2.00±0.100a | 0.67±0.333a | 35.00±0.578a | 9.67±0.333a |
Values expressed are mean±SE of ten samples.
In parallel to neutrophils, Table 3 and Fig. 4D showed that lymphocytes percentage in faeces-inhaled group (30.67±0.333) is lower than both the native group (35.33±0.333) and the garlic-treated group (35.00±0.578). On contrast, the percent of monocytes in faeces-inhaled group (14.67±0.333) is higher than the garlic-treated group, recording 9.67±0.333 (Table 3 and Fig. 4E). Statistical analysis showed that there is a significant difference in leukocyte count between garlic-treated group and either control or faeces-treated group, P<0.05.
DiscussionThis work includes two aspects, namely, (1) mites annual prevalence and (2) allergenic disease. Occupational asthma is common in individuals exposed to flour and grain.18 Possible explanations for this are the development of hypersensitivity reactions to proteins in the flour and grain themselves, or to moulds and mites in the flour and grain when stored.19 One of the most significant findings of this study was a stigmatid mite, A. siro. It was found in all collected samples from visited flour mills monthly for four seasons (from winter 2009 to autumn 2010). This species had been also reported as the most allergenic stored product mite.20
In the present study, this species developed population densities of over 200 mites per 10g of dry sample weight. Prostigmatid and mesostigmatid mites were also found in the examined samples but in much lower numbers.
Moisture and temperatures are known to affect the population growth of stored product mites.21 Optimum temperature and relative humidity of A. siro population are 20–25°C and 75–85%, respectively.22 The above-recorded temperature and relative humidity are similar to those recorded during the beginning of spring and the end of autumn and are in accordance with this study, where the population peaks of A. siro were in spring and autumn. Similar population fluctuation patterns of A. siro complex were previously observed in wheat bulks stored for prolonged periods.23
In addition to the mite population, we have investigated whether the most dominant mite species faeces antigen is important in the development of asthma or not, where mite faeces break up into many small pieces and combine with dust particles to form an allergenic dust.24
The potential of certain pollutants to elicit allergic airway disease has been established in both experimental models and in humans.25 Short exposure (10 days) to HDM alone or to HDM and OVA resulted in equivalent levels of airway inflammation due to a robust inflammatory response in the lung. Moreover, it has been recently shown that intranasal administration of HDM daily for 10 days induces allergic sensitisation and Th2 airway inflammation, both of which are substantially reduced by concurrent treatment with anti–GM-CSF antibodies.26 This prompted us to expose the tested animals for about 10 days.
In addition to the dominance of A. siro in flour mills and stored products, it was also detected in house dust. Mechanistically, there is evidence that house dust mites disrupt cell monolayer and degrade tight junctions in the airway epithelium in vitro.27 This would facilitate their penetration across the airway epithelial barrier and, presumably, increase the accessibility to antigen presenting cells located in the sub-epithelial compartment.28
The prevalence of allergic disorders has been rising in recent years. It has been demonstrated that IgE antibodies play an important role in mediating type I hypersensitivity in humans.29 In both food and inhalant allergy it is accepted that food- or HDM-specific IgE binds to high-affinity Fc RI on mast cells, basophils, macrophages, and dendritic cells, as well as to low-affinity Fc RII on macrophages, monocytes, lymphocytes, eosinophils, and platelets.30 When mite allergens penetrate mucosal barriers of the gastrointestinal or respiratory tract and contact IgE antibodies bound to mast cells or basophils, histamine and other mediators that induce symptoms of immediate hypersensitivity are released.31 Other studies have also suggested that cleavage of CD23 (low-affinity IgE receptor) may promote and enhance an IgE immune response.32
Another factor to consider is the cytokine production. Indeed, it has been suggested that HDM may privilege the generation of a Th2-polarised response by modulating the balance between IL-4 and IFN-γ.33 In addition, various dust mite allergens act on bronchial epithelial cells in vitro to elicit the production of a number of cytokines.34
IL-4 is a key anti-inflammatory cytokine and plays an important role in the cytokine network and in the Th1/Th2 balance. It is generated by helper T cells, and possibly in the early adaptive immune response by mast cells and basophiles.35 In this study, the low level of IL-4 in the faeces-treated group may be due to the negative feedback that is exerted by histamine itself to inhibit IL-4.31
So, this study tended to measure the levels of INF-γ, IL-4 and IgE that are involved in allergenic disease. Repeated exposure to A. siro allergenic faeces caused an increase in both INF-γ and IgE, and a decrease in IL-4. The high level of both INF-γ and IgE, and the low level of IL-4 may participate in airway disorder. After garlic injection, the increase of IL-4 and the decrease of both INF-γ and IgE were maintained, and this may play an important role in the airway remodelling processes. The immunomodulatory effects on INF-gamma, IL-4 and IgE balance were tested by others.36
With respect to the numbers of leukocytes, this study showed that the numbers of basophils (main leukocyte responsible for allergy) in mite faeces-treated group to be higher than those recorded in the other groups. These results are in accordance with the previous data.37 The role of basophils in allergenic immune response, especially their function in IgE, IL-4 and INF-γ balance has also been studied by others.38
However, several pathways leading to the inhibition of basophil functions, mainly in terms of cytokine production, have recently been reported, namely: leukocyte Ig-like receptor (LIR3) signalling that becomes inhibitory upon co-legation with LIR7 or Fc¿RI, decreasing IL-4 production, histamine release and CysLT generation by basophils; the SHIP pathway that represses IL-3-induced IL-4 secretion by basophils; the inhibition by flavonoids, such as fisetin or lutein, that decrease IL-4 and other TH2-type cytokines produced by basophils; STAT1 signalling that regulates IL-4 production by pulmonary basophils in a model of primary respiratory syncytial virus infection; the lyn pathway that controls IL-4 production by basophils.39
Garlic in small amounts applied as a food condiment does not usually pose an important risk, aside for rare specific allergic reactions in hypersensitive people.40 In contrast, this study showed that some allergenic disorder could be modulated by garlic.
In conclusion, these data demonstrated that A. siro was the most dominant species detected in collected flour mills samples in El-Minia Governorate. Its population peaked in spring and autumn. Repeated exposure to A. siro allergenic faeces caused an increase in both the pro-inflammatory cytokine INF-γ and the main allergenic immunoglobulin IgE, and a decrease in the anti-inflammatory cytokine IL-4. The high level of both INF-γ and IgE, and the low level of IL-4 may participate in airway disorder. After garlic treating, the increase of IL-4 and the decrease of both INF-γ and IgE were maintained, and this means that garlic may play an important role in the amelioration of the airway immune response.
Conflict of interestThe authors have no conflict of interest to declare.
I thank Prof. Dr. El-Feki M. A. (Zoology Department, Faculty of Science, El-Minia University, El-Minia, Egypt) for revising the manuscript. I also thank Dr. Shaban H. A. (Zoology Department, Faculty of Science, El-Minia University, El-Minia, Egypt) for his efforts in data analysis.