Renal failure in cirrhotic patients is a very severe condition. Hepatorenal syndrome has the worst prognosis among all causes of kidney failure in such patients. Hepatorenal syndrome is diagnosed especially in cirrhotic patients with ascites who develop loss renal function, despite diuretic suspension and volume expansion with albumin and for whom other causes of kidney injury have been excluded. Patients with hepatorenal syndrome should be treated with a vasoconstrictor in combination with albumin as a bridge to receiving a liver transplant. The vasoconstrictor of choice is terlipressin or noradrenaline. In spite of higher drug-related costs associated to terlipressin, initial evidence demonstrates that, considering all direct medical costs involved, the treatment strategy using terlipressin is probably more economical than that using noradrenaline.
Cirrhosis is the final stage of chronic liver disease and is detected in 4.5 to 9.5% of all necropsies. In 2001, it was the fourteenth most frequent cause of death worldwide and was considered responsible for 771,000 deaths. It is expected that cirrhosis will be the twelfth most frequent cause of death by 2020.1 Most cirrhosis-related deaths are related to decompensation. The complications of cirrhosis have been used to describe its natural history in four stages2 and more recently as five or six stages, the last of which includes sepsis and/or renal failure.3,4
Renal failure has a poor prognosis in cirrhotic patients.4 Its impact on prognosis is so dramatic that it is the only single-organ failure used to determine the diagnosis of acute-on-chronic liver failure.5 The diagnosis and classification of renal failure in cirrhotic patients have been studied thoroughly. It is believed that the classical criterion based solely on a creatinine level > 1.3-1.5 mg/dL is not an ideal marker of kidney injury in these patients. This is because their creatinine level can be affected by renal dysfunction as well as changes in its production and distribution volume.6
The RIFLE classification (RIFLE: risk, injury, failure, loss of kidney function, and end-stage kidney disease), for instance, has been studied in critically ill cirrhotic patients. It was demonstrated that patients without renal impairment had an in-hospital mortality of 32.1%, while mortality rates were 68.8%, 71.4% and 94.8% for patients classified as RIFLE-R, RIFLE-I and RIFLE-F respectively (P < 0.001).6
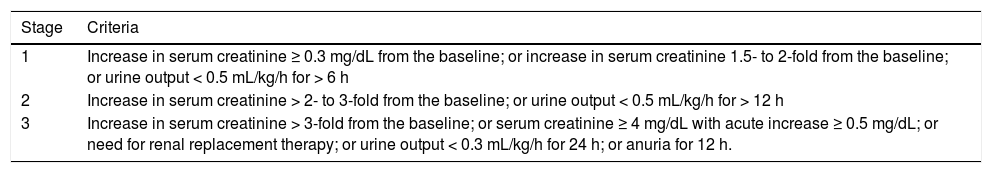
The acute kidney injury network (AKIN) criteria (Table 1)7 have been applied in cirrhotic patients in a much wider context.8–12 In the outpatient environment, cirrhotic patients diagnosed with kidney injury using the AKIN criteria have worse survival compared with controls, regardless of whether they recover normal renal function.9 Among hospitalized cirrhotic patients, mortality was greater for patients who lost renal function during hospitalization than for those who were already admitted with kidney injury (36% and 21% respectively, p = 0.01).10 Moreover, the progression of renal failure according to AKIN classification was independently associated with a greater mortality. On the other hand, in this study, the mortality of patients who did not progress beyond AKIN stage 1 was much lower than that of patients who reached AKIN stages 2 or 3 (2%, 15% and 44% respectively).10 Such a low mortality among patients who did not progress beyond AKIN stage 1 is noteworthy because it implies the need to weigh the advantages of using more-sensitive criteria for the diagnosis of renal failure in cirrhotic patients with the disadvantages of using less-specific criteria for assessing the prognosis of these patients.
Acute kidney injury network (AKIN) criteria for staging acute kidney injury.
| Stage | Criteria |
|---|---|
| 1 | Increase in serum creatinine ≥ 0.3 mg/dL from the baseline; or increase in serum creatinine 1.5- to 2-fold from the baseline; or urine output < 0.5 mL/kg/h for > 6 h |
| 2 | Increase in serum creatinine > 2- to 3-fold from the baseline; or urine output < 0.5 mL/kg/h for > 12 h |
| 3 | Increase in serum creatinine > 3-fold from the baseline; or serum creatinine ≥ 4 mg/dL with acute increase ≥ 0.5 mg/dL; or need for renal replacement therapy; or urine output < 0.3 mL/kg/h for 24 h; or anuria for 12 h. |
An Italian study11 also called attention to the same matter, when authors evaluated the role of AKIN criteria on defining the prognosis of in-hospital cirrhotic patients with ascites and compared it to that of the conventional diagnostic criterion for renal failure (≥ 50% increase in creatinine concentration to > 1.5 mg/dL). Acute kidney injury was diagnosed in 26% of patients according to AKIN criteria and in only 12% according to the conventional criterion. Normal renal function was recovered by 50.8% of the former patients and only by 35.7% of the latter patients. Besides, a cutoff creatinine level of 1.5 mg/dL was associated to progressive renal failure, which was strongly associated to mortality. In this study, there was no difference between mortality rates for patients without renal dysfunction compared with patients with AKIN stage 1 kidney injury, and the conventional criterion was a better predictor of mortality than the AKIN criteria.11 A Spanish study that evaluated the use of these criteria in in-hospital cirrhotic patients reported similar results. This study confirmed that patients diagnosed with AKIN stage 1 with a creatinine level ≤ 1.5 mg/dL had a similar survival as that of patients with a normal renal function (84% and 88%, respectively; P = 0.52). These survival rates were higher than that of patients diagnosed with AKIN stage 1 with a creatinine level > 1.5 mg/dL (68%; P < 0.05).12
Another study evaluated 337 hospitalized cirrhotic patients with infection. Thirty-day mortality was higher for the 166 patients who developed renal dysfunction diagnosed using the AKIN criteria than for those who did not develop kidney injury (34% and 7%, respectively; P < 0.001).13 In a reanalysis of the data, the authors confirmed that 30-day survival was lower in patients who developed acute kidney injury using the AKIN criteria and had a creatinine level ≤ 1.5 mg/dL than in those who did not lose renal function (81% and 93%, respectively; P = 0.038) and was similar to that of patients with a creatinine level > 1.5 mg/dL (81% and 63%, respectively; P = 0.09). The authors concluded that using the conventional criterion of serum creatinine concentration > 1.5 mg/dL to diagnose acute kidney injury could lead to detrimental clinical decisions.14
Hepatorenal SyndromeAmong the causes of renal failure in cirrhosis, hepatorenal syndrome (HRS) has the worst prognosis. In a prospective study of cirrhotic patients with loss of renal function, 3-month survival for patients with parenchymal nephropathy, hypovolemia-associated renal failure, renal failure associated with infection, or HRS were 73%, 46%, 31%, and 15%, respectively (P < 0.0005).15
HRS is a severe, yet potentially reversible, complication in patients with cirrhosis and ascites, severe acute liver failure, or severe alcoholic hepatitis.16 Among cirrhotic patients with ascites, the incidence of HRS development is 18% at 1 year and 39% at 5 years.17 Known since the 19th Century, HRS is characterized by mainly functional renal failure that is not usually related to significant injuries in kidney anatomy or histology. The etiopathogenic substrate of HRS consists of an important vasoconstriction of renal arterial system.16,18
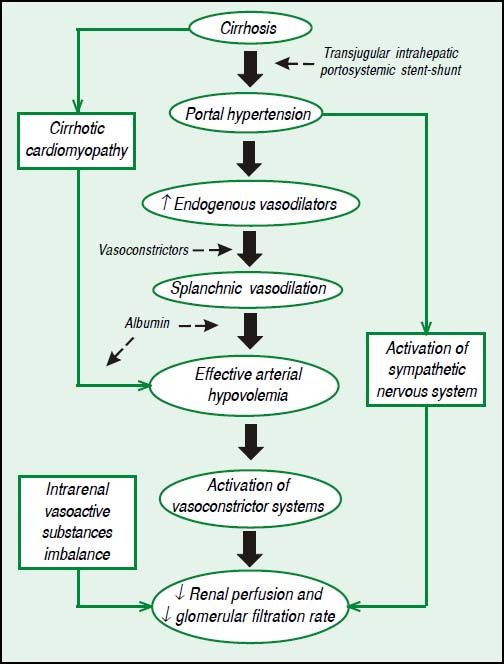
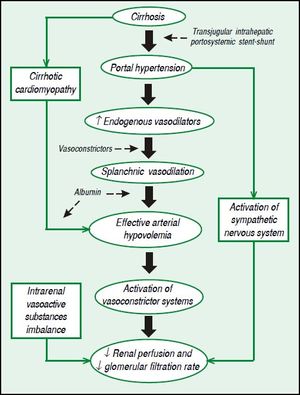
Pathophysiology of HRSThe process begins with severe liver dysfunction, a restriction to blood flow to the liver, a reduction in the production of vasodilators by the liver and an increase in the contractility of stellate cells. The resultant portal hypertension leads to increased tension of the walls of splanchnic vessels and, therefore, to increased production of vasodilators, such as nitric oxide, causing splanchnic vasodilation. With the progression of portal hypertension and the development of portosystemic collaterals, blood flow and vasodilators are redirected to systemic circulation, leading to systemic vasodilation, effective arterial hypovolemia and a hyperdynamic circulation. In order to compensate for effective arterial hypovolemia, vasoconstrictor systems are activated, such as renin-angiotensin-aldosterone system, as well as sympathetic nervous system, leading to a reduction of renal perfusion and to a decrease of glomerular filtration rate, once kidneys in cirrhotic patients are unable to produce proper quantity of vasodilators, such as prostaglandins and kallikrein. Renal hypoperfusion also increases the production of renal vasoconstrictors, such as angiotensin II and endothelin. Finally, cirrhotic patients have a limited cardiac reserve, and the increased cardiac output associated to a hyperdynamic circulation is not sufficient to compensate for an excessively low systemic vascular resistance. This leads to systemic arterial hypotension and undermine even more renal perfusion.8Figure 1 shows the pathophysiology of HRS with therapeutic targets.
Classification and diagnosis of HRSHRS is classified as types 1 and 2. Type 1 HRS is rapidly progressive renal failure, in which the creatinine level more than doubles and may reach > 2.5 mg/dL in < 2 weeks. It is usually associated with a precipitating factor, often an infection.16,19 Type 1 HRS has a worse prognosis and, if left untreated, the median survival from type 1 HRS is < 2 weeks, and the survival rates are 25% and 10% at 1 and 3 months, respectively.17 Type 2 HRS is characterized by moderate renal dysfunction and a creatinine level of 1.5-2.5 mg/dL. Type 2 HRS evolves slowly and, usually, spontaneously, and is typically associated with refractory ascites.16,19 The 3-month survival is 70%.17
Since 2007, the diagnosis of HRS has been based on increased creatinine concentration to > 1.5 mg/dL in a patient with cirrhosis and ascites who does not recover after 48 h of suspension of diuretics and volume expansion with albumin (1 g/kg/day up to a maximum of 100 g/day). The diagnosis requires the exclusion of shock, recent use of nephrotoxic drugs, and parenchymal kidney disease, as suggested by a 24-h urinary protein > 500 mg, urinary sediment with an erythrocyte count > 50 cells per high-power field, or an abnormal renal ultrasound.16,20 The International Club of Ascites has recently proposed a revision of these diagnostic criteria. In their new suggestion, instead of using a fixed cutoff of creatinine concentration of 1.5 mg/dL, the HRS diagnosis should be based on an increase ≥ 0.3 mg/dL (or ≥ 50%) over the baseline.21 As previously discussed, this may increase the sensitivity of the diagnosis, but we believe that the impact of this proposal on the assessment of the prognosis of patients diagnosed with HRS needs to be evaluated further.
Biomarkers have been studied in attempts to improve the differential diagnosis of the causes of renal failure in cirrhosis. Urinary neutrophil gelatinase-associated lipocalin (uNGAL) seems to be the most promising biomarker at this time.20,22,23 In a recent study of 241 cirrhotic patients, uNGAL levels were higher in patients with acute tubular necrosis compared with those with prerenal acute kidney injury, chronic kidney disease, or HRS (P < 0.001).22 In another prospective study of cirrhotic patients with renal dysfunction, some biomarkers, including uNGAL, were significantly elevated in patients with acute tubular necrosis.23
Prophylaxis of HRSThe most frequent triggering factors for HRS are infection, digestive bleeding, and large-volume paracentesis without volume expansion with albumin.16 Some measures can help prevent the development of HRS. When treating infections, in addition to proper treatment with antibiotics, albumin administration can have a major preventive role. In treating spontaneous bacterial peritonitis, 1.5 g/kg albumin on day 1 and 1 g/kg on day 3 reduced the incidence of HRS and mortality.24 A recent meta-analysis confirmed that patients with spontaneous bacterial peritonitis treated with albumin have lower rates of renal failure and death, but the analysis could not define whether albumin should be reserved for high-risk patients (those with creatinine level > 1 mg/dL or bilirubin > 4 mg/dL) or should be used in all patients.25 Lower doses of albumin have been used in a pilot study, apparently with good results,26 but such doses need to be evaluated further before being recommended. Two trials have evaluated the use of albumin in treating infections other than spontaneous bacterial peritonitis and reported divergent results.27,28 Therefore, the use of albumin in the treatment of other infections cannot be recommended at this point and needs further study.
The use of norfloxacin as primary prophylaxis for spontaneous bacterial peritonitis in patients with some characteristics of advanced cirrhosis and ascitic protein < 1.5 g/dL has been shown to reduce the incidence of HRS and to increase survival.29 Whether the use of beta-blockers should be suspended in cirrhotic patients with spontaneous bacterial peritonitis is debated because these drugs might increase the risk of developing HRS and reduce transplant-free survival.30
In large-volume paracentesis in which more than 5 L of ascites is removed, volume expansion with 8 g/L albumin is recommended to avoid postparacentesis-induced circulatory dysfunction.19,20,31 A meta-analysis of 1,225 cirrhotic patients with tense ascites showed that albumin was significantly superior to alternative treatments for reducing postparacentesis-induced circulatory dysfunction, hyponatremia, and mortality.32. In relation to refractory or recurrent ascites, another recent meta-analysis compared large-volume paracentesis with transjugular intrahepatic portosystemic stent-shunt and confirmed that the latter decreases the risk for HRS and increases transplant-free survival, but increases the risk of hepatic encephalopathy.33 Nevertheless, the quality of the evidence seems insufficient to state that treatment with a transjugular intrahepatic portosystemic stent-shunt increases survival and that it should be recommended as a first-line treatment for such patients.
Treatment of HRSLiver transplantation is the definitive treatment for HRS because renal failure is functional and liver disease is the actual cause of the problem.16,19 A study of patients with type 1 HRS showed that the 6-month survival rates were 4% in those who did not respond to clinical treatment, 47% in those who responded to clinical therapy, and 97% in those who received a transplant (P < 0.001).34 Nevertheless, considering the high mortality rate associated with HRS and the limitations related to the shortage of liver grafts, clinical treatment for HRS remains vital to allow patients to reach transplantation.16,35 Clinical therapy may be the only option for patients who are not candidates for liver transplantation.34,35
Clinical treatment of HRS is currently based on vasoconstrictors and albumin.16,20 A meta-analysis reported increased survival of patients with HRS treated with vasoconstrictors.36 Another study reported that improved creatinine level occurred in parallel with the increased mean arterial pressure caused by vasoconstrictors.37 Terlipressin, noradrenaline, or midodrine (in combination with octreotide) are the vasoconstrictor drugs recommended for the treatment of HRS (Table 2).16,20 In addition to treatment with a vasoconstrictor and albumin, transjugular intrahepatic portosystemic stent-shunt seems to have a role in improving renal function in some patients and warrants further study.16,19,20,38 Dialysis should be reserved for urgent situations or as a bridge to liver transplantation because, without a transplant, the chance of survival for patients receiving dialysis is dismal.19,20,39
Vasoconstrictor drugs used in hepatorenal syndrome.
| Drug | Posology |
|---|---|
| Terlipressin (vasopressin analog) | Begin with 0.5-1.0mg intravenously every 4-6 hours. Increase twofold every 2 days to a maximum of 12mg/day if creatinine does not decrease by >25% of the initial level. |
| Noradrenaline (α-adrenergic agonist) | 0.5-3.0mg/hour intravenously (continuous infusion) -aim at increasing mean arterial pressure in 10 mmHg. |
| Midodrine and Octreotide (α-adrenergic agonist and somatostatin analog) | 7.5-12.5mg orally every 8 hours (midodrine) and 100-200 µg subcutaneously every 8 hours (octreotide) -aim at increasing mean arterial pressure in 10 mmHg. |
Terlipressin is the most studied drug in the context of HRS.16,19 It is a synthetic analog of lysine-vasopressin and acts as a potent vasoconstrictor by binding to vasopressin receptor V1.40,41 Because its effect on vascular receptor V1 is much greater than that on renal receptor V2, its vasoconstrictive action is greater in the splanchnic circulation than in the renal circulation.42 Initial studies have shown the efficacy and safety of terlipressin in reversing type 1 HRS43,44 and type 2 HRS,45 especially when given with albumin.46 Four later well-conducted randomized controlled trials confirmed its efficacy in the treatment of patients with type 1 HRS40,47,48 and in a sample of patients with either type of HRS.41 Systematic reviews have corroborated the role of terlipressin in HRS reversal42,49-51 and even in decreasing mortality.36,52,53 Finally, a recently published study of the largest sample of type 1 HRS patients ever studied in a multicenter, randomized, double-blind, placebo-controlled trial found that patients using terlipressin combined with albumin had a significantly greater decrease in creatinine level than did those using placebo combined with albumin (P < 0.001). The change in creatinine level was significantly associated with survival (P < 0.001).54
The association of terlipressin and albumin has also been evaluated in the context of sepsis-related HRS type-1, in which it has led to the recovery of renal function in 67% of cases.55 Moreover, it has been shown by another study that, in patients with HRS type-1 associated to an infection who were not early treated for HRS, 67% did not recover renal function, despite the use of antibiotics.56
The recommended strategy for the treatment of HRS with terlipressin is to use doses of 0.5-1.0 mg every 4-6 h and to double the dosage every 2 days if the creatinine concentration does not decrease by > 25% of the initial level. The maximum dosage is 12 mg/day. Albumin should be used at 1 g/kg (up to 100 g) on the first day, followed by 20-40 g/day on following days. Treatment can be extended for up to 2 weeks, but could be suspended after 7 days of the full dosage if the creatinine concentration does not decrease by ≥ 50% or after 3 days if there is no reduction in creatinine level.16 If HRS recurs after treatment, it can be retreated the same way.16,19 Terlipressin should not be used in patients with ischemic heart, cerebrovascular, or peripheral vascular diseases.19,39 Recently, it has been proposed that terlipressin should be administered as continuous infusion instead of boluses. A randomized controlled trial compared both strategies and found a lower rate of adverse events with continuous infusion (35.29% vs. 62.16%; P < 0.025), which may be associated with the lower initial dose of terlipressin in the continuous infusion. There was a tendency toward a higher 3-month transplant-free survival with terlipressin given in boluses (69% vs. 53%; P > 0.05).57 We propose that this issue should be studied further before a recommendation supporting continuous infusion is made.
Noradrenaline compared with terlipressinBecause terlipressin is a costly drug and is unavailable in many countries, noradrenaline has been studied for the treatment of HRS. Noradrenaline is a catecholamine with predominantly α-adrenergic activity, which has a well-documented vasoconstrictor activity with limited effects on the myocardium. It is thought that noradrenaline can correct the low systemic vascular resistance that is typical of HRS. In the first study to evaluate noradrenaline for the treatment of HRS, it was used in addition to albumin and furosemide, and HRS was reversed in 10 of 12 treated patients.58 The recommended treatment dose of noradrenaline is 0.5-3.0 mg/h combined with albumin.19
The efficacies of terlipressin and noradrenaline for the treatment of HRS have been compared in four randomized controlled trials.59-62 The first evaluated 22 cirrhotic patients with type 1 or 2 HRS. HRS reversal occurred in 10 of 12 patients treated with terlipressin and in seven of 10 patients treated with noradrenaline, and these rates did not differ significantly. The study reported one death in the terlipressin group and two in the noradrenaline group during a follow-up of 1 month, which also was not significantly different.59
Two other trials evaluated patients with type 1 HRS.61,62 One evaluated 20 patients in each treatment arm and found no significant differences in the rates of HRS reversal between terlipressin and noradrenaline (10 patients in each group) or 1-month survival (11 patients in each group).62 The other study evaluated 23 patients in each treatment group and found no significant differences between drugs for HRS reversal (nine patients in the terlipressin group and 10 in the noradrenaline group) or survival (nine patients using terlipressin and 11 using noradrenaline were alive after 15 days, and seven and eight, respectively, after 30 days).61 The fourth trial investigated only patients with type 2 HRS. Of 23 patients receiving terlipressin, 17 achieved HRS reversal, 19 were alive at 15 days, and 17 were alive at 30 days. Of 23 using noradrenaline, 17 achieved HRS reversal, 18 were alive at 15 days, and 17 were alive at 30 days. There were no significant differences between treatment arms.60
There is concern about the benefits of treating type 2 HRS patients with vasoconstrictors and albumin before liver transplantation. A recent study evaluated 56 patients listed for transplantation who did or did not receive terlipressin for type 2 HRS. There were no significant differences in mortality on the waiting list between treatment groups or in post-transplant renal function and survival between patients with normal or abnormal kidney function before transplantation.63 This was a small retrospective study and probably should have evaluated post-transplant renal function and survival according to whether the patients were or were not treated for HRS instead of according to kidney function at the time of transplantation. We believe that this matter should be evaluated further and that patients with type 2 HRS should still be considered for clinical treatment.
Some systematic reviews also have compared terlipressin and noradrenaline for the treatment of HRS, and there is no evidence of superiority of one over the other regarding HRS reversal or survival.36,51,64,65 Considering the absence of evidence of significant differences in the efficacy of these drugs, the facts that terlipressin is more expensive than noradrenaline and that noradrenaline requires administration in an intensive care setting, whereas terlipressin can be used in a regular ward, an economic evaluation was required. A recent study performed this evaluation by considering all direct medical costs associated with the different treatment strategies. The study showed that the treatment strategy using terlipressin was more economical than that using noradrenaline, under both the private and under public health systems.65 In this context and considering that high occupancy in intensive care units is a universal problem, it should also be taken into consideration the possibility of sparing an intensive care unit bed by using terlipressin to treat patients with HRS.
Midodrine compared with terlipressinFinally, the association of midodrine, an a-adrenergic agonist, and octreotide, a somatostatin analog, has also been compared with terlipressin for the treatment of HRS in a randomized trial. However, the study was interrupted prematurely because of the significant superiority of terlipressin over the combination of midodrine and octreotide in terms of HRS reversal (55.5% and 4.8% respectively; P < 0.001).66
ConclusionIn conclusion, HRS is a dramatic complication of advanced liver disease and should be treated with a vasoconstrictor, especially terlipressin or noradrenaline, and albumin, as a bridge to liver transplantation. Physicians should pursue this diagnosis especially in cirrhotic patients with ascites and renal function loss, and establish prompt treatment to save lives.
Abbreviations- •
AKIN: acute kidney injury network.
- •
HRS: hepatorenal syndrome.
- •
RIFLE: risk, injury, failure, loss of kidney function, and end-stage kidney disease.
- •
uNGAL: urinary neutrophil gelatinase-associated lipocalin.
None.
Conflicts of InterestThe authors declare that there is no conflict of interest regarding the publication of this paper.