The burden of alcoholic liver disease continues to be a major public health problem worldwide. The spectrum of disease ranges from fatty liver to cirrhosis and hepatocellular carcinoma. Alcoholic hepatitis (AH) is a type of acute-on-chronic liver failure and the most severe form of alcoholic liver disease. Severe AH carries a poor short-term prognosis and its management is still challenging, with scarce advances in the last decades. Corticosteroids are still the first line of therapy in severe cases. Unfortunately, many patients do not respond and novel targeted therapies are urgently needed. Liver transplantation has shown extraordinary results in non-responders to corticosteroids however; its applicability is very low. This review summarizes the epidemiology, natural history, risk factors and pathogenesis of alcoholic liver disease with special focus on the latest advances in prognostic stratification and therapy of patients with alcoholic hepatitis.
Alcoholic liver disease (ALD) is a major cause of chronic liver disease worldwide, leading to fibrosis and cirrhosis.1 The burden of alcohol related morbidity is well documented in a recent report from the World Health Organization, indicating that 3.3 million deaths (6% of all global deaths) were attributable to alcohol use, and that alcohol misuse accounts for 50% of cirrhosis. In Europe, in the last decade, 38% of liver transplants for cirrhosis were caused by ALD.2
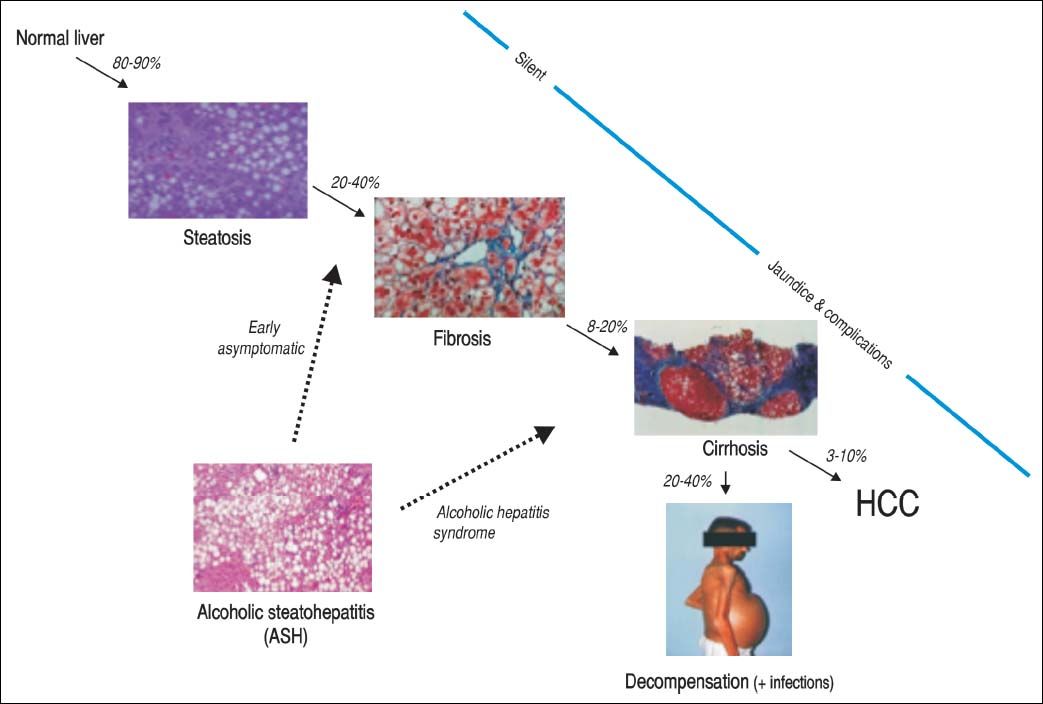
ALD presents as a broad spectrumof disorders ranging from fatty liver, steatohepatitis (ASH), progressive fibrosis, end-stage cirrhosis and superimposed hepatocellular carcinoma. Alcoholic hepatitis (AH) develops in patients with excesive alcohol comsuption with healthy livers and/ or underlying advanced fibrosis or cirrhosis; the latest form can evolve into an acute-on-chronic liver failure (ACLF) syndrome.3
The heterogeneity of AH nomenclature has lead to confusion between physicians worlwide. Additionally, the lack of reproducible animal models has difficulted advances in novel therapeutic strategies. Currently no major breakthroughs in AH therapy are described after 40 years of corticosteroids use.
To review the natural history, pathogesis and risk factors of ALD, with a special focus the prognostic assessment, therapeutic advances and future directions on AH.
Alcoholic Liver Disease: Natural History, Risk Factors and PathogenesisNatural historyALD comprises a broad spectrum of diseases ranging from fatty liver, ASH, progressive fibrosis, end-stage liver disease and superimposed hepatocellular carcinoma (Figure 1). Nearly 90% of patients with heavy alcohol consumption have some degree of liver steatosis;4 alcoholic fatty liver is the initial phase and can be considered as a mild or moderate stage in the spectrum of ALD. Histology is characterized by early-mild steatosis in zone 3 (perivenular) hepatocytes; it can also affect zone 2 and even zone 1 (periportal) hepatocytes when liver injury is more severe.5 These changes are usually asymptomatic and rapidly reversible with alcohol abstinence.
The natural history of ALD is not homogenous for all patients. Only one third will progress to advanced forms of the spectrum. Additionally, patients with underlying ALD and continued heavy alcohol consumption may develop episodes of superimposed AH and accelerate the progression to end-stage liver disease, increasing morbidity and mortality.
Risk factorsCurrently described risk factors for ALD development in alcoholics patients are age, drinking patterns, sex, obesity, dietary factors, genetic factors and cigarrette smoking. Abusive alcohol consumption is a serious health issue worldwide. High-risk drinking is defined in sex-specific terms as drinking 20 g per day or more of pure alcohol on average for females and 40 g on average for males. Age at onset of high-risk alcohol consumption is an independent risk factor for alcoholic cirrhosis. A higher risk of alcoholic cirrhosis onset was observed for an older age at the beginning of high-risk alcohol consumption.6 Female sex is a widely known risk factor due to lower levels of gastric alcohol dehydrogenase, higher body fat levels than men and the presence of estrogens. The prevalence of malnutrition is high in alcoholics with clinically severe liver disease and these nutritional deficiencies may modulate the risk of developing alcohol-related liver damage. Genetic factors might also influence susceptibility to advanced ALD, however little data are available especially in Caucasians. Variations in genes that encode antioxidant enzymes, cytokines and other inflammatory mediators, and alcohol-metabolizing enzymes, could have a role. Recently, the patatin-like phospholipase domain-containing 3 (PNPLA3) rs738409, a genetic variation consisting of an isoleucine to methionine substitution at position 148 (I148M), has been found to be associated with an increased risk of both alcohol-related cirrhosis and hepatocarcinoma. A 50% increase in the risk of cirrhosis onset was observed for each 148 M allele. Interestingly, the effect of the PNPLA3 148M allele was even more pronounced in individuals with younger compared to older age at onset of high-risk alcohol consumption.6–9
Finally, the co-existence of other hepatic insults such as chronic hepatitis B, and hepatitis C infection, non-alcoholic fatty liver disease, autoimmune liver diseases and hemocromatosis can also accelerate fibrosis.10
PathogenesisAlcoholic steatosis is a complex process manifested through several mechanisms. The main pathogenetic factors underlying this process are increased fatty acid (FA) and triglyceride synthesis, enhanced hepatic influx of free fatty acids from adipose tissue and of chylomicrons from the intestinal mucosa, increased hepatic lipogenesis, inhibited lipolysis, and damaged mitochondria and microtubules, all of which result in accumulation of very low-density lipoproteins.11 Recent studies suggest that alcohol up-regulates sterol regulatory element-binding protein 1c (SREBP-1c), a master transcription factor that stimulates expression of lipogenic genes and subsequently promotes fatty acid synthesis in the hepatocyte.1 In addition, alcohol down-regulates adenosine monophosphateactivated protein kinase (AMPK). AMPK inactivates acetyl-CoA carboxylase 1 leading to reduced fatty acid synthesis and increased fatty acid oxidation, promoting steatosis.12
Several molecular mechanisms contribute to the development of steatohepatitis. Alcohol abuse results in changes in the colonic microbiota and promotes the translocation of bacteria from the gastrointestinal lumen to the portal vein, where they bind to the lipopolysaccharide-binding protein.13 In Kupffer cells, lipopolysaccharide (LPS) binds to CD14, which combines with toll-like receptor (TLR)-4 activating multiple cytokine genes with inflammatory cytokine production, decreasing the expression of signal transducer and activator of transcriptions (STATs) leading to reduced liver regeneration.14,15 More recent translational studies showed that CXCL chemokines, rather than TNF-α, are markedly overexpressed in AH and correlate with survival.16 Other inflammatory mediators such as Fn14, CXC20 and osteopontin have been identified as potential targets for therapy in AH.17–19 Longterm alcohol consumption alters the intracellular balance of antioxidants with subsequent decrease in the release of mitochondrial cytochrome c and expression of Fas ligand, leading to hepatic apoptosis through the caspase-3 activation pathway.20 Besides inflammation, bile stasis and liver fibrosis are major histological components of AH and are associated with a poor prognosis. Initially, the fibrotic tissue is typically located in pericentral and perisinusoidal areas with expansion of collagen bands and bridging fibrosis in advanced stages, which precedes the development of regeneration nodules and liver cirrhosis.21
Although the cellular and molecular mechanisms of fibrosis are not completely understood, alcohol metabolites (e.g. acetaldehyde) can directly activate hepatic stellate cells. Hepatic stellate cells are activated in a paracrine fashion by activated Kupffer cells, damaged hepatocytes and polymorphonuclears.22,23 Finally, Interleukin-22 (IL-22) has shown hepatoprotective and anti-fibrotic functions, with relatively few known side effects in animal and translational studies.24–26 IL-22 treatment has also showed protective effects on bacterial infection and kidney injury, two major complications associated with severe AH.27,28 Currently, the effects of IL-22 in AH are under investigation in preclinical and clinical studies.
Alcoholic Hepatitis: Clinical Presentation and Diferential DiagnosisThe diagnosis of AH should be suspected in all patients with excessive alcohol consumption and the coexistence of clinical and/or biological features suggestive of liver injury.
AH should be considered as a clinical syndrome defined by the abrupt onset of jaundice and/or liver decompensation in a patient with history of chronic high-risk alcohol consumption. Patients with AH may also present with fever, infection, weight loss, malnutrition, and tender hepatomegaly. In its severe forms, AH is frequently associated with liver decompensation (e.g. ascites, encephalopathy, gastrointestinal bleeding, acute kidney injury or bacterial infection development). Thus, a high level of suspicion for AH diagnosis is mandatory in patients presenting liver decompensation and risky alcohol comsumption. Recently, an AH prevalence of 80% was reported in cirrhotics with gastrointestinal bleeding and recent development of jaundice, modified discriminant function (mDF) ≥ 32 and active alcohol intake.29
Analytical evaluation typically shows increased AST levels (up to 2-6 times the upper normal limit) and an AST/ ALT ratio > 2. The proposed mechanisms accounting for this feature is explained by the reduced hepatic alanine aminotransferase activity, alcohol-induced depletion of hepatic pyridoxal 5’-phosphate, and increased hepatic mitochondrial aspartate aminotransferase. Increased bilirubin, elevation of cholestatic enzimes (e.g. alkaline phophatase and gamma-glutamyl transpeptidase) and neutrophilia are also commonly observed features. Liver sinthetic function is usually altered. Serum albumin is frequently decreased, prothrombin time prolonged and the international normalized ratio (INR) may be increased.
The presence of AH can be highly suspected based on clinical and analytical criteria as described above, however, histological confirmation is recommended for severe cases. Liver biopsy using transjugular approach is preffered due to the presence of coagulopathy in most cases. This recommendation is supported by the European Association for the Study of the Liver (EASL) latest guidelines.30 In most cases, advanced liver fibrosis (mainly cirrhosis) and superimposed ASH is found. ASH is defined by the coexistence of steatosis, hepatocyte ballooning and/or Mallory-Denk bodies, and an inflammatory infiltrate with neutrophils. Other typical features include presence of megamitochrondria and bilirubinostasis. Of note, the presence of canalicular and/or ductular bilirubinostasis has been associated with bacterial infections (overt or covert) in these patients.32
Differential diagnosis with other possible liver damage entities such as drug-induced liver disease, ischemic hepatitis (especially in patients with concomitant cocaine consumption), foamy liver degeneration, septic liver, progressive ALD and malignancies, require histological sampling.
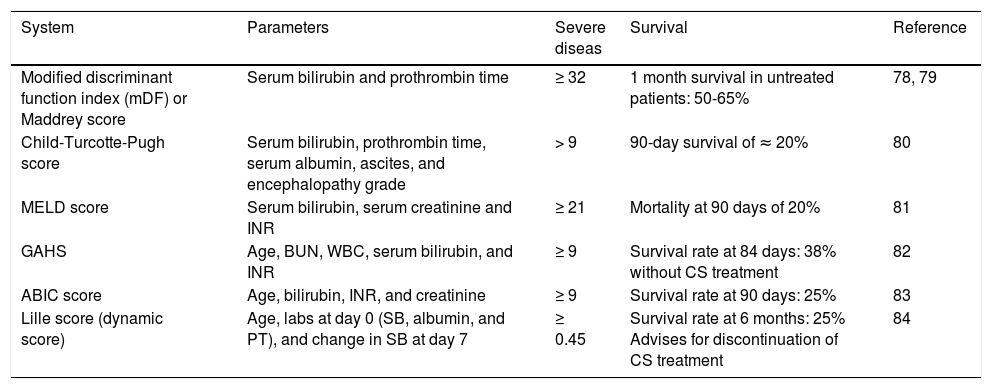
Prognostic AssessmentPrognostic scoring systemsPrognostic scores are useful to assess severity of an AH episode and the need for a specific therapy to improve clinical outcomes in patients with severe disease. There are non-specific scores and disease-specific scores for this purpose. Many scoring systems have been developed for use in clinical practice (Table 1).
Scoring systems for severity assessment in alcoholic hepatitis.
| System | Parameters | Severe diseas | Survival | Reference |
|---|---|---|---|---|
| Modified discriminant function index (mDF) or Maddrey score | Serum bilirubin and prothrombin time | ≥ 32 | 1 month survival in untreated patients: 50-65% | 78, 79 |
| Child-Turcotte-Pugh score | Serum bilirubin, prothrombin time, serum albumin, ascites, and encephalopathy grade | > 9 | 90-day survival of ≈ 20% | 80 |
| MELD score | Serum bilirubin, serum creatinine and INR | ≥ 21 | Mortality at 90 days of 20% | 81 |
| GAHS | Age, BUN, WBC, serum bilirubin, and INR | ≥ 9 | Survival rate at 84 days: 38% without CS treatment | 82 |
| ABIC score | Age, bilirubin, INR, and creatinine | ≥ 9 | Survival rate at 90 days: 25% | 83 |
| Lille score (dynamic score) | Age, labs at day 0 (SB, albumin, and PT), and change in SB at day 7 | ≥ 0.45 | Survival rate at 6 months: 25% Advises for discontinuation of CS treatment | 84 |
ABIC: age, bilirubin, INR, creatinine. GAHS: Glasgow alcoholic hepatitis score. INR: International Normalized Ratio. MELD: Model for End-Stage Liver Disease. PT: prothrombin time in seconds. SB: serum bilirubin in mg/dL. WBC: white blood count in 109/L. CS: corticosteroids.
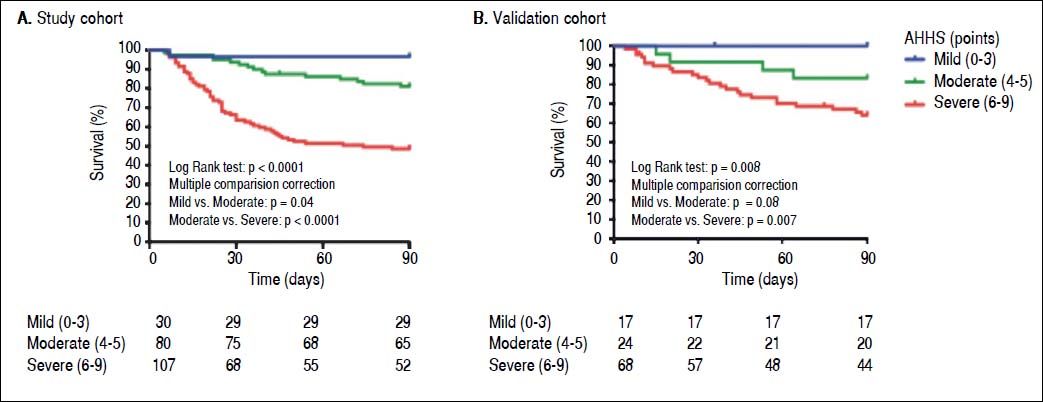
Recently, we performed a large multicentre study to develop a histological scoring system capable of predicting short-term survival in patients with AH. The resulting Alcoholic Hepatitis Histological Score (AHHS) includes 4 parameters that are independently associated with patients’ survival: stage of fibrosis, degree of neutrophil infiltration, type of bilirubinostasis, and presence of megamitochondria. The combination of these histological features in a semiquantitative manner, was able to stratify patients into 3 different risk groups of death at 90 days (Figure 2).31
Which is the most accurate scoring system to predict survival in AH?Several comparative studies have tried to answer this question. One cross-validation study in 71 biopsy-proven AH patients found that there were no significant accuracy differences in the area under the receiver characteristic curves (AUROCs) of the evaluated scoring systems relative to 30-day/90-day mortality: model for end-stage liver disease (MELD) 0.79/0.84, DF 0.71/0.74, Glasgow Alcoholic Hepatitis Score (GAHS) 0.75/0.78, age, bilirubin, INR, creatinine (ABIC) score 0.71/0.78, MELD-Na 0.68/ 0.76, United Kingdom Model for End-Stage Liver Disease (UKELD) 0.56/0.68. In the same study, one-week rescoring yielded a trend towards improved predictive accuracies for all scoring systems. In addition, when evaluating patients with mDF ≥ 32 (n = 31), response to corticosteroids according to early change in bilirubin levels at 7 days (defined as a 25% fall in bilirubin levels at day 7 vs. admission levels) and the Lille model yielded AUROCs of 0.73/ 0.73, 0.78/0.72 and 0.81/0.82 for a 30-day/90-day outcome, respectively. All models showed excellent negative predictive values (NPVs; range: 86-100%).32 A similar trend was described in a recent study including 274 patients with clinical diagnosis of AH in Denmark. The AUROCs generated to predict mortality at 28/84/180 days for MELD, MELD-Na, GAHS, Lille-model, and the ABIC scoring systems yielded non significant differences between them with improved performances when re-scoring one week after admission.33
Importantly, a recent multinational multicenter study including a large cohort of patients with AH showed that combining static scoring systems (e.g.mDF; ABIC; and MELD) with dynamic models (e.g. Lille model), results in enhanced outcomes prediction when compared with either model alone.34 In this study, authors evaluated 3 jointeffect models’ capacity (Maddrey+Lille, MELD + Lille, and ABIC + Lille) to predict patient survival after 2 and 6 months. All of them, in the derivation and validation cohorts, predicted outcomes significantly better than either static or dynamic models alone (P < 0.01 for all compari-sons).34
Other key events with prognostic impact- •
Acute kidney injury (AKI): Another factor associated with mortality in AH is the development of acute kidney injury (AKI). In a recent study AKI, defined as an absolute increase of serum creatinine of 0.3 mg/dL or a 50% increase above baseline, was associated with a marked decrease in 90-day survival.35 Interestingly, patients with systemic inflammatory response syndrome (SIRS) at admission developed AKI in much higher proportion. Moreover, a recent study associated the development of AKI in severe AH with non-selective beta-blocker therapy.36
- •
Systemic inflammatory response syndrome and multiple organ dysfunction. Patients with AH often show criteria of SIRS even in the absence of an infection at the time of admission. Recent data correlated SIRS with increased mortality, multiple organ failure (odds ratio [OR] = 2.69, p = 0.025) and acute kidney injury development (OR 2.9, p < 0.001).37,38 Additionally procalcitonin, but not high-sensitivity C-reactive protein, serum levels at admission can identifiy those patients with SIRS and ongoing infection (overt or covert). Finally, LPS serum levels have been associated with development of multiorgan failure (MOF) during admission and the response to prednisolone at day 7.37
- •
Infections. A systematic screening for infections is recommended. In patients with severe AH (SAH), infection prevalence at admission is about 25.6% according to a prospective study. Interestingly, a 23.7% of infections developed after steroid treatment. Infection occurred more frequently in nonresponders than in responders (42.5 vs. 11.1%, respectively; p < 0.001) and are associated with a higher short and long-term mortality.39 A recent study has shown that the probability of developing infection is lower for patients with concomitant SAH and gastrointestinal bleeding (24.1 vs. 44.7%, p = 0.04). This observation suggests a benefitial role for prophylactic antibiotics.29
Infections are the second cause of death in the first 84 days after a first episode of AH according to a recent large Danish registry analysis.40
Finally, a recent meta-analysis assessing mortality from infections in SAH did not find an increased occurrence or mortality from bacterial infections in patients with severe alcoholic hepatitis and steroid treatment.41 However these authors found an increased rate for fungal infections during admission. On this regard, a recent Belgian study has identified invasive aspergillosis (IA) as a frequent complication in patients with SAH and carries a very high risk of mortality,42 subsecuently recommending systematic screening for IA in patients with SAH.
Nutritional support includes providing adequate calories and protein, as well as vitamin (e.g., thiamine, folate, and pyridoxine) and mineral (e.g., potassium, phosphate, magnesium) repletion. Vitamin B1 supplementation is advisable due to the high risk of Wernicke encephalopathy development. Vitamin K is usually given to patients with prolonged prothrombin time, even though this regimen is often ineffective because the coagulopathy is more a reflection of the severity of liver failure than real vitamin K deficiency. Oral intake should be started as soon as possible and approximately a daily dose of 1.5 g/kg of body weight of protein is recommended. Low calorie intake was associated with mortality in a recent randomized trial, at least 21.5 kcal/kg/day should be guaranteed.43 Hepatic encephalopathy should be treated with non-absorbable disaccharides. Rifaximin can be added if treatment with non-adbsorbable disaccharides is not effective after 24-48 h.44 Precipitating factors must be recognized and controlled.
Intensive care unit admission is recomended in unstable patients (patients with severe sepsis, septic shock, grade III-IV encephalopathy, acute variceal bleeding or withdrawal syndrome unable to be controlled with standard measures). Clinical outcomes from septic shock and unstable cirrhotic patients admitted to intensive care units have improved in the latest years, which supports the arguments to offer intensive care and monitoring to patients with severe forms of AH.45 Airway management with orotracheal intubation may be necessary in cases with high grades of hepatic encephalopathy or uncontrolled agitation (e.g. severe alcohol withdrawal syndrome). Finally, benzodiazepines are generally contraindicated in patients with AH, but might be necessary in the case of severe alcohol withdrawal.
Since patients with AH are prone to develop infections that negatively impact their prognosis, a complete work-up to rule out infection is mandatory. Diagnostic paracentesis, blood cultures, urine and sputum cultures should be done and repeated in case of clinical deterioration. Although not contemplated in current guidelines, antibiotic prophylaxis might have a beneficial role in AH. A recent study showed that infection development was lower in patients with AH receiving antibiotics after gastrointestinal bleeding compared to those without it.29 Trials addressing this issue are undergoing (NCT02116556).
Specific therapies- •
Corticosteroids. The rationale for use of corticosteroids in AH is derived from their immunological and anti-inflammatory effects. Despite conflicting data,46-49 corticosteroids are still the first-line of treatment in SAH (mDF > 32).50,51 Data from a recent network meta-analysis including 22 randomized controlled trials (RCT) (2,621 patients), comparing 5 different interventions, supports the use of corticosteroids alone (relative risk [RR], 0.54; 95% credible interval [CrI], 0.39-0.73) or in combination with pentoxifylline (RR, 0.53; 95% CrI, 0.36-0.78) or NAC (RR, 0.15; 95% CrI, 0.05-0.39), to reduce short-term mortality in patients with AH. However, no treatment showed efficacy in reducing medium-term mortality. Moreover, in the interaction analysis, the authors found that the addition of N-acetylcysteine (NAC), but not pentoxifylline (PTX), to corticosteroids may be superior to corticosteroids alone to reduce short term mortality.52
Recently a large double-blind, factorial (2 × 2) designed multicentric trial (steroids or pentoxifylline for alcoholic hepatitis -STOPAH- trial), randomized 1,093 patients with SAH in 63 centers from the UK, to one of four groups of treatment:
- °
Group A. Placebo/placebo.
- °
Group B. Placebo/prednisolone.
- °
Group C. Pentoxifylline(PTX)/placebo.
- °
Group D. PTXprednisolone.
The primary endpoint was mortality at 28 days, secondary endpoints being mortality at 90 days and 1 year. For PTX the OR for mortality at 28 days was 1.07 (95% confidence interval [CI] 0.77-1.49; p = 0.68) and for prednisolone 0.72 (95% CI 0.52-1.01; p = 0.056). After adjustment for baseline severity and prognostic factors (e.g., age, INR, urea, WBC, creatinine and hepatic encephalopathy) the OR in the prednisone treated group was 0.61 (95% CI 0.41-0.90; p = 0.01). No significant differences were found between treatment groups for secondary outcomes. In conclusion, pentoxifylline did not improve survival in patients with AH and prednisolone was associated with a reduction in 28-day mortality with no improvement in outcomes at 90 days or 1 year.53.
- °
- •
Pentoxifylline. Pentoxifylline is a phosphodiesterase inhibitor with modulatory effect on TNF-α transcription. Previous experimental and human data revealed increased TNF-α levels in AH suggesting a possible role in its physiopathology. The seminal study with PTX therapy in AH showed a 25% improvement on mortality rate associated to hepatorenal syndrome reduction.54 However, data from the STOPAH trial did not reproduce this survival benefit with PTX in a large series of patients treated with this drug.53 Combination of PTX with corticosteroids has been also evaluated in a RCT with no evidence of survival improvement at 6-months when compared to corticosteroids alone.55 Finally, rescue treatment with PTX in patients with AH not responding to corticosteroids has showed no benefit on 2-month survival.56 In conclusion pentoxifylline does not seem to be effective in SAH treatment according to current data.
- •
N-acetylcysteine. The combination of NAC, an antioxidant, with prednisolone has been studied for the treatment of severe AH. In this trial, 174 patients were randomized to receive prednisolone plus NAC (n = 85) or prednisolone alone (n = 89). Combination therapy with prednisolone plus NAC improved 1-month survival (p = 0.006) without differences at 6-months (p = 0.07).57 However, given the trend toward an increased survival in those treated with NAC plus corticosteroids, additional confirmatory studies using this strategy are warranted.
- •
Biological anti-TNF-αtherapies. Both infliximab (a chimeric mouse-human anti-TNF antibody) and etanercept (a competitive inhibitor of TNF-α to its cell surface receptors) have been tested in AH patients. Both molecules increased mortality due to an increased rate of severe infections.
In consecuense, biological anti-TNF-α therapies do not have a current role in AH treatment.58,59
Anabolic-androgenic steroids like oxandrolone have been tested in AH. Oxandrolone had efficacy only in those with moderate malnutrition,60 however a Cochrane systematic review could not demonstrate any significant beneficial effects on any clinically important outcomes.61 Metadoxine, a drug with antioxidant properties, has been also evaluated. Results from a single center study have shown that additiion of metadoxine to corticosteroid treatment improves the short-term survival of patients with SAH (74.3 vs. 45.7%, P = 0.02).62 Other antioxidants such as vitamin E and silymarin have not shown any survival benefit in patients with AH.63,64 Propylthiouracil also showed conflicting results in AH treatment,65,66 however a systematic review did not find any efficacy.67
Artifical and bioartificial liver support systemsMolecular adsorbent recirculating system (MARS) and Prometheus devices have been tested in patients with ACLF (some of them triggered by superimposed AH). The MARS device, but not the Prometheus device, showed a significant attenuation of hyperdynamic circulation in ACLF, presumably by a difference in the removal rate of certain vasoactive substances.68 In addition, treatment with MARS also produces an acute reduction on portal pressure and improves encephalopathy in patients with AH.69,70 Currently a novel bioartificial liver support system (ELAD) is being evaluated for the treatment of patients with AH not responding to corticosteroids (ClinicalTrials.gov Identifier NCT01829347).
Liver transplantation in AHLiver transplantation (LT) is not widely contemplated for patients with AH due to active alcoholism. Currently, the most widely accepted rule for LT enrollment is 6-months abstinence prior to waiting list admission. A multicenter multinational European case-control study has challenged this “golden rule” enrolling 26 highly selected patients with a first episode of severe AH and failure to respond to corticosteroids, to receive LT as a salvage therapeutic maneuver. All included patients had good family support, no severe coexisting conditions, and positive commitment to alcohol abstinence. Not surprisingly, the cumulative 6-month survival was higher among patients who received early transplantation than those who did not (77 ± 8% vs. 23 ± 8%, p < 0.001). This benefit was maintained through 2 years of follow-up (hazard ratio, 6.08; p = 0.004). Importantly, only less than 2% of evaluated patients in all participating centers with an episode of AH were listed for a LT. Although the follow-up was limited, the rate of alcohol relapse was extremely low (3 out of 26 patients).71 More recenlty, an American case-control study aimed to reproduce this strategy, including 9 (9.6% of the potential candidates) carefully selected patients with a first-epidose of severe AH refractory to corticosteroids to receive “early LT”. The 6-month survival rate was higher among those receiving early LT compared with matched controls (89 vs. 11%, p < 0.001) with 8 out of 9 recipients alive at 735 days of follow-up and 1 patient with alcohol relapse.72 Although, “early LT” for severe AH has shown excellent clinical outcomes with low impact on the donor pool and low rates of alcohol relapse in highly selected patients, this “rescue treatment” needs extensive validation with larger series of patients and should be only performed at specialized LT centers.
Alcohol Misuse InterventionsAbstinence from alcohol is the key and most effective treatment of patients with ALD. Cessation of alcohol misuse is of paramount importance in the management of patient with AH. Data from a long term follow-up study has identified abstinence as the only independent predictor of long-term survival in patients with severe AH, with alcohol relapse occurring in two-thirds of patients during follow-up.73 Currently, we do not have any validated tool to predict alcohol abstinence after an episode of AH.
Behavioral therapy must be a central component of alcoholism treatment. Brief motivational interventions are encouraged during hospitalization, although this approach is might be limited by poor mental status in some patients. To succesfully accomplish abstinence, a multidisciplinary team approach involving addiction specialits, social workers and hepatologists is required. Pharmacological therapies (e.g. anticraving drugs) may be used, however data in patients with liver diseases are scarce. Baclofen, an alphaaminobutyric acid beta-receptor agonist, has been successfully tested in patients with cirrhosis. In a RCT, cumulative abstinence duration was two-fold higher in patients allocated to baclofen than in those assigned to placebo (mean 62.8 [SE 5.4] vs. 30.8 [5.5] days; p = 0.001). No liver side-effects were recorded.74 Baclofen tolerability and safety for patients with AH has been also studied in a recent retrospective study with promising results.75 Further prospective and randomized studies exploring the dose-dependent efficacy and safety of Baclofen are needed. Finally, naltrexone and acamprosate may be useful in mantaining abstinence, however such drugs have not been tested in patients with advanced liver disease.76,77 Disulfiram is unsafe in this setting due to its high risk of fulminant hepatitis.
Current Situation and Future DirectionsAlthough there are no dramatic improvements in survival rates with available pharmacological therapies for AH, much progress has been made in understanding the pathogenesis of ALD, resulting in improved prevention of complications and promising molecular targets for more effective treatments such as IL-22. Future studies targeting amelioration of liver damage with hepatoprotective and antifibrogenic molecules, alone or in combination with current treatments, are warranted. Early LT for AH has excellent clinical outcomes with low impact on the donor pool and low rates of alcohol relapse. However, this therapeutic approach can only be offered to a small group of highly selected patients (≈ 2-% of patients with AH) in LT centers with extensive experience. Antibiotic prophylaxis for bacterial infections might be a good strategy to reduce complications and death in patients with AH and studies are undergoing (NCT02116556, NCT02326103, NCT02281929). Finally, prospective studies testing pharmacological treatments for sustained abstinence are urgently needed.
Conflict Of InterestVP, JC, VV, RB and JA declare no conflic of interest.
Grant Support and AcknowledgmentsThis work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA)(1U01AA021908-01). JA wishes to express his gratitude to the Mexican National Council of Science and Technology (CONACyT, Mexico City, Mexico) for partially supporting his predoctoral stay at IDIBAPS. We thank Monica Cruz-Lemini for her assistance with final editing, reviewing and improving the use of English in this manuscript.