The novel coronavirus disease 2019 (COVID-19) has affected more than 5 million people globally. Data on the prevalence and degree of COVID-19 associated liver injury among patients with COVID-19 remain limited. We conducted a systematic review and meta-analysis to assess the prevalence and degree of liver injury between patients with severe and non-severe COVID-19.
MethodsWe performed a systematic search of three electronic databases (PubMed/MEDLINE, EMBASE and Cochrane Library), from inception to 24th April 2020. We included all adult human studies (>20 subjects) regardless of language, region or publication date or status. We assessed the pooled odds ratio (OR), mean difference (MD) and 95% confidence interval (95%CI) using the random-effects model.
ResultsAmong 1543 citations, there were 24 studies (5961 subjects) which fulfilled our inclusion criteria. The pooled odds ratio for elevated ALT (OR = 2.5, 95%CI: 1.6-3.7, I2 = 57%), AST (OR = 3.4, 95%CI: 2.3-5.0, I2 = 56%), hyperbilirubinemia (OR = 1.7, 95%CI: 1.2-2.5, I2 = 0%) and hypoalbuminemia (OR = 7.1, 95%CI: 2.1-24.1, I2 = 71%) were higher subjects in critical COVID-19.
ConclusionCOVID-19 associated liver injury is more common in severe COVID-19 than non-severe COVID-19. Physicians should be aware of possible progression to severe disease in subjects with COVID-19-associated liver injury.
The novel Coronavirus Disease 2019 (COVID-19) has affected more than 5 million patients globally, causing more than 300,000 death to date [1]. The symptoms of COVID-19 range from mild respiratory symptoms to acute respiratory distress syndrome with multiorgan failure and death, particularly among the elderly with multiple comorbidities. Extra-pulmonary symptoms such as COVID-19 associated liver injury has been reported [2–4]. COVID-19 associated liver injury is defined as any liver damage in patients with COVID-19, with or without the pre-existing liver disease [5]. The possible mechanisms of COVID-19 associated liver injury include immune-mediated damage and ischemic hepatitis secondary systemic inflammatory response syndrome in severe COVID-19, drug-induced liver injury, as well as reactivation of underlying chronic liver disease [6]. Besides, the direct virus-induced cytopathic effect has also been postulated as a result of viral replication within the infected hepatocytes [7].
The data on the prevalence and severity of COVID-19 associated liver injury are conflicting. While growing evidence suggested a higher incidence of liver injury among severe COVID-19, such findings are not consistent [8–10]. Recent position statement highlighted our gaps in understanding this novel disease [11–13]. In particular, patients with non-alcoholic fatty liver disease may have co-existing comorbidities which put them at a higher risk of severe COVID-19 [11]. Furthermore, COVID-19 associated liver injury is associated with prolonged hospitalization [6]. Given this premise, it is of scientific interest to expand our understanding of the clinical outcome of patients with COVID-19 associated liver injury. However, the severity of COVID-19 patients was often poorly defined by including subjects ranging from tachypnea to intubation or death. As the literature in COVID-19 is expanding exponentially, keeping up-to-date with scientific progress has become increasingly challenging for physicians. Therefore, we systematically reviewed and summarized the existing literature on COVID-19 associated liver injury among adult patients. Our meta-analysis aims to compare the risks and clinical outcomes of COVID-19 associated liver injury among adults with severe and non-severe COVID-19.
2Methods2.1ObjectiveThe objective of this meta-analysis is to compare the risk and clinical outcome of COVID-19 associated liver injury between COVID-19 patients with severe and non-severe COVID-19.
2.2Types of participantsWe included all adults with COVID-19 associated liver disease, regardless of their underlying chronic liver disease or the severity of COVID-19. Only studies that report outcome data between severe and non-severe COVID-19 were included, regardless of the pattern or the severity of the liver injury.
2.3Study OutcomesOur primary outcome was the pooled risk of serum alanine aminotransferase (ALT) elevation in adult patients with severe and non-severe COVID-19.
Our secondary outcomes were the pooled risk of the following parameters in adult patients with severe and non-severe COVID-19: (1) serum aspartate aminotransferase (AST) elevation, (2) hyperbilirubinemia and (3) hypoalbuminemia. We also assessed the pooled mean difference (MD) of Gamma-Glutamyl transferase (GGT) from the included studies. We defined the elevation of serum ALT or AST as levels beyond 40 U/L. We defined hyperbilirubinemia as total bilirubin level higher than 17 mmol/L. We defined hypoalbuminemia as serum albumin level below 40 g/L.
2.4Search strategyWe conducted a comprehensive search of three electronic databases, PubMed/MEDLINE, EMBASE and Cochrane Library (earliest inception to 24th April 2020). Keywords used in the search included a combination of “Coronavirus disease 2019”, “COVID19”, “SARS-CoV-2” and “Coronavirus infection/complications [MESH]". We restricted our search to adult human studies. An experienced medical librarian helped with our literature search. In addition, we manually searched all references of selected articles for additional relevant articles. We used PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) to report all studies identified using a pre-defined search protocol detailed in Appendix 1.
2.5Study selectionIn this meta-analysis, we included all studies that met the following inclusion criteria: (1) population: adult patients infected with the COVID-19, (2) reported outcome data on liver enzymes derangement (3) reported outcome data on the risk or severity of liver injury between severe and non-severe COVID-19. All studies, regardless of language, geography, publication dates or publication status are included so long as they provided data relevant to this analysis. Our exclusion criteria were:
- 1
Any study with subjects < 21 years old,
- 2
Case report or case series with less than 20 subjects,
- 3
Review articles, editorial and guidelines.
Severe COVID-19 was defined based on the definition used in the respective study. We further defined subjects as “critical COVID-19” based on the need for admission to the intensive care unit, mechanical ventilation, or death.
Three authors (MT, JL, RK) independently reviewed all titles and abstracts of the studies identified in the primary search. Studies that were duplicates or did not address the research question based on pre-defined criteria were excluded. We subsequently reviewed the full texts of all remaining articles to determine if they contained relevant information. Any discrepancy in the article selection was resolved by consensus with a co-author (WYJ).
2.6Data extractionWe extracted data on the demographic of study populations (age, gender, sample size, the proportion of subjects with baseline chronic liver disease and the use of Lopinavir/ritonavir) as well as the pattern of COVID-19 associated liver injury (ALT, AST, bilirubin, albumin and GGT) from all included studies. The data from each study were independently extracted by into a standardized form by two authors (WYJ, RK). We contact the corresponding author through email for any missing data. We did not encounter multiple reports from the same author or study population in this meta-analysis.
2.7Risk of Bias assessmentAll included studies were independently reviewed by 2 authors (MT, JL) to assess for quality and risk of bias using the modified Newcastle-Ottawa scale for cohort studies [14]. The scoring consist of 8 questions: representation of average adult in community (Population-based study = 1 point ; multicenter = 0.5 point; single center = 0 point); cohort size (more than 100 subjects = 1 point, between 50 to 99 subjects = 0.5 point, fewer than 50 subjects = 0 point); reported information on percentages and pattern of liver injury (information with clarity = 1 point, information derived from percentages = 0.5 point, unclear = 0); reported percentages of subjects with chronic liver disease at baseline (yes = 1 point, no = 0 point); assessed factors between mild and severe COVID-19 (yes = 1, no = 0); adequate clinical assessment (yes = 1, no = 0), sufficient follow-up period for outcome to occur (yes = 1 point, unclear = 0 point); adequate follow-up (all subjects were followed-up = 1 point, > 50% subjects were followed-up = 0.5 points, < 50% subjects were followed-up = 0 point). The final decision on the overall risk of bias was made through discussion. All differences were resolved by discussions with third author (WYJ). We considered studies with a score of ≥6, 3-4, < 3 as high-quality, medium-quality and low-quality, respectively.
2.8Statistical analysisWe used Review Manager Software version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to perform our meta-analysis to estimate the pooled odds ratio (OR), mean difference (MD), and 95% confidence interval (95%CI). We used the random-effects model and validated our results using sensitivity analysis and heterogeneity assessment across the included studies. A p-value of less than 0.05 was considered to be statistically significant. The statistical heterogeneity was evaluated using the I2 statistics. We defined substantial heterogeneity across study as low, moderate, substantial and considerable with a I2 value of <30%, 31% to 60%, 61% to 74% and > 75% [15]. We performed subgroup analysis among subjects with critical COVID-19 (defined as subjects needing admission to intensive care unit, mechanical ventilation, or death) to minimize the impact of heterogeneity in the definition of severe COVID-19 on our results. As case-control studies are generally considered to have a higher risk of bias and more susceptible to selection and recall bias [16], we performed subgroup analysis based on study design (cohort study versus case-control study).
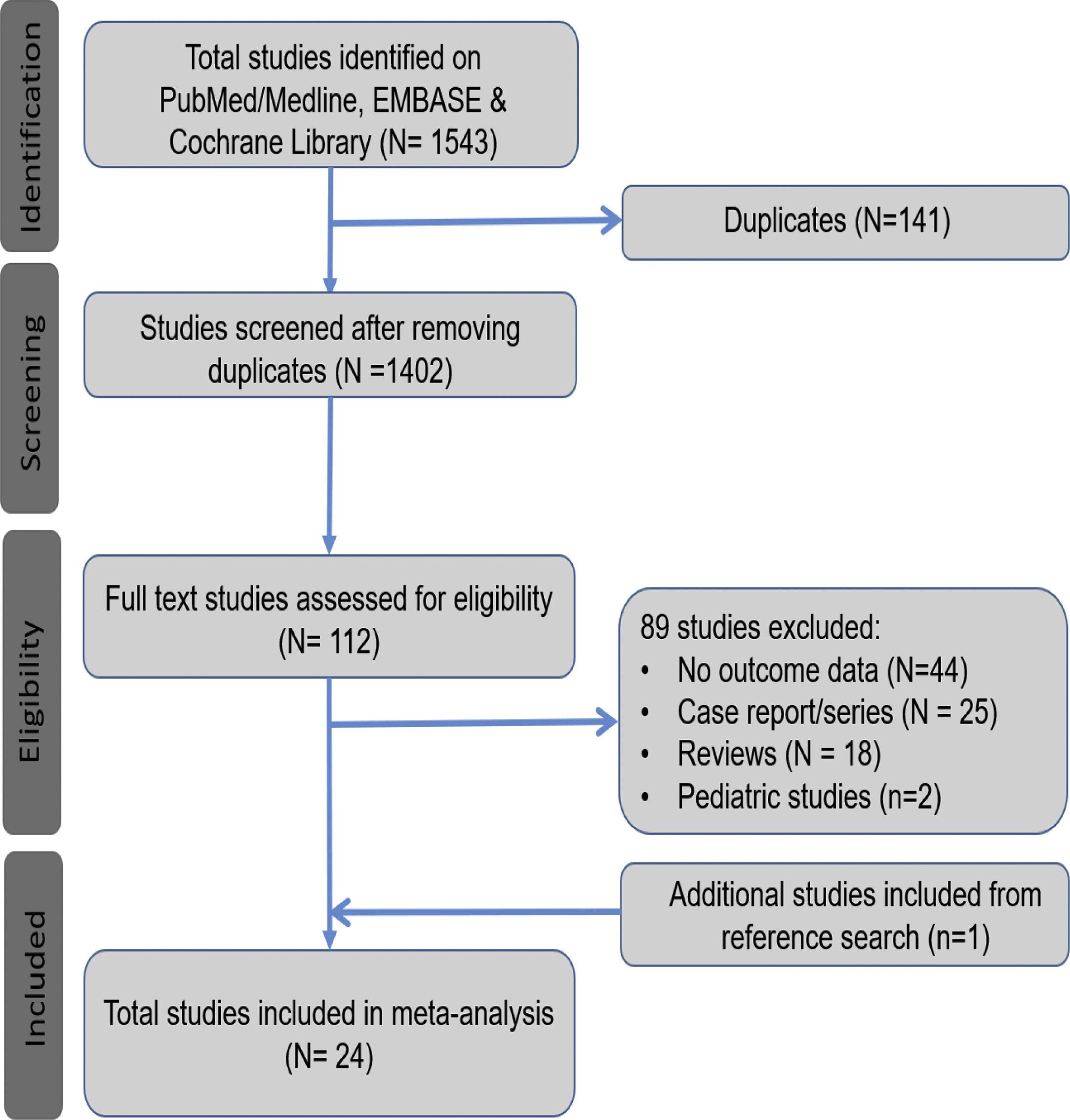
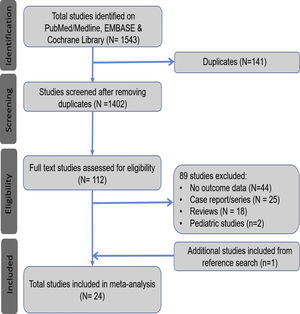
3Results3.1Search results and population characteristicsFrom an initial total of 1543 citations identified by using our search strategy, we identified a total of 112 studies. Of these, 89 were excluded for the following reasons: no outcome data reported (n = 44), case report or case series fewer than 20 subjects (n = 25), review article (n = 18) and pediatric study (n = 2). We manually search the references of all the included studies and included one additional study [10]. A total of 24 studies met our inclusion criteria [10,17–39]. (Fig. 1).
3.2Characteristics and quality of the studiesThe characteristics of all included studies are presented in Table 1. Most studies were retrospective (15 case-control studies and 8 cohort studies) in nature except for one prospective cohort study [24]. Seven studies reported multicenter data, while the remaining reported single-center data. All studies were from Asia. The definition of severe COVID-19 was heterogeneous across all the included studies. The commonest definition of severe COVID-19 was based on clinical criteria (n = 15), followed by death (n = 5) and ICU admission (n = 3). All studies were published as a full manuscript. Overall, seven studies were considered to be of high quality, while the rest were considered to be medium quality (SupplementaryTable 1).
Characteristics of included studies.
| No | Author, year | Country | Sample size | Age (Median/IQR) | Male (n, %) | Definition of Severe Disease | Lopinavir/ritonavir (%) | Baseline CLD (%) | Liver injury (%) | Non-severe | Severe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cai Q, 2020 | Shenzhen, China | 417 | 47 (34-60) | 48 | Clinical | 80 | 5 | 76 | 225 | 192 |
| 2 | Cao J, 2020 | Wuhan, China | 102 | 54 (37-67) | 52 | Death | 27.5 | 2 | 33 | 85 | 17 |
| 3 | Cao W, 2020 | Xiangyang, China | 128 | NR | 47 | Clinical | NR | NR | NR | 107 | 21 |
| 4 | Chen G, 2020 | Wuhan, China | 21 | 56 (50-65) | 81 | Clinical | NR | NR | 29 | 10 | 11 |
| 5 | Chen T, 2020 | Wuhan, China | 274 | 62 (44-70) | 62 | Death | 80 | 4 | 5 | 161 | 113 |
| 6 | Deng Y, 2020 | Wuhan, China | 225 | Recovered: 40, Death 69 | 55 | Death | NR | NR | NR | 116 | 109 |
| 7 | Du R, 2020 | Wuhan, China | 109 | *70.7 (10.9) | 68 | ICU | NR | 2 | 46 | 58 | 51 |
| 8 | Guan W, 2020 | China | 1099 | 47 (35-58) | 58 | Clinical | NR | 2 | 22 | 926 | 173 |
| 9 | Huang C, 2020 | Wuhan, China | 41 | 49 (41-58) | 73 | ICU | NR | 2 | 37 | 28 | 13 |
| 10 | Jin X, 2020 | Zhejiang, China | 651 | *45.2 (14.4) | 51 | Clinical | 89 | 4 | 10 | 577 | 74 |
| 11 | Lian J, 2020 | Zhejiang, China | 788 | 44.8 (13.4) | 52 | Clinical | 27.3 | 4 | 10 | 710 | 78 |
| 12 | Liu W, 2020 | Wuhan, China | 78 | 38 (33-57) | 50 | Clinical | 30.8 | NR | NR | 67 | 11 |
| 13 | Mo P, 2020 | Wuhan, China | 155 | 54 (42-66) | 56 | Clinical | 17.4 | 5 | NR | 70 | 85 |
| 14 | Wan S, 2020 | Chongqing, China | 135 | 47 (36-55) | 53 | Clinical | 100 | 2 | NR | 95 | 40 |
| 15 | Wang D, 2020 | Wuhan, China | 138 | 56 (42-68) | 54 | ICU | NR | 3 | NR | 102 | 36 |
| 16 | Wang Z, 2020 | Wuhan, China | 69 | 42 (35-62) | 46 | Clinical | NR | 1 | 33 | 55 | 14 |
| 17 | Wu C, 2020 | Wuhan, China | 201 | 51 (43-60) | 64 | ARDS | 15 | 4 | 30 | 84 | 117 |
| 18 | Xu X, 2020 | Zhejiang, China | 62 | 41 (32-52) | 56 | Clinical | 89 | 11 | 16 | 29 | 33 |
| 19 | Yang X, 2020 | Wuhan, China | 52 | *59.7 (13.3) | 67 | Death | 13.5 | NR | 29 | 32 | 20 |
| 20 | Zhang G, 2020 | Wuhan, China | 95 | 49 (39-58) | 56 | Clinical | NR | NR | 55 | 63 | 32 |
| 21 | Zhang X, 2020 | Zhejiang, China | 654 | *45.3 (14.3) | 50 | Imaging | NR | 4 | 12 | 72 | 573 |
| 22 | Zhang Y, 2020 | Wuhan, China | 115 | *49.5 (17.1) | 43 | Clinical | NR | NR | 15 | 84 | 31 |
| 23 | Zheng F, 2020 | Changsha, China | 161 | 45 (34-57) | 50 | Clinical | NR | 3 | 14 | 131 | 30 |
| 24 | Zhou F, 2020 | China | 191 | 56 (46-67) | 62 | Death | 21 | NR | 31 | 137 | 54 |
Abbreviations: *mean (SD); NR = not reported; CLD = chronic liver disease; CT = computer tomography; NR = Not reported.
Twenty-four studies, with a total of 5952 patients (4024 in the severe group and 1928 in the non-severe group), were included in the final analysis [10,17–39]. The patients' characteristics were detailed in Table 1. The median age of subjects ranged from 38 to 71 years-old. The proportion of male ranged from 43% to 81%. The proportion of subjects with admission to an intensive care unit ranged from 1% to 100%. The prevalence of baseline chronic liver disease was low (ranged from 1% to 11%) [17–18,20,22–26,28–33,36,38]. The liver injury occurred in 5%-76% of subjects with COVID-19 [17–20,22–26,31–39].
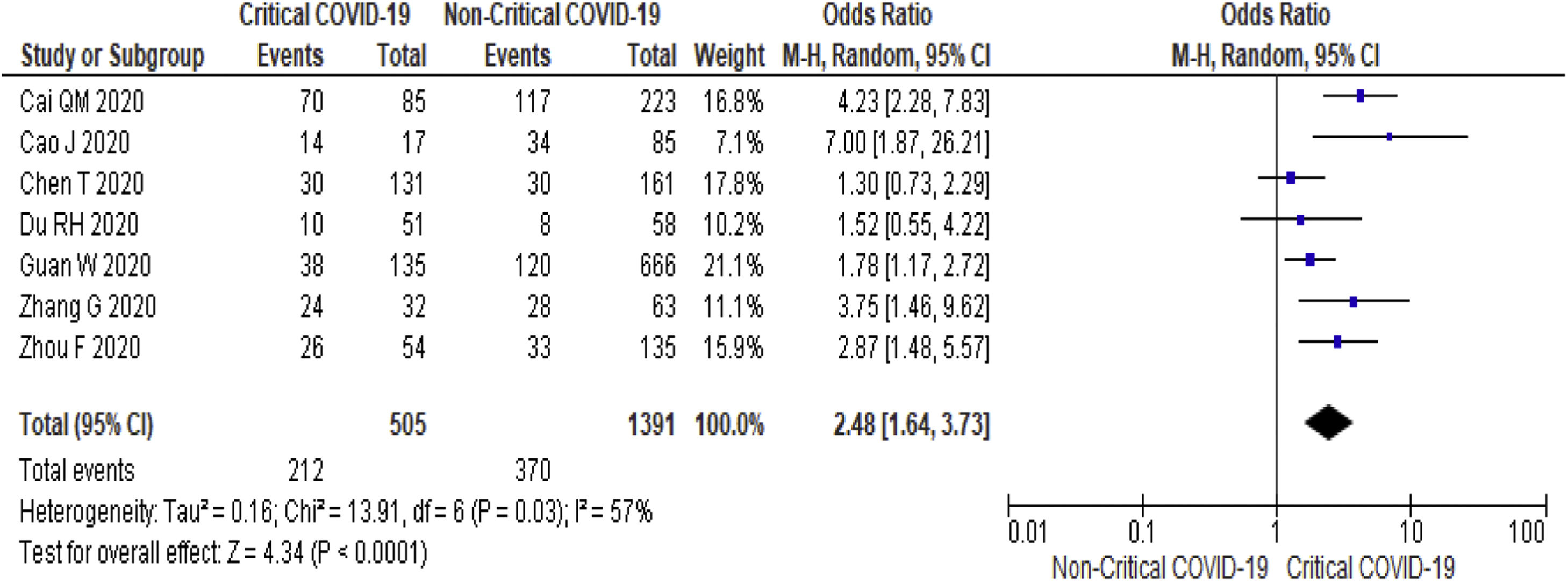
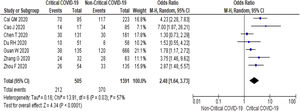
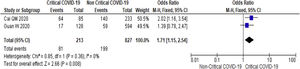
3.3Study Outcome3.3.1Serum Alanine Aminotransferase (ALT)Twenty-three studies (5900 subjects) reported outcome data on ALT [10,17–33,35–39]. The mean level of ALT is higher in severe COVID-19 when compared to non-severe group (31.9 U/L vs 23.5 U/L, 95%CI = 5.3-12.8, p < 0.001), with high heterogeneity (I2 = 99%, p < 0.001) (SupplementaryTable 2). The pooled risk of ALT elevation was higher in severe COVID-19 with moderate heterogeneity (OR = 2.8, 95%CI: 1.8-4.3, I2 = 63%) (Table 2). When the stratified analysis was performed within the subgroup of patients with critical COVID-19, the pooled risk of ALT elevation remained higher among subjects with critical COVID-19, with less heterogeneity (OR = 2.5, 95%CI = 1.6-3.7, I2 = 57%) (Fig. 2).
Subgroup analysis.
| Outcome | Subgroups | No. of studies | Effect size (Odds ratio / mean difference) | I2 | |
|---|---|---|---|---|---|
| ALT elevation (>40 U/L) | Overall | 8 | 2.8 (1.8-4.3) | 63 | |
| Critical COVID-19 | 7 | 2.5 (1.6-3.7) | 57 | ||
| Lopinavir/ritonavir usage | High usage (≥ 80%) | 1 | 4.6 (2.5-8.6) | NA | |
| Low usage (< 80%) | 7 | 2.5 (16-3.9) | 56 | ||
| Cohort | 6 | 2.5 (1.5-4.2) | 66 | ||
| Case-control | 2 | 4.4 (1.4-13.4) | 55 | ||
| AST elevation (>40 U/L) | Overall | 9 | 3.4 (2.3-5.1) | 59 | |
| Critical COVID-19 | 7 | 3.4 (2.3-5.0) | 56 | ||
| Non-survivor | 1 | 5.9 (3.4-10.5) | NA | ||
| Lopinavir/ritonavir usage | High usage (≥ 80%) | 3 | 3.3 (1.6-6.8) | 53 | |
| Low usage (< 80%) | 6 | 3.5 (2.1-5.9) | 66 | ||
| Cohort | 5 | 3.3 (2.0-5.3) | 70 | ||
| Case-control | 4 | 3.7 (1.7-8.4) | 49 | ||
| Hyperbilirubinemia (>17 mmol/L) | Overall | 3 | 1.9 (1.1-3.1) | 30 | |
| Critical COVID-19 | 2 | 1.7 (1.2-2.5) | 0 | ||
| Lopinavir/ritonavir usage | High usage (≥ 80%) | 1 | 2.0 (1.2-3.5) | NA | |
| Low usage (< 80%) | 2 | 2.2 (0.6-7.7) | 61 | ||
| Cohort | 2 | 1.7 (1.1-2.5) | 0 | ||
| Case-control | 1 | 5.2 (1.2-23.2) | NA | ||
| Hypoalbuminemia (<40 g/L) | Overall | 3 | 8.8 (4.1-19.0) | 46 | |
| Critical COVID-19 | 2 | 7.1 (2.1-24.1) | 71 | ||
| Lopinavir/ritonavir usage | High usage (≥ 80%) | 0 | NA | NA | |
| Low usage (< 80%) | 3 | 8.8 (4.1-19.0) | 46 | ||
| Cohort | 2 | 7.1 (2.1-24.1) | 71 | ||
| Case-control | 1 | 13.1 (3.7-46.4) | NA | ||
| GGT level | Overall | 3 | 46.7 (-4.1-97.6) | 96 | |
| Critical COVID-19 | 2 | 36.6 (-5.2 -78.4) | 100 | ||
| Lopinavir/ritonavir usage | High usage (≥ 80%) | 1 | 98.5 (75.3-121.6) | NA | |
| Low usage (< 80%) | 2 | 15.3 (13.7-16.8) | 0 | ||
| Cohort | 2 | 56 (-25 - 137.6) | 98 | ||
| Case-control | 1 | 28.4 (2.1-54.8) | NA |
Abbreviation: ALT = Alanine Aminotransferase; AST = Aspartate aminotransferase; GGT = Gamma-Glutamyl Transferase; NA = Not available.
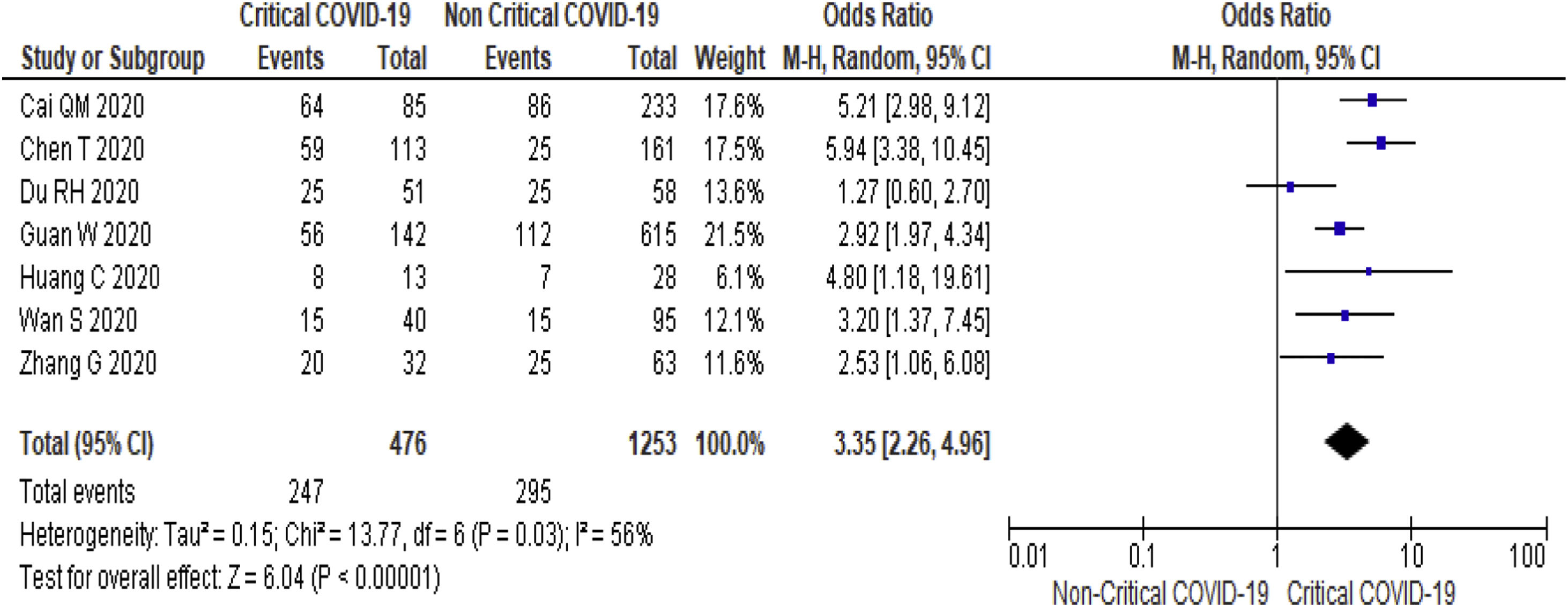
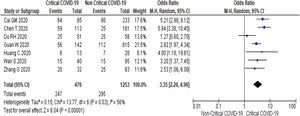
Twenty-one (5292 subjects) reported outcome data on AST [10,17,19–33,35–38]. The mean level of AST is higher in severe COVID-19 when compared to non-severe group (35.1 U/L vs 26.6 U/L, 95%CI = 8.7-14.2, p < 0.001), with high heterogeneity (I2 = 99%, p < 0.001) (SupplementaryTable 2). The pooled risk of AST elevation was higher in severe COVID-19 with moderate heterogeneity (OR = 3.4 95%CI: 2.3-5.1, I2 = 59%) (Table 2). Stratified analysis among subjects with critical COVID-19 showed a higher pooled risk of AST elevation, with less heterogeneity (OR = 3.4, 95%CI: 2.3-5.0, I2 = 56%) (Fig. 3). One study reported a higher risk of AST elevation in non-survivor (OR = 5.9, 95%CI = 3.4-10.5, p < 0.001) (Table 2). [20]
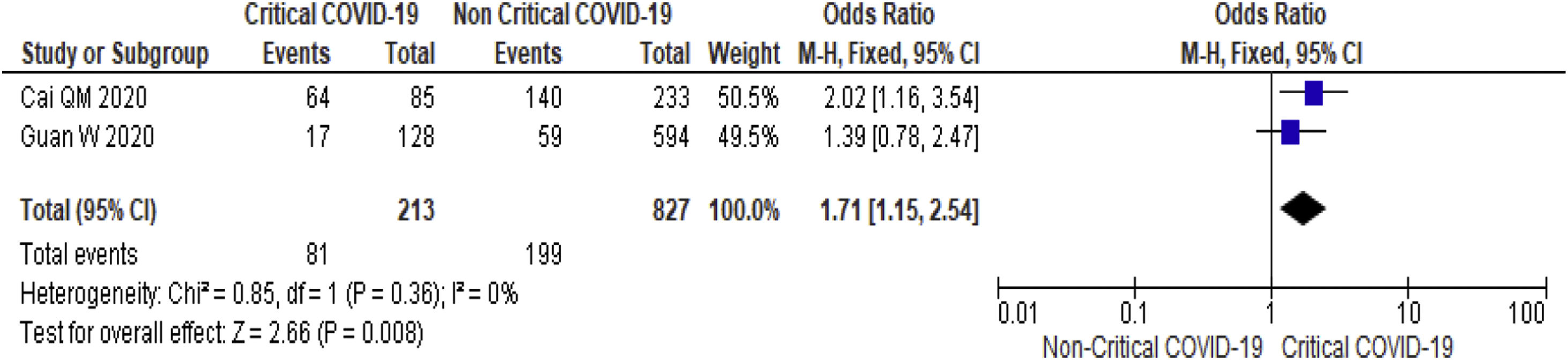
3.3.3Serum bilirubinThirteen studies (3540 subjects) reported outcome data on serum bilirubin [17,19–20,23–26,29,32,30,36–38]. The mean level of bilirubin is higher in severe COVID-19 when compared to non-severe group (12.2 mmol/L vs 10.5 mmol/L, 95%CI = 1.5-3.5, p < 0.001), with high heterogeneity (I2 = 98%, p < 0.001) (SupplementaryTable 2). The pooled risk of hyperbilirubinemia (serum bilirubin > 17 mmol/L) is higher in subjects with severe (OR = 1.9, 95%CI: 1.1-3.1, I2 = 30%) (Table 2) and critical COVID-19 (OR = 1.7, 95%CI: 1.2-2.5, I2 = 0%) (Fig. 4).
3.3.4Serum albuminThirteen studies (3404 subjects) reported outcome data on serum albumin [19–20,24–29,32,36–37,39]. The mean level of albumin is lower in severe COVID-19 when compared to non-severe group (37.2 G/L vs 40.1 G/L, 95%CI= -6.0, -2.9, p < 0.001), with high heterogeneity (I2 = 100%, p < 0.001) (SupplementaryTable 2). The pooled risk of hypoalbuminemia (serum albumin <40 g/L) was higher in subjects with severe COVID-19 (OR = 8.8, 95%CI: 4.1-19.0, I2 = 46%) and critical COVID-19 (OR = 7.1, 95%CI: 2.1-24.1, I2 = 71%) (Table 2).
3.3.5Serum Gamma-Glutamyl Transferase (GGT)Three studies (699 subjects) reported outcome data on serum GGT [17,20,37]. There is a trend towards higher GGT is higher in severe COVID-19 (68.5 U/L vs 36.8 U/L, p = 0.07) and critical COVID-19 (56.9 U/L vs 28.5 U/L, p < 0.001), with high heterogeneity (I2 = 96% and 100% respectively, p < 0.001) (SupplementaryTable 2). The mean difference of GGT did not significantly differ in subjects with severe or critical COVID-19 as compared to non-severe/non-critical COVID-19 patients (Table 2).
3.3.6Subgroup analysisWe performed a subgroup analysis based on the types of studies and the proportion of subjects with Lopinavir/ritonavir usage.
We performed a subgroup analysis based on study design by comparing a cohort study and case-control study. We found that the effect size for the case-control study was bigger as compared to cohort study for ALT elevation, AST elevation, hyperbilirubinemia and hypoalbuminemia (Table 2).
The proportion of subjects using Lopinavir/ritonavir (ranged from 14% to 100%) was reported in 12 studies [17–18,20,25–29,32–34,38]. We arbitrarily defined high Lopinavir/ritonavir usage as a study with ≥ 80% of their subjects treated with Lopinavir/ritonavir. Among the four studies with high Lopinavir/ritonavir usage [17,25,29,33], we observed a higher pooled odds risk for ALT and GGT elevation (Table 2) when compared to studies with low Lopinavir/ritonavir usage. When the analysis was stratified among subjects with critical COVID-19, high Lopinavir/ritonavir usage group had a higher level of ALT (41.5 U/L vs 36.9 U/L, p < 0.001) and bilirubin (16.7 mmol/L vs 14.0 mmol/L, p < 0.001) as compared to low Lopinavir/ritonavir usage group (Supplementary Table 4).
3.4Validation of meta-analysis results3.4.1Sensitivity analysisWe performed a sensitivity analysis to assess whether an individual study had a dominant effect on the overall pooled results. This was performed by serially removing one study at a time while repeating the analysis. The pooled risk of ALT elevation ranged from 2.2 to 2.8, which is still within our reported 95%CI. Besides, we also performed repeated analysis using the fixed-effect model. Using the fixed-effect model, the level of GGT is higher in severe COVID-19 as compared to non-severe group (MD = 14.6, 95%CI: 14.1-17.2, I2 = 96%). All the remaining findings were similar in the sensitivity analysis (SupplementaryTable 2).
3.4.2HeterogeneityWe assessed heterogeneity based on the percentages of I2 for each reported outcome. We consider the heterogeneity on the observed risk and severity of COVID-19 associated liver injury as moderate and substantial, respectively. This heterogeneity is likely the result of a different definition of severe disease used in various studies.
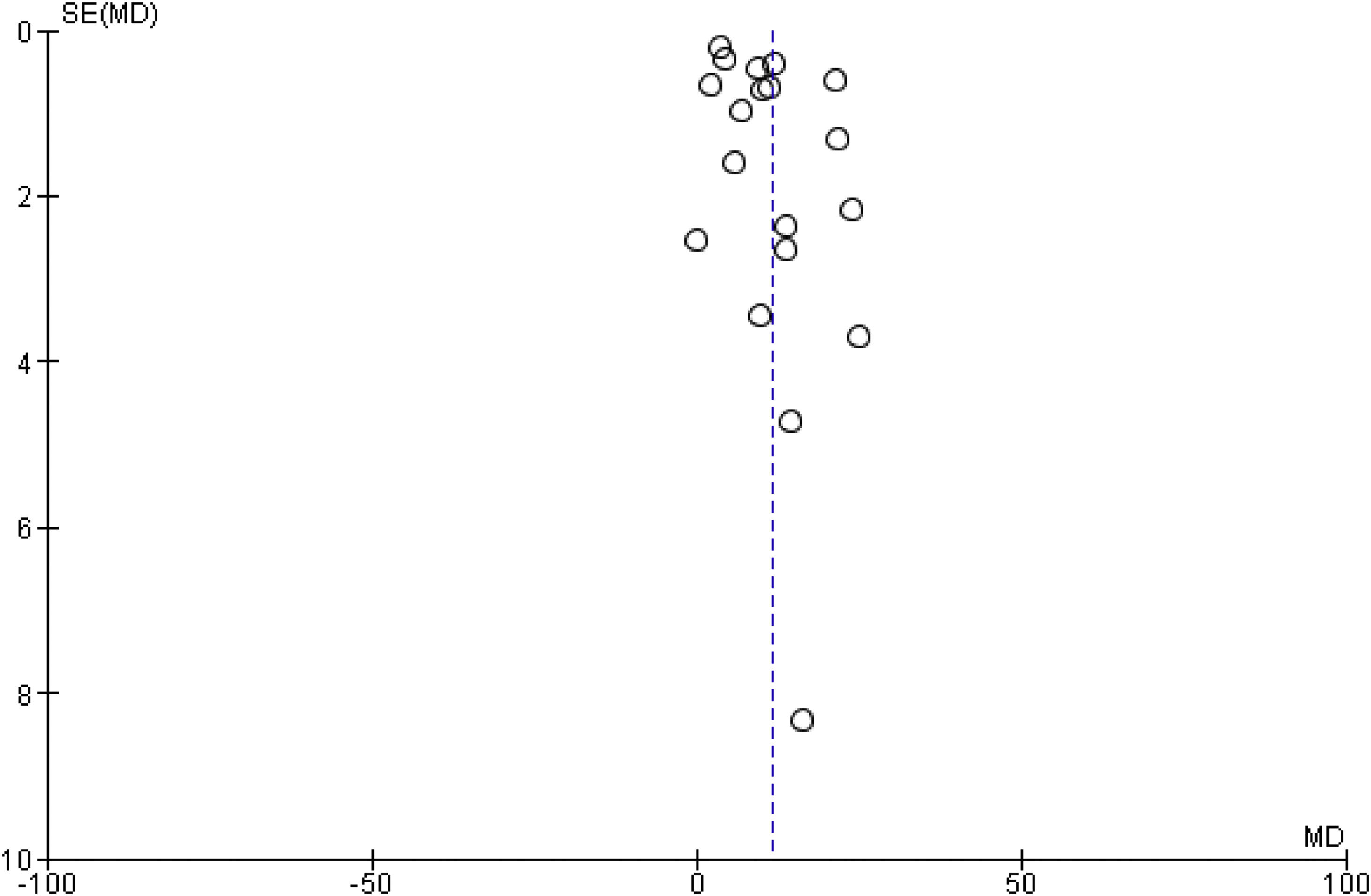
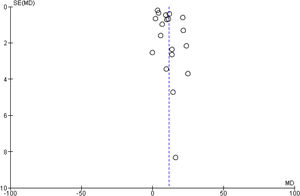
3.4.3Publication biasThere was no evidence of publication bias based on visual inspection of the funnel plot (Fig. 5).
4DiscussionCOVID-19 associated liver injury is defined as any liver damage in COVID-19 patients, with or without the pre-existing liver disease [5]. While the exact mechanism remains unknown, several potential mechanisms have been postulated [6–9]. Severe systemic inflammatory response during severe COVID-19 can lead to immune-mediated damage or ischemic hepatitis from the severe systemic inflammatory response. Current treatment options such as Lopinavir/ritonavir, hydroxychloroquine and Remdesivir are potentially hepatotoxic and may cause drug-induced liver injury (DILI) [11]. Other immunosuppressive alternative such as tocilizumab or steroid can result in reactivation of underlying chronic hepatitis B. While direct cytotoxicity due to viral replication within infected hepatocytes has been postulated, viral inclusions for SARS-COV-2 has not been found in the liver [40]. The current data on the severity and prevalence of COVID-19 associated liver injury remains conflicting due to the heterogeneity in study populations. To address these issues, we systematically reviewed the current literature on liver injury in COVID-19 and performed a meta-analysis on the severity and risk of COVID-19 associated liver injury in these patients.
At the point when this meta-analysis was conducted, most studies did not specifically report the prevalence of COVID-19 patients with baseline chronic liver disease. None of the included studies reported outcomes of COVID-19 patients based on their underlying liver disease. As all included studies reported a low incidence of chronic liver disease (CLD), our findings will be more reflective of the general population.
Our findings were consistent with current literature that adult patients with severe COVID-19 have a higher risk of liver injury. Most liver injuries were mild. To date, there is only one reported case of death from liver failure in COVID-19 patients without pre-existing liver disease. In a recently published international registry of COVID-19 among patients with chronic liver disease, only 12% of death was attributed to liver disease [41]. That having said, the mortality risk among patients with the pre-existing liver disease did increase with the severity of underlying liver disease (13% in chronic liver disease, 24% in Child-Pugh’s A cirrhosis, 43% in Child-Pugh’s B cirrhosis and 63% in Child-Pugh C cirrhosis) [41]. Based on our analysis, the pooled OR of ALT elevation was 2.5, AST elevation was 3.4, and hyperbilirubinemia was 1.7 among COVID-19 patients who were critically ill. We found that indirect markers for liver injury, such as hypoalbuminemia increased by seven-fold in patients with severe COVID-19. While we are mindful that hypoalbuminemia could be confounded by factors such as systemic inflammation, malnutrition and the timing of presentation to hospital, similar findings were reported in other studies [42–43]. Therefore, physicians should be alert about potential clinical deterioration (intubation, ICU admission and death) when COVID-19 associated liver injury was observed.
In this meta-analysis, the pooled risk of liver injury was higher among studies with high usage of Lopinavir/ritonavir. In a recent retrospective study involving 417 COVID-19 patients [17], Lopinavir/ritonavir was associated with 7-fold higher risk of liver injury. In this meta-analysis, we found a higher level of GGT and ALT elevation in studies with high Lopinavir/ritonavir usage, suggesting that Lopinavir/ritonavir might be hepatotoxic (Table 2). Although Lopinavir/ritonavir did not significantly increase the risk of liver injury in a recent open-labelled randomized trial, this trial excluded subjects with abnormal baseline liver functions. We are unable to exclude preferential treatment of severe COVID-19 patients using Lopinavir/ritonavir from this meta-analysis. Based on our findings, physicians may consider monitor for liver injury when Lopinavir/ritonavir is being used for COVID-19 patients.
While it was postulated that SARS-COV-2 can bind to ACE-2 positive cholangiocytes, elevation in cholestatic markers such as GGT is rarely reported in COVID-19. We observed a trend of higher GGT level among subjects with critical COVID-19. However, due to limited published literature on GGT in COVID-19, a statistical significance was not achieved, further studies are required to shed light on this.
This meta-analysis adds value to the current literature by summarizing the prevalence and pattern of liver injury in adults with severe COVID-19. Prior to this, the prevalence of COVID-19 associated liver injury was confounded by the heterogeneity of study population, and study design as pertinent information such as the proportion of subjects’ baseline chronic liver disease or ICU admission were often not reported [5]. In this meta-analysis, we found that the severity of most COVID-19 associated liver injury was considered mild, suggesting liver failure is uncommon among critical COVID-19. Nevertheless, physicians should be mindful of potential deterioration among COVID-19 patients liver injury as it is associated with ICU admission, mechanical ventilation and death.
There are several strengths in our meta-analysis. To our best knowledge, this is the most comprehensive description of the liver manifestation of COVID-19 in comparison. Earlier meta-analysis had either included fewer studies evaluating liver manifestation among COVID-19 patients [43–49] or included a wide range of “severe” COVID-19 patients ranging from the presence of tachypnoea to death [43–46]. In this meta-analysis, we conducted a systematic search of the literature using a pre-defined inclusion and exclusion criteria; including a large number of studies to allow assessment of publication bias and subgroup analysis, detailed extraction of data on study outcomes; subgroup analysis to define a more homogenous study population for sensitivity analysis; rigorous evaluation of study quality; and the use of various statistical methods to evaluate the validity of our findings.
Our meta-analysis was limited by the heterogeneity in the study population of included studies. We attempted to mitigate this limitation by performing subgroup analysis based on the characteristics of each study. Most of the included studies were limited by their retrospective nature of study design and small sample size. Because of this, we cannot estimate the incidence and comparative risk of drug-induced liver injury across different treatment with high confidence. While liver injury is more likely to occur in adults with critical COVID-19, we cannot be certain of its prognostic value as most of the studies were retrospective in nature.
In conclusion, COVID-19 associated liver injury is generally mild. As liver injuries are more common in patients with severe COVID-19, physicians should be mindful about the potential risk of clinical deterioration in these patients.
AUTHORS CONTRIBUTIONAll authors worked in all 4 aspects of authorship as per ICJME guidelines.
FundingWe declare that there is no funding support for this study
Conflict of interestAll authors declared no conflict of interest pertaining to this work
KEY SUMMARY- •
COVID-19 associated liver injury is generally mild. The commonest liver injury associated with COVID-19 is hypoalbuminemia (OR = 7.1) followed by elevated AST (OR = 3.4) and ALT (OR = 2.5)
- •
COVID-19 associated liver injury is common in patients requiring intensive care, mechanical ventilation and death. Physicians should be mindful about the potential risk of clinical deterioration in these patients.
We wish to thank and acknowledge the contributions of Mr. Steve Tam Yew Chong in the current work.
| Search | Query | Items found |
|---|---|---|
| #1 | Search "COVID-19" Filters: Abstract; Humans | 562 |
| #2 | Search “Coronavirus disease 2019” Filters: Abstract; Humans | 148 |
| #3 | Search “SARS-CoV-2” Filters: Abstract; Humans | 200 |
| #4 | Search ((("COVID-19" AND hasabstract[text] AND Humans[Mesh])) OR (“Coronavirus disease 2019” AND hasabstract[text] AND Humans[Mesh])) OR (“SARS-CoV-2” AND hasabstract[text] AND Humans[Mesh]) Filters: Abstract; Humans | 565 |
| #5 | Search "COVID19" Filters: Abstract; Humans | 7 |
| #6 | Search (((((("COVID-19" AND hasabstract[text] AND Humans[Mesh])) OR (“Coronavirus disease 2019” AND hasabstract[text] AND Humans[Mesh])) OR (“SARS-CoV-2” AND hasabstract[text] AND Humans[Mesh])) AND hasabstract[text] AND Humans[Mesh])) OR ("COVID19" AND hasabstract[text] AND Humans[Mesh]) Filters: Abstract; Humans | 565 |