Among the complications of cirrhosis, hepatorenal syndrome (HRS) is characterized by having the worst survival rate. HRS is a disorder that involves the deterioration of kidney function caused primarily by a systemic circulatory dysfunction, but in recent years, systemic inflammation and cirrhotic cardiomyopathy have been discovered to also play an important role. The diagnosis of HRS requires to meet the new International Club of Ascites-Acute Kidney Injury (ICA-AKI) and Hepatorenal Syndrome-Acute Kidney Injury (HRS-AKI) criteria after ruling out other causes of kidney injury. At the time of diagnosis, it is important to start the medical treatment as soon as possible where three types of vasoconstrictors have been recognized: vasopressin analogs (ornipressin and terlipressin), alpha-adrenergic agonists (norepinephrine and midodrine) and somatostatin analogues (octreotide); all should be combined with albumin infusion. Among them, terlipressin and albumin are the first lines of treatment in most cases, although terlipressin should be monitor closely due to its adverse events. The best treatment of choice is a liver transplant, because it is the only definitive treatment for this disease.
Liver cirrhosis is classified into two stages: compensated and decompensated liver cirrhosis. Decompensated cirrhosis is characterized by the presence of ascites, variceal bleeding, and hepatic encephalopathy, with a difference of 2 years of survival versus 12 years of compensated disease [1]. The main cause of the transition from a compensated to a decompensated stage is portal hypertension [2]. The occurrence of further decompensation (i.e. refractory ascites, spontaneous bacterial peritonitis, hepatorenal syndrome (HRS), and recurrence of hepatic encephalopathy and variceal bleeding) shortens the survival. The hepatorenal syndrome is the one characterized by a very poor prognosis [3], therefore it is necessary to identify it early and understand the syndrome so other causes of renal failure can be excluded so a medical treatment or transplant can start as soon as possible.
2DefinitionHepatorenal Syndrome is defined as a deterioration of kidney function that takes place in the context of severe chronic liver diseases, such as advanced cirrhosis or acute liver failure [3,4]. HRS is described by a decrease in kidney perfusion due to a severe reduction of effective circulating volume and massive activation of vasoactive endogenous system [5,6] that causes renal vasoconstriction in the context of systemic and splanchnic arterial vasodilation [7].
HRS is an exclusion diagnosis and is believed to be a functional pathology due to its reversibility after liver transplantation, although there are still certain controversies in this regard [8]. Traditionally, two types of HRS were described. Type-1 HRS is characterized by a rapid deterioration of renal function with a doubling of serum creatinine (sCr) to values above 2.5 mg/dl within 2 weeks, while type-2 HRS is characterized by a slower increase in sCr to values above 1.5 mg/dl. The main clinical characteristic of Type-1 HRS is acute renal failure, while the main characteristic of type-2 HRS is refractory ascites [9,10]. The diagnosis of HRS required the exclusion of other causes of kidney injury (e.g. no improvement after diuretic withdrawal and plasma volume expansion with 1 g/kg of albumin for 2 days; absence of shock and/or treatment with nephrotoxic drugs; no proteinuria >500 mg/24 h; no haematuria >50 erythrocytes per high power field in the urine; normal renal ultrasonography). Many concepts have changed in recent years regarding HRS: First, the acceptance of the new definition of Acute Kidney Injury (AKI) by the Hepatology Community and the International Club of Ascites (ICA) [11]; second, the systemic inflammation induced by Pathogen-Associated Molecular Patterns or by Damage Associated Molecular Patterns was found to play important role in acute decompensation in patients with cirrhosis [5].
2.1Criteria for Acute Kidney InjuryThe traditional definition of HRS, although useful, had multiple limitations leading to a potential delay in its diagnosis [1] and treatment. In the last decade, the Kidney Disease Improving Global Outcomes (KDIGO) criteria redefined AKI based on small sCr changes. In the new definition of the KDIGO “Improving Global Outcomes” guidelines, AKI is defined by any of the following criteria (see Table 1). Some studies showed that sCr based criteria is useful in estimating the mortality rate in patients with cirrhosis [11–16]. Therefore, the International Club of Ascites accepted the KDIGO criteria and updated the definition of type-1 HRS which is now named HRS-AKI, while type-2 HRS is now known as Hepatorenal Syndrome-Chronic Kidney Disease [5]. Nevertheless, the urine output criteria were not broadly accepted due to the lack of available data [17]. The acceptance of KDIGO criteria aim to: (1) Improve and increase awareness related to AKI in patients with cirrhosis; (2) provide a timely and early diagnosis; and (3) stratify the mortality risk of patients with liver cirrhosis according to AKI stage [1,18].
Table of the new diagnosis criteria for AKI. KDIGO Criteria of AKI acc epted by ICA for the new classification of AKI according to the subtle increase in serum creatinine and depending on the prognosis of overcome in 3 months [11–16]. The subdivision of AKI [1] in 2 stages depends on the different prognosis betwen creatinine <1.5 mg/dl or >1.5 mg/dl [1].
⇧ Increase, ⇩ reduction, AKI. Acute Kidney Injury, sCr. Serum Creatinine, ICA. International Club Ascitis.
*In the last 7 days.
Acute kidney injury is subdivided into 3 types according to the sCr and its prognosis. The reasons for dividing AKI 1 into AKI 1A and AKI 1B are the difference in prognosis (see Table 1) and the variation in the etiology: In 1A, hypovolemia is more common, and in stage 1B, tubular necrosis with the HRS is more frequent. Considering diuresis in HRS was excluded because cirrhotic patients are usually oliguric without having AKI or polyuric because of diuretic use, therefore this criterion is not applicable [8].
Currently, HRS-AKI is considered a stage greater or equal to 2 of the ICA-AKI criteria diagnosed after other causes of AKI were excluded (for example, hypovolemia, shock, kidney parenchymal diseases, urinary tract obstruction, or nephrotoxins) [6].
A baseline creatinine is important to diagnose AKI. There can be two scenarios [1]:
- 1
First scenario: where AKI is developed in hospitalized patients. This scenario is easy to identify because of the sCr values present at the moment of admission, which can be used as baseline sCr [1].
- 2
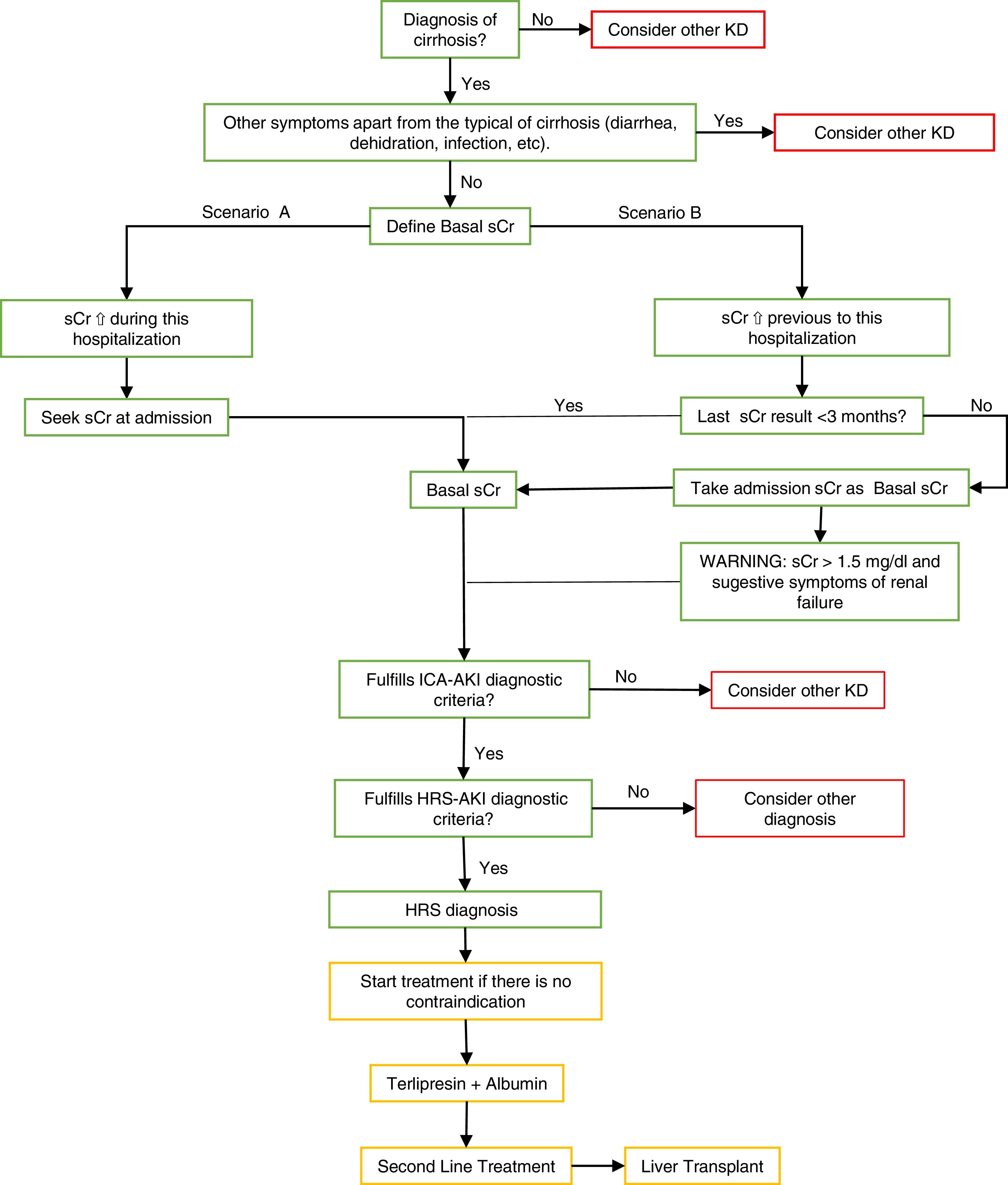
Second scenario: where patients develop AKI before hospitalization (which is a large percentage of patients) and show high values of sCr at the moment of admission. In these cases, basal serum creatinine values should be used in the tests before hospitalization [1]. See Fig. 1.
Fig. 1.Summary algorithm of the diagnosis and treatment of the Hepatorenal Syndrome.
The algorithm shows the way to make a differential diagnosis of other diseases or an HRS diagnosis using the two scenarios to take the basal sCr and the treatment’s summary.
Scenario B: The ICA-AKI criteria define the baseline sCr as the value closest to the date of hospitalization in the last 3 months [1]. In patients who do not have baseline sCr 3 months before hospitalization, serum creatinine should be considered as the first baseline assessment of admission, however, if high values are identified without previous basal values the diagnosis may be dismissed. It is recommended to pay close attention in these patients where their initial serum creatinine is >1.5 mg/dl, where clinical experience indicates renal failure and they should be treated as AKI if it is highly suspected. MDRD formula is not recommended in patients with cirrhosis to calculate baseline serum creatinine levels [1,19].
⇧ increase, ⇩ reduction, HRS. Hepatorenal Syndrome, AKI. Acute Kidney Injury, sCr. Serum Creatinine, KD. Kidney Disease, ICA. International Club Ascitis, MDRD. Modification of Diet in Renal Disease equation.
Green: diagnosis algorithm based on the criteria; yellow: treatment; red: other considerations.
(0.44MB).
HRS occurs mostly because of circulatory dysfunction, however, in recent years other influencing factors in its development have been found, such as systemic inflammation and cirrhotic cardiomyopathy. HRS-AKI is characterized by rapid deterioration caused by precipitating events that lead to the failure of one or more organs, aggravating the patient's central hypovolemic state [8].
3.1Systemic circulatory dysfunction and renal factorsArteriolar splanchnic vasodilation is as a key factor for the pathophysiology of HRS.
In an initial stage of cirrhosis, there is a modest increase in portal hypertension along with a decrease in systemic resistance caused by the vasodilation of the organs. This vasodilation, which is the main cause of HRS, is triggered by the overproduction of vasodilator substances (nitric oxide, carbon monoxide, and endocannabinoids) and its low degradation due to increased portal hypertension and the leak of these substances by the portosystemic shunts into the general circulation. As a compensatory effect, the body increases cardiac output and heart rate and activates powerful vasoconstrictor systems (such as the sympathetic nervous system), the renin-angiotensin-aldosterone system, and the non-osmotic secretion of vasopressin. In compensated cirrhosis these mechanisms control vasodilation and blood pressure keeping it in normal ranges, however, with the development of complications in decompensated cirrhosis, these systems are not efficient enough, therefore they cause renal flow impairment and general deterioration. Even in very advanced stages of cirrhosis there is a decreased cardiac output that conditions the perfusion to the main organs [7,8].
The mentioned consequences are associated with the retention of sodium and free water with the accumulation of ascites and edema. This retention leads to kidney vasoconstriction, decrease glomerular filtration rate (GFR), and the final development of HRS. Otherwise, if renal vasoconstriction is not reversed in time, it will cause kidney parenchyma damage followed by acute tubular necrosis [7,8,20].
3.2Systemic inflammationSystemic inflammation plays a primary role in the pathophysiology of HRS [8]. Systemic inflammation occurs as a result of a rise in intestinal permeability causing pathological bacterial translocation from the gut to the systemic circulation [21], changes in quantity and quality of the microbiome [22], and cirrhosis associated immune dysfunction [23]. Translocation of viable bacteria or Pathogen-Associated Molecular Patterns activates antigen-presenting cells (monocytes, macrophages, and dendritic cells) that induce an inflammatory response by releasing proinflammatory cytokines and vasodilators causing an increase in vasodilation and impairment in cardiac contractility. Bacterial infections are one of the main triggers of HRS-AKI. Increased inflammatory cytokines also have been found in patients with HRS-AKI [7].
3.3Cirrhotic cardiomyopathyCirrhotic cardiomyopathy affects the pathophysiology of HRS, mainly because it greatly alters kidney function. Some studies have shown that HRS tends to develop more in patients with low cardiac output and because there is a direct association with HRS as cardiac output decreases [24,25]. Cirrhotic heart disease is characterized by systolic dysfunction due to physical or pharmacological stress, with diastolic dysfunction caused by relaxation and electromechanical irregularities. This hypothesis will have a great impact on the development of new treatments for HRS [8,26].
4EpidemiologyAKI is one of the most common complications of decompensated cirrhosis with a frequency of 20–50% in cirrhotic patients admitted to hospital for complications of the disease, and 50% of cirrhotic patients who die develop it [20,27]. Approximately 20% of patients with advanced cirrhosis will develop HRS after the first year of diagnosis, and 40% will develop it during the next 5 years [27]. HRS-AKI represents 15–30% of all cases of AKI but is the type of AKI characterized by the worst prognosis [28].
5Diagnostic criteriaTo establish a diagnosis of HRS, it is necessary to meet the ICA-AKI and HRS-AKI criteria [8], which has been summarized in Table 2.
Criteria to diagnosis of Hepatorrenal Syndrome.
| 1. Cirrhosis and ascites |
| 2. Diagnosis of AKI according to its ICA-AKI criteria |
| 3. Absence of shock |
| 4. No response to diuretic withdrawal and plasma volume expansiona |
| 5. No recent use of nephrotoxic drugs |
| 6. No macroscopic signs of structural kidney injury |
“No macroscopic signs of structural kidney injury” refers to [8]:
1.- There is no presence of proteinuria (>500 mg/dl) [8].
2.- There is no presence of microhematuria (>50 red blood cells per high power field) [8].
3.- Normal findings on renal ultrasonography [8].
HRS. Hepatorenal Syndrome, AKI. Acute Kidney Injury, sCr. Serum Creatinine, KD. Kidney Disease, ICA. International Club Ascitis.
The current criteria are not very accurate to exclude a parenchymal kidney injury in patients with cirrhosis. Indeed, histopathological studies found common signs of parenchymal kidney injury from renal biopsies in patients with AKI and the absence of proteinuria/hematuria [29]. In current clinical studies, there is an increase of markers to assess kidney damage in AKI. Among them, Interleukin-18, Kidney Injury Marker, Liver-Type Fatty Acid-Binding Protein, and Neutrophil-Gelatinase Associated Lipocalin, better known as NGAL, are the most widely investigated. NGAL and Interleukin-18 were the most promising in performing a differential diagnosis between Acute Kidney Injury-Acute Tubular Necrosis and HRS-AKI [30–33]. The first one also predicts mortality in these patients. In the future, these biomarkers are candidates to be included in the differential diagnosis of AKI [34].
7TreatmentIt is important to start medical treatment at the diagnosis of HRS-AKI [20]. Liver transplantation is widely recognized as the best treatment of HRS-AKI, but it is not immediately available.
7.1Vasoconstrictors and albuminThree classes of vasoconstrictors are currently used: Vasopressin analogues (ornipressin and terlipressin) that cause smooth muscle vasoconstriction and reduce portal pressure; Alpha-adrenergic agonists (norepinephrine and midodrine) that lead smooth muscle vasoconstriction and increase in systemic vascular resistance; and the somatostatin analogs (such as octreotide) that inhibit the release of systemic vasodilators [20]. All vasoconstrictors should be combined with human albumin infusion given at a dose of 20–40 grams per day [7]. Table 3 summarizes the treatment with terlipressin and albumin.
First Line Treatment. The table explains how to use terlipressin plus albumin as first line treatment in HRS and additional comments to consider. Terlipressin should be started at the dose of 2–4 mg per day and if there is no improvement in the first 2–3 days of treatment (that is a decreased of serum creatinine >25% of the values before the administration), the dose should be increased in a stepwise fashion up to 12 mg/day [38]. This drug requires continuous monitoring because it is a potent vasoconstrictor related to adverse ischemic or cardiovascular effects in 20% of patients requiring its suspension [7,42]. In case the adverse effects are not severe, the dose should be decreased and treatment continued, but always with the patient monitored [7,20].
| Treatment | Recomended drug | Dosis | Ea/Warnings | Consider |
|---|---|---|---|---|
| First Line | Terlipressin | IV infusion: initial dose 2–4 mg/d. | Abdominal pain, diarrea, cardiovascular ischemia, arrythmia, heel cyanosis | If reduction is not achieved in 2–3 days (Cr > 25%), ⇧ up to 12 mg/d h until complete response (⇩ <1.5 sCr) or to baseline values. It requires continous monitoring. |
| First Line | Albumin | IV infusion: 20–40 g/d. | Pulmonary edema, anaphylaxia, headache, hyperthermia | Discontinue if central venous pressure increase >15 mmHg. |
| Anaphylaxis, headache, hyperthermia |
⇧ increase, ⇩ reduction, HRS. Hepatorenal Syndrome, IV. intravenous, EA. adverse effects.
Vasopressin-like vasoconstrictors and alpha-adrenergic agonists, and albumin, are the pharmacological treatment of choice in HRS-AKI. These drugs augment systolic blood pressure and the arterial volume, increasing the renal perfusion, and in consequence, producing systemic and splanchnic vasoconstriction [20]. Various studies have shown that terlipressin and albumin improve HRS-AKI by 40–50%, and also patient survival [7,35–40].
Factors associated with HRS reversal are: lower serum creatinine at the start of the medications, lower serum bilirubin, and longer grade of Acute on Chronic Liver Failure [7,40,42,43].
7.2Other vasoconstrictorsOther vasoconstrictors can be used when terlipressin is not available. In some studies, norepinephrine showed to be as effective as terlipressin in the treatment [7,20,41], except in patients with HRS-AKI and Acute on Chronic Liver Failure, where norepinephrine is less effective [44]. Norepinephrine needs to be administered under continuous monitoring and its use needs to be done in a critical care unit. Another option is the combination of oral midodrine plus octreotide with albumin, but this treatment is less effective than terlipressin [4,37].
4.3Liver TransplantLiver transplant is the treatment of choice because it represents the definitive treatment for HRS-AKI and liver disease. Due to the poor prognosis that these patients present, they should be considered as candidates and priorities in liver transplant, since in many cases they die waiting for a donor. However, due to the new MELD classification (Model for End-Stage Liver Disease) more candidates have been considered compared to the previous period to the MELD [45]. Liver transplantation alone is preferred only to simultaneous liver and kidney transplantation because HRS-AKI is reversible with liver transplantation in most cases. However, simultaneous liver and kidney transplantation should be considered when the patient has chronic kidney disease and meets the appropriate criteria [7,20,41]:
- 1
GFR (with the Modification of Diet in Renal Disease of 6 Variables, better known as MDRD6 equation) ≤40 mL/min or measured GFR with iothalamate clearance ≤30 mL/min [7].
- 2
Proteinuria >2 g/d [7].
- 3
Kidney biopsy showing >30% global glomerulosclerosis or >30% interstitial fibrosis [7].
- 4
An inherited metabolic disease or and HRS-AKI that does not respond to pharmacological therapy (that has required renal replacement therapy >4 weeks or GFR ≤ 35 mL/min or GFR ≤ 25 mL/min ≥4 weeks) and has a low probability of kidney function recovery [7].
Another option for HRS-AKI is placing a portosystemic intrahepatic transjugular shunt, better known as TIPS, since it improves circulation and reduces endogenous vasoconstrictors that enhances renal function by 60%. However, it is not recommended because there are not enough studies that support these treatments and the applicability is low since patients with HRS-AKI have many contraindications for this therapy [7].
Recirculatory Reabsorption System (MARS) and the Absorption and Separation of Fractionated Plasma (Prometheus) have been proposed, but there is not enough evidence to recommend it yet [7].
Renal replacement therapy is an option in patients who show no improvement to medical treatment and developing pulmonary edema, severe hyperkalemia, and metabolic acidosis and or complications of uremia. However, it does not solve the underlying cause of HRS-AKI and results are quite disappointing in patients who are not candidates for liver transplant [46].
8ConclusionsHRS develops as a complication of cirrhosis and portal hypertension with high morbidity and mortality. It is an exclusion diagnosis and treatment should be administered as soon as the diagnosis of this disorder is suspected. The first-line treatment is with volume expanders (albumin) and vasopressin analogs (terlipressin). The definitive treatment is liver transplantation.
Conflict of interestThe authors have no conflicts of interest to declare.










![Summary algorithm of the diagnosis and treatment of the Hepatorenal Syndrome. The algorithm shows the way to make a differential diagnosis of other diseases or an HRS diagnosis using the two scenarios to take the basal sCr and the treatment’s summary. Scenario B: The ICA-AKI criteria define the baseline sCr as the value closest to the date of hospitalization in the last 3 months [1]. In patients who do not have baseline sCr 3 months before hospitalization, serum creatinine should be considered as the first baseline assessment of admission, however, if high values are identified without previous basal values the diagnosis may be dismissed. It is recommended to pay close attention in these patients where their initial serum creatinine is >1.5 mg/dl, where clinical experience indicates renal failure and they should be treated as AKI if it is highly suspected. MDRD formula is not recommended in patients with cirrhosis to calculate baseline serum creatinine levels [1,19]. ⇧ increase, ⇩ reduction, HRS. Hepatorenal Syndrome, AKI. Acute Kidney Injury, sCr. Serum Creatinine, KD. Kidney Disease, ICA. International Club Ascitis, MDRD. Modification of Diet in Renal Disease equation. Green: diagnosis algorithm based on the criteria; yellow: treatment; red: other considerations. Summary algorithm of the diagnosis and treatment of the Hepatorenal Syndrome. The algorithm shows the way to make a differential diagnosis of other diseases or an HRS diagnosis using the two scenarios to take the basal sCr and the treatment’s summary. Scenario B: The ICA-AKI criteria define the baseline sCr as the value closest to the date of hospitalization in the last 3 months [1]. In patients who do not have baseline sCr 3 months before hospitalization, serum creatinine should be considered as the first baseline assessment of admission, however, if high values are identified without previous basal values the diagnosis may be dismissed. It is recommended to pay close attention in these patients where their initial serum creatinine is >1.5 mg/dl, where clinical experience indicates renal failure and they should be treated as AKI if it is highly suspected. MDRD formula is not recommended in patients with cirrhosis to calculate baseline serum creatinine levels [1,19]. ⇧ increase, ⇩ reduction, HRS. Hepatorenal Syndrome, AKI. Acute Kidney Injury, sCr. Serum Creatinine, KD. Kidney Disease, ICA. International Club Ascitis, MDRD. Modification of Diet in Renal Disease equation. Green: diagnosis algorithm based on the criteria; yellow: treatment; red: other considerations.](https://static.elsevier.es/multimedia/16652681/000000220000000C/v3_202107300613/S1665268120301411/v3_202107300613/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




