The prostaglandin D2 receptor (PTGDR) gene has been associated to asthma and related phenotypes by linking and association studies. Functional studies involving animal models and other expression studies based on in vitro cell models also point to a possible role of polymorphisms in the promoter region, in the differential binding of transcription factors, and thus in PTGDR expression, which appear to be associated to the development of asthma or of susceptibility to the disease.
Prostaglandin D2 (PGD2) is a lipid mediator of autocrine and paracrine functions derived from arachidonic acid.1 In the central nervous system it intervenes in processes related to sleep, body temperature, olfactory function and hormone release. In turn, at peripheral level, PGD2 inhibits platelet aggregation, promotes vasodilatation and smooth muscle relaxation, while at pulmonary level it causes bronchoconstriction.2 It is regarded as an important inflammatory mediator in different allergic diseases such as asthma, allergic rhinitis, or atopic dermatitis.1
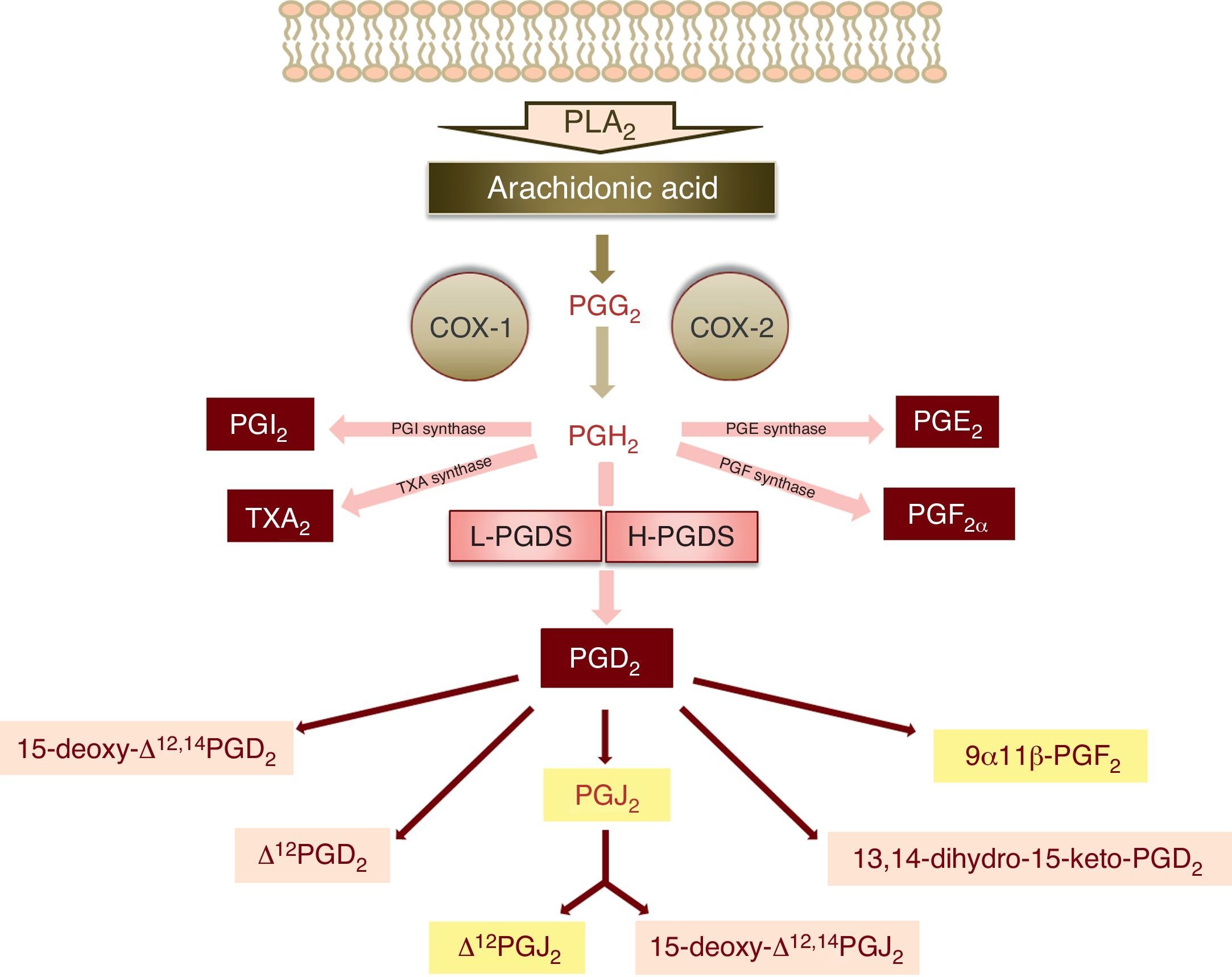
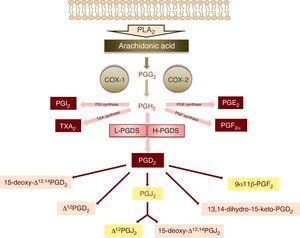
The synthesis of PGD2 starts with the activity of phospholipase A2, which catalyses the hydrolysis of arachidonic acid of the membrane phospholipids through physiological or pathological stimuli.3 The enzymatic action of cyclooxygenases 1 and 2 (COX-1 and COX-2) upon arachidonic acid produces a cyclic endoperoxide, PGG2, which in turn gives rise to PGH2 (Fig. 1). PGH2 is a substrate of the different enzymes that generate the prostanoids: thromboxanes and prostaglandins – including PGD2 (Fig. 1).
Schematic representation of the metabolism of PGD2 (PLA2: phospholipase A2, PGG2: prostaglandin G2, PGH2: prostaglandin H2, COX: cyclooxygenase, PGI2: prostaglandin I2, TXA2: thromboxane A2, PGE2: prostaglandin E2, PGF2α: prostaglandin F2α, L-PGDS: lipocalin-type prostaglandin synthase, H-PGDS: haematopoietic prostaglandin synthase, PGD2: prostaglandin D2, PGJ2: prostaglandin J2). The main metabolites of prostaglandin D2 are shown over a light grey background.
There are two isoforms of PGD2 synthase: the lipocalin isoform (L-PGDS), fundamentally expressed in the brain, and the haematopoietic isoform (H-PGDS), present in mast cells and Th2 lymphocytes, among other immune cells1,3 (Fig. 1).
Once synthesised, PGD2 is released from the cell by the prostaglandin transporters (PGTs) and by other transporters that remain to be characterised. Its half-life is very short, with rapid metabolisation to prostaglandins of the J2 series (15-deoxy-Δ12,14PGJ2 and Δ12PGJ2), which are synthesised spontaneously and not enzymatically, depending on the presence of serum albumin.4
The biological functions and actions of PGD2 are mediated by the specific transmembrane receptors, PTGDR and CRTH2.3 In addition, PGD2 binds to the thromboxane A2 receptor, TP, and to one of the receptors of prostaglandin E2, EP3.4 On the other hand, some metabolites of PGD2, such as 15-deoxy-Δ12,14PGJ2, can participate in intracellular processes, interacting with transcription factors by binding to nuclear receptors–specifically PPARγ (peroxisome proliferator-activated receptor-gamma)–or via mechanisms independent of such receptors.1
Structure of prostaglandin D2 receptorThe PGD2 receptor, known as prostanoid D receptor (PTGDR) or prostanoid D2 receptor 1 (PTGDR1), is encoded by the PTGDR gene, located on the long arm of chromosome 14 (14q221), and which the literature also refers to as DP, DP1, AS1, or ASTR1.
The PTGDR molecule is composed of 359 amino acids, and its molecular mass is 40.28kDa. It possesses seven transmembrane domains–a structure characteristic of the receptors associated to signal transmission heterotrimeric G proteins.2
PTGDR has three possible N-glycosylation sites, located in the amino-terminal extremity and in the first and third extracellular loops, respectively. It also contains two sites amenable to phosphorylation by protein kinase C (PKC), located in the first two cytoplasmic loops. On the other hand, additional phosphorylation sites have been described in serine and threonine residues at the carboxyl-terminal extremity of the receptor, which could be implicated in its desensitisation, and hence in its homeostatic capacity in cell activation processes.2
Phylogenetic studies of sequence homology with other members of the prostanoid receptor family have established a high degree of similarity with IP, the receptor of prostaglandin I2, and with EP2 and EP4, two receptors of prostaglandin E2,2 regarded as relaxing receptors, and which are generally coupled to a stimulator G protein such as Gs.
Functions of PTGDRThe PTGD2 receptor is activated by PGD2 and by some of its metabolites, including Δ12PGD2 and PGJ2,3 though these latter two metabolic products are scantly active.1 On the other hand, many studies use different compounds that behave as agonists or antagonists of the receptor. In this context, mention should be made of BW245C, a selective PTGDR agonist, with a binding affinity even somewhat greater than that of PGD2 itself,2 and of BW868C, MK-0524 and S-5751, as selective PTGDR antagonists.4
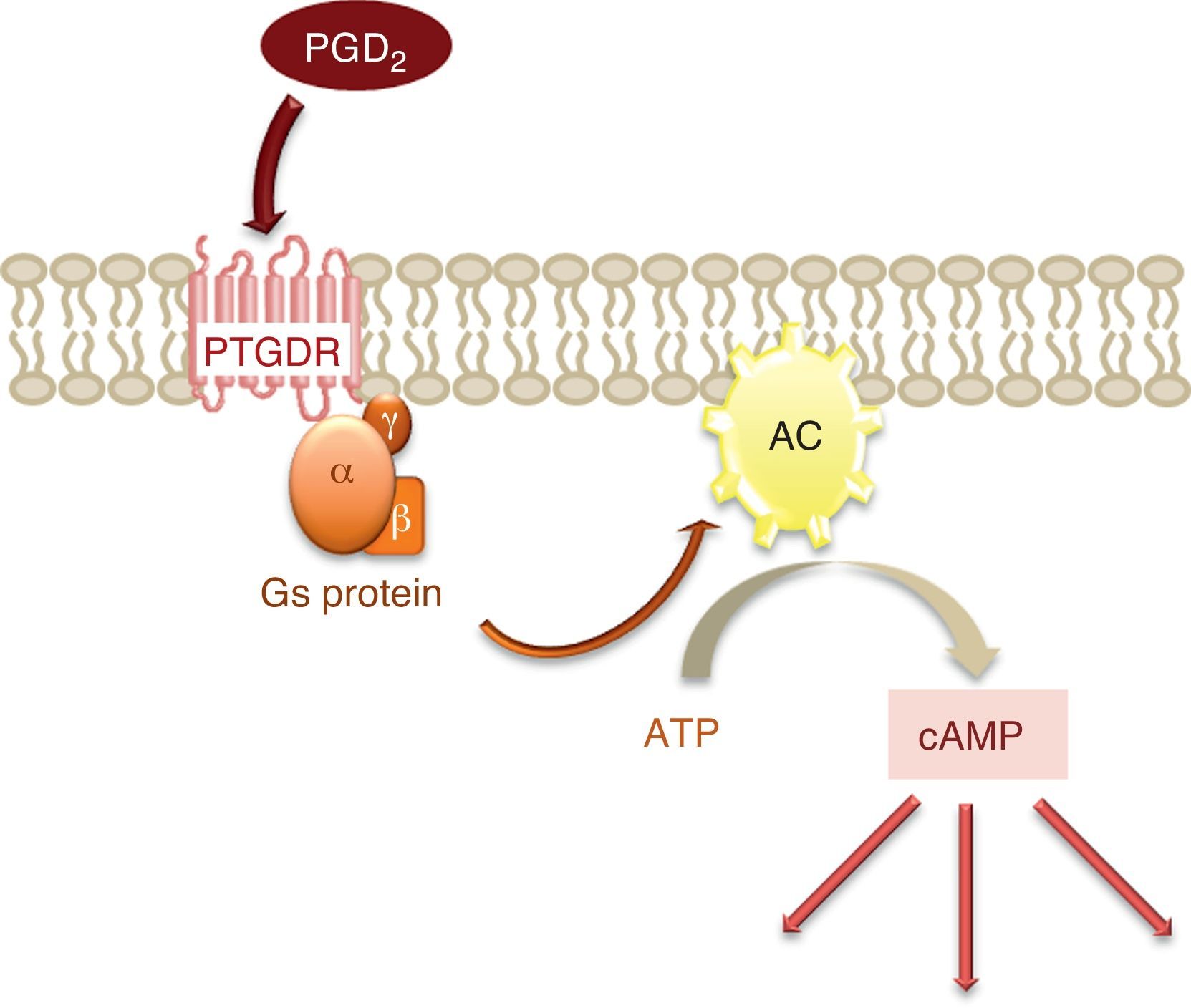
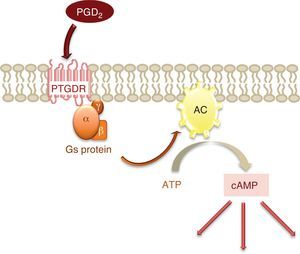
The activation of this receptor gives rise to an increase in the intracellular levels of the messenger cyclic adenosine monophosphate (cAMP).2 cAMP is synthesised from adenosine triphosphate (ATP) by the enzyme adenylate cyclase (AC), located in the cell membrane and activated by the α subunit of protein Gs, to which PTGDR is coupled (Fig. 2). This increase in intracellular cAMP would imply the activation of protein kinase A (PKA), although it has been shown that signal transmission via PTGDR is independent of PKA in certain cell types such as the NK lymphocytes and dendritic cells.5
Depending on the signal and cell type involved, cAMP can either enhance or suppress the participation of calcium (Ca2+) in signal transmission.5 A transient increase in the concentration of intracellular Ca2+ has been described in the signal transmission process through intracellular deposits and extracellular influx.5 On the other hand, the stimulation of PTGDR could induce the activation of proteins of the MAP kinase (MAPK) family and protein kinase C (PKC), and thus of another signal transmission pathway.3
Although PTGDR is a scantly abundant receptor, its expression is not limited to haematopoietic and inflammatory cells as in the case of CRTH2. In effect, the receptor is also found in NK, Th1 and Th2 lymphocytes, dendritic cells, eosinophils, neutrophils and basophils. In fact, some of these cells co-express both receptor forms.1,3 PTGDR is also found on the platelet surface, in concrete regions and cells of the central nervous system, in smooth muscle, in ciliary and non-ciliary epithelial cells of the bronchioles, in type II and type I alveolar epithelial cells (although in this latter type of cell DP expression is moderate),2,3,6 and in B lymphocytes.7 The activation of this receptor by PGD2 or its agonists facilitates smooth muscle relaxation and vasodilatation, reduces ocular tension, inhibits platelet aggregation, contributes to the regulation of pain perception and sleep, and plays an important role in the immune response found in allergic diseases.1
Association to asthmaAsthma is an inflammatory disorder resulting from the secretion of different intermediary molecules that promote the accumulation of Th2 cells, eosinophils and basophils. Among these molecules, PGD2 is the arachidonic acid metabolite produced in greatest abundance in response to environmental allergens.6 In asthmatic individuals, the levels of this prostanoid and of its main metabolite, 9α,11β-PGF2, rise sharply within minutes after exposure to the allergen–increasing up to 150 times above the levels prior to exposure, in bronchoalveolar lavage (BAL) fluid.3 Likewise, an increase in the concentration of this metabolic product is detected in the urine of patients with asthma induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs), following bronchial provocation with these substances.8
It has been shown that mast cell activation is required for the synthesis and secretion of PGD2, and that these cells are responsible for most of the production of this prostaglandin in the context of allergic response – despite the fact that other cell types such as Th2 lymphocytes, dendritic cells, macrophages, fibroblasts, bronchial smooth muscle cells and bronchial epithelial cells also have this capacity.3
The biological responses attributed to this prostanoid in asthma comprise bronchoconstriction, mucosal secretion, vasodilatation, increased vascular permeability and inflammatory cell chemotaxis referred particularly to Th2 cells, as well as modulation of the production and secretion of cytokines and chemokines – all mediated by its PTGDR, CRTH2 and TP receptors.1
The interaction of PGD2 with PTGDR promotes the accumulation of cAMP, which in general is associated to inhibition of the effector functions of Th1 lymphocytes, NK cells and other immune cells.3 IL-2 and IFN-γ production in CD4+ and CD8+ T cells,3 and IL-12 production in dendritic cells, are inhibited through signal transmission mediated by this receptor.5 The migration and functions of NK lymphocytes, as promoters of the Th1 type immune response, are also inhibited via DP.5 On the other hand, the interaction of PGD2 with the CRTH2 receptor promotes the recruitment and activation of Th2 cells, eosinophils and basophils, as well as the production of Th2 cytokines (IL-4, IL-5 and IL-13) on the part of these lymphocytes.1 Through PTGDR, PGD2 would suppress the Th1 response and produce a favourable environment for Th2 cell polarisation which, in coordination with the action of CRTH2, would contribute to establishing the dominant Th2 state characteristic of allergic inflammation.1,3
Other functions attributed to PTGDR are vasodilatation and increased vascular permeability,9 which facilitate the extravasation of inflammatory cells, increased survival on the part of eosinophils10 and even other cell types such as Th2 lymphocytes and mast cells,11 and epithelial cell stimulation for the production of macrophage derived chemokine (MDC or CCL22),9,10 which induces the recruitment and activation of Th2 lymphocytes,9 and the synthesis of arachidonic acid metabolites – including PGD2 itself, through a PTGDR-mediated autocrine mechanism.3
PGD2, and therefore its receptors, would constitute an important link between immediate and late allergen response, directing the initial cellular processes of the immune response to the recruitment and activation of Th2 lymphocytes, eosinophils and the rest of inflammatory cells–with the consequent associated physiopathological results.3
However, there have been some contradictory findings referring to the function of PTGDR in asthma disease, including even the proposal of an anti-inflammatory role for this receptor. Thus, it has been described that its activation at basophil surface level inhibits the migration and IgE-dependent degranulation of these cells.3 Regarding the role of the receptor in eosinophils, some studies have produced contradictory data: while some describe an increase in the survival of these cells,10 others have reported the inhibition of eosinophils activation and migration via PTGDR.3 Likewise, there have been descriptions of the inhibition of dendritic cell activation and migration to the lymph nodes, mediated by DP.3
Studies in animal modelsComplete deletion of the PTGDR gene in mice with asthma induced through sensitisation with ovoalbumin (OVA) results in inhibition of the inflammatory response in the airway, mediated by this allergen.6 Loss of the receptor does not affect the immediate immune response; accordingly, the levels of IgE against OVA are similar to those found in control mice that still carry the gene. However, the concentrations of Th2 cytokines (IL-4, IL-5 and IL-13), as well as the lymphocyte and eosinophil counts, decrease markedly in BAL fluid following exposure to the allergen and in the absence of this receptor.6
In mice with asthma induced by OVA, the administration of PGD2 prior to contact with the allergen not only induces an increased Th2 response referred to cell infiltration and the production of cytokines in the BAL sample, but also an increased expression of MDC.9
In this model, over-expression in the lungs of L-PGDS, the synthase specific of PGD2, increases the concentrations of IL-4 and IL-5, and eosinophil infiltration in BAL fluid.6 In the same model of asthma, but involving guinea pigs, the inhalation of S-5751, a selective PTGDR antagonist, produces a decrease in eosinophil infiltration of the lungs.1
On the other hand, mice deficient in uteroglobin – an anti-inflammatory protein that binds to PGD2 and which in turn prevents the latter from binding to PTGDR – show increased levels of this prostaglandin in BAL fluid and an increased expression of COX-2 in epithelial cells, fibroblasts and muscle cells through PTGDR promoted signal transmission.3
In other animal species such as dogs, the administration of PGD2 in the airway induces marked eosinophil recruitment6 and a transient but important reduction in the presence of circulating eosinophils.1
Genetic studiesIn the search for genes associated to asthma and atopic disease, linking studies have made it possible to associate different gene markers (in this case located in chromosome 14) to asthma or related phenotypes.12 One such study focusing on the regulation of serum IgE levels identified two new asthma-linked markers, and thus a new chromosome region, that might contain genes implicated in asthma disease. Although the authors of the article did not originally consider PTGDR, the fact is that this gene is located close to one of the mentioned associated markers, D14S63.12
Following the association of this gene with asthma and allergy on the basis of its gene location,12 and considering the results obtained from functional studies in animal models,6 a series of positional cloning and association studies between asthma disease and certain polymorphisms present in the promoter region and in the gene coding region in different populations13 confirmed its association to asthma and allergy. Different associations have been identified between the polymorphisms present in the promoter region of PTGDR and the asthmatic phenotype.7,13–15
However, the association studies have generated some controversy, and in a number of cases the results obtained have not been reproducible in some populations.4 There have been descriptions of similar haplotypic frequencies resulting from the combination of polymorphisms −549T>C, −441C>T and −197T>C of PTGDR among Caucasians, Afro-Americans and individuals from Puerto Rico, which could constitute a group in which several associations of PTGDR to asthma and related phenotypes have been described. In contrast, in a second group composed of Asians and Mexicans, PTGDR would not be a candidate gene for asthma.4,7
On the other hand, in association studies, laboratory quality control measures are essential, in the same way as strict statistical control, and the control group and patient selection criteria used.7 Lastly, in some cases the problem is that the studies are only fundamented on descriptive aspects. In addition, definition of the possible functional effects of the gene variants offers valuable information with which to contrast the genetic association findings, as has been established by a number of studies.7,13
Control of the expression of a gene is mainly carried out through regulatory elements in its promoter region. The variants of PTGDR statistically associated to asthma and related phenotypes are located in transcriptional factor binding sites. In fact, descriptions have been made of their capacity to alter the binding of these transcription control elements.13 Furthermore, certain haplotypic combinations of three of them have been detected (−549T>C, −441C>T and −197T>C), associated to increased transcriptional efficacy and thus to increased PTGDR expression. In contrast, other combinations are associated to a protective effect against asthma disease secondary to lesser binding of the transcription factors and to a decrease in the expression of the receptor.13
In this line, our group recently published a study integrating genetic association analysis and a functional study with the epigenetic aspects of the PTGDR promoter in patients with asthma, in a Caucasian population.7 Results were obtained referring to the associations of the individual PTGDR polymorphisms and their haplotypic and diplotypic combinations with allergic asthma, completing previously published findings.13–15 In addition, we have detected the capacity of the variant −613C>T to modify the binding pattern of nuclear proteins to DNA,7 and based on an in silico analysis of this promoter region, the binding of the transcription factors whose interaction is dependent upon the nucleotide present, has been postulated.7 Lastly, we have described the hypomethylation state of the PTGDR promoter in the B cells of a group of patients with asthma versus the control group, as well as its increased expression in studies involving messenger RNA.7
All these results in relation to PTGDR and asthma disease underscore its possible role as a therapeutic target in dealing with the disorder. In addition, in relation to new clinical trials, the usefulness of stratifying the participating patient population is suggested – identifying those individuals carrying haplotypes associated to increased PTGDR expression, in which improved benefits could be expected from therapy guided to inhibit the receptor.
This work was supported by the Spanish “Fondo de Investigación Sanitaria” (FIS) grants PS09/02068 and PI10/01706; by the “Sociedad Española de Alergología e Inmunología Clínica”; and by the “Fondo Caja de Burgos de ayudas a la Investigación Clínica”.