Hepatorenal syndrome (HRS) is a serious complication of cirrhosis treated with various medications. We aim to evaluate terlipressin and albumin's effectiveness and safety compared to albumin and noradrenaline in adult hepatorenal disease patients.
Materials and MethodsClinical trials from four databases were included. Cochrane's approach for calculating bias risk was utilized. We rated the quality evaluation by Grading of Recommendations Assessment, Development, and Evaluation (GRADE). We included the following outcomes: serum creatinine (mg/dl), urine output (ml/24 h), mean arterial pressure (mmHg), reversal rate of HRS, mortality rate, blood plasma renin activity (ng/ml/h), plasma aldosterone concentration (pg/ml), urine sodium (mEq/l), and creatinine clearance (ml/min).
ResultsOur analysis of nine clinical studies revealed that the noradrenaline group was associated with higher creatinine clearance (MD = 4.22 [0.40, 8.05]), (P = 0.03). There were no significant differences in serum creatinine levels (MD = 0.03 [-0.07, 0.13]), urinary sodium (MD = -1.02 [-5.15, 3.11]), urine output (MD = 32.75 [-93.94, 159.44]), mean arterial pressure (MD = 1.40 [-1.17, 3.96]), plasma renin activity (MD = 1.35 [-0.17, 2.87]), plasma aldosterone concentration (MD = 55.35 [-24.59, 135.29]), reversal rate of HRS (RR = 1.15 [0.96, 1.37]), or mortality rate (RR = 0.87 [0.74, 1.01]) between the two groups (p-values > 0.05).
ConclusionsNoradrenaline is a safe alternative medical therapy for HRS.
Hepatorenal Syndrome (HRS) is the impairment of renal function that occurs in 7–15 % of patients with decompensated cirrhosis [1]. There are three components of HRS: liver dysfunction, abnormalities in circulation, and progressive renal failure [2]. According to the updated 2021 American Association for the Study of Liver Diseases (AASLD) guidance on Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis, and HRS, the revised definition of HRS -acute kidney injury (HRS-AKI) emphasizes the role of acute kidney injury (AKI) in its pathogenesis. HRS-AKI is a functional renal failure that occurs in patients with decompensated cirrhosis and is caused by systemic hemodynamic abnormalities. The new management recommendations are expected to improve the outcomes of patients with HRS-AKI [3]. There are two types of HRS type 1 and type 2. HRS type 1 (HRS-1) is more severe than HRS type 2 (HRS-2) because it exhibits multi-organ failure, a fast doubling of serum creatinine to more than 2.5 mg/dl in less than two weeks, and a gradual and rapid decline in renal function [4,5]. Within two weeks of the diagnosis, HRS-1 kills approximately 50 % of the patients [6]. Liver transplantation is is effective in HRS-AKI, without any transplant contraindications, although it is not always feasible [7]. In liver diseases, large amounts of vasoactive substances are released, causing a reduction of blood flow to the kidneys. This reduction leads to stimulation of the juxtaglomerular apparatus, causing activation of the renin-angiotensin system with increased renin secretion, which results in vasoconstriction of systemic vessels, especially renal vessels [8].
Renal vasoconstriction is the cornerstone of HRS physiology, which occurs to compensate for the cardiac underfilling that results from arterial vasodilatation [9]. Many drugs used to antagonize the mechanism of renal damage includes albumin for volume expansion plus vasoactive medications such as midodrine, octreotide, terlipressin, norepinephrine, and dopamine [10–12]. The use of vasoactive drugs in combination with albumin has been shown to help restore renal perfusion [13,14]. Terlipressin is a vasopressin analog used with albumin to improve renal function and increase median survival time [15,16]. Terlipressin may improve renal processes by causing dilatation of the intrahepatic vessels and reduction of intrahepatic resistance and portal pressure [17].
Furthermore, the current clinical practice guidance supports the use of terlipressin for gastroesophageal variceal bleeding and HRS in liver cirrhosis [18]. Noradrenaline, a catecholamine with predominately alpha-adrenergic activity is an alternative to terlipressin [19]. Terlipressin is not available in many countries or is very expensive. However, Noradrenaline is widely available and is inexpensive [10].
This study aims to compare terlipressin's efficacy and safety with noradrenaline's for managing HRS.
2Materials and methodsThe Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were used to conduct this meta-analysis [20], as well as the recommendations listed in the Cochrane Handbook for Systematic Reviews of Interventions [21].
2.1Eligibility criteriaThe inclusion criteria were: population: adult patients with HRS, intervention: terlipressin, comparator: noradrenaline, including clinical trials, and Primary outcomes: serum creatinine (mg/dl), urine output (ml/24 h), mean arterial pressure (mmHg), reversal rate of HRS and mortality rate.Secondary outcomes are blood plasma renin activity (ng/ml/h), plasma aldosterone concentration (pg/ml), urine sodium (mEq/l), and creatinine clearance (ml/min). All secondary publications, including meta-analyses, reviews, animals studies, conference abstracts, and studies with insufficiently reported data were disregarded.
2.2Information sourcesWe searched databases including PubMed, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) until January 2023 for articles meeting inclusion criteria.
2.3Search and study selectionIn doing our search, we employed the following search strategy: ((terlipressin) OR (glypressin)) AND ((noradrenaline) OR (noradrenalin) OR (norepinephrine)) AND ((HRS) OR (hepatorenal syndrome)). The included articles were reviewed in three stages. The initial phase involved transferring the outcomes from digital databases to a Microsoft Excel [22] sheet utilizing the EndNote software [22). The articles entered into the Excel sheet were screened for titles and abstracts in the second stage. The included citations from the second step were subjected to full-text screening in the third stage. In addition, we manually checked the references for the publications included for any potential overlooked investigations.
2.4Data collectionFrom each study that we included, we gathered three types of information: baseline and demographic information about the participants, including the author, year, sample size, age, gender, and Model for End-Stage Liver Disease (MELD) score; and Child-pugh score and serum creatinine (mg/dl), serum albumin (g/dl), prothrombin time test (INR), serum sodium (mEq/l), mean arterial pressure (mmHg), urinary sodium (mEq/dl), urinary volume (ml/min), creatinine clearance (ml/min), alcohol percentage, urea (mg/dl), serum bilirubin (mg/dl), and plasma renin activity (ng/ml/h) are some of the other measurements that can be made. Serum creatinine (mg/dl), urine output (ml/24 h), mean arterial pressure (mmHg), reversal rate of HRS, mortality rate, and other analysis results were mainly included in the second group. Plasma aldosterone concentration (pg/ml), plasma renin activity (ng/ml/h), serum sodium concentration (mEq/l), urinary sodium concentration (mEq/l), and creatinine clearance (ml/min). The third category contained information for determining the danger of bias. Data gathering was carried out using Microsoft Excel [22].
2.5Risk of bias assessmentIn judging this study's merit, we adhered to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Guidelines [23]. Using Cochrane's risk of bias methodology for clinical trials, two writers evaluated the risk of bias among the included papers [23]. The instrument evaluates patient randomization, allocation concealment, and sufficient blinding through seven domains. Each domain is assigned a "low," "unclear," or "high" probability of bias [23].
2.6AnalysisUsing Review Manager Software, we conducted the meta-analysis for this study [24]. Both continuous and binary outcomes were included in our study. We used mean difference (MD) and 95 % confidence interval (CI) to analyze continuous data and risk ratio (RR) and 95 % CI to evaluate dichotomous data. When data were homogenous, the fixed-effects model was employed; when data were heterogeneous, the random-effects model was utilized. We used the I2 and p-value of the Chi-square tests to assess the consistency between the studies [21]. The heterogeneity was significantly indicated by P ≤ 0.1 or I2 > 50 % values. Utilizing Cochrane's leave-one-out technique, we attempted to resolve the inconsistent results of varied outcomes [21].
3Results3.1Summary of included studiesA PRISMA flow diagram of our literature search is shown in Fig. 1. Patients' data from nine trials were analyzed for our investigation [2,10–12,25–29]. The patients allocated to receive triplessin were 244 patients. The patients assigned to receive noradrenaline were 242 patients. The mean age of the terlipressin group was 51.4 ± 11.6 years, while that of the noradrenaline group was 51.5 ± 12.8. The participants' demographic information, sample size, age, gender, MELD score, and other details are summarized in Tables 1, 2, 3. Child-pugh score and serum creatinine (mg/dl), serum albumin (g/dl), INR, serum sodium (mEq/l), mean arterial pressure (mmHg), urinary sodium (mEq/dl), urinary volume (ml/min), creatinine clearance (ml/min), alcohol percentage, urea (mg/dl), serum bilirubin (mg/dl), and plasma renin activity (ng/ml/h) are some of the other measurements that can be made.
Shows a detailed summary of the included participants and their demographic data.
Shows a detailed summary of the included participants, INR, serum sodium, serum potassium, serum albumin and child-pugh score.
A detailed summary of the included participants, MAP, urinary sodium, urinary volume, alcohol, creatinine clearance and urea.
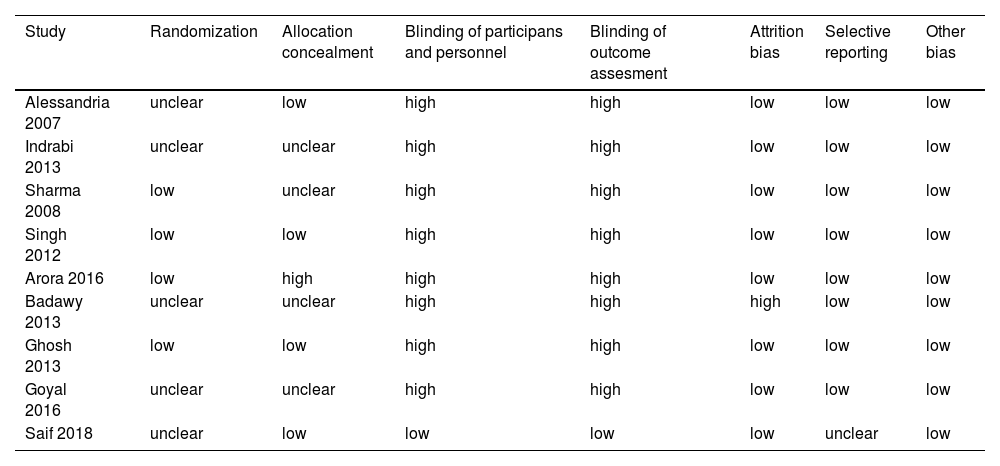
According to Cochrane's technique, the outcome of the risk of bias evaluation of randomized clinical trials revealed an overall low risk of bias. Randomization results from four studies [12,25–27] were low risk, and five studies [2,10,11,28,29] did not provide sufficient data. Four studies on allocation concealment [10,25,27,28] were at low risk, and one study (26) was at high risk, but the rest of the studies [2,11,12,29] did not provide sufficient data. Except for Saif et al. 2018, all investigations were not conducted with participants and staff in the dark [28] becoming blind. All of the trials except Saif et al. 2018 were not blinded to outcome assessment. Fig. 2 illustrates a summary of the included trials' risk of bias. An exhaustive risk of bias analysis is shown in Table 4[28].
A detailed risk of bias assessment.
Seven studies [2,9–11,24,26,27] reported the serum creatinine outcome. Between the two groups, there was no discernible difference (MD = 0.03 [−0.07, 0.13]; P = 0.6). Fig. 3 shows that the pooled analysis was homogenous (P = 0.14; I2 = 37 %). Based on the dose of terlipressin, we performed a subgroup analysis, and there was no discernible difference between the two groups.
3.3.2. Urinary sodium (mEq/l)The urinary sodium outcome was reported by four studies (9,10,24,26). Between the two groups, there was no discernible difference (MD = −1.02 [−5.15, 3.11]; P = 0.63). I2 = 27 %; the pooled analysis was homogenous (P = 0.25) Fig. 4. We performed a subgroup analysis based on terlipressin dose, which yielded the same conclusion.
3.3.2.1. Urine output (ml/24 h)Eight studies [2,9–11,24–27] reported the urine output outcome. The overall mean difference showed that there was no significant difference between both groups (MD = 32.75 [−93.94, 159.44]), (P = 0.6). We conducted a subgroup analysis based on the time of this outcome assessment. Six studies [2,9–11,24,26] measured the outcome at day 15. The overall mean difference showed that there was no significant difference between both groups (MD=−71.27 [−198.83, 56.29]), (P = 0.27). Pooled analysis was heterogeneous (P = 0.08); I² = 50 % Fig. 5A. We solved the heterogeneity by excluding Goyal et al. [10]. The mean difference showed that there was no significant difference between both groups (MD = −38.96 [−149.97, 72.04]), (P = 0.49). Pooled analysis was homogeneous (P = 0.18); I² = 36 %. Two studies measured the urine output on day 7 [25,27]. The mean difference showed that the noradrenaline group was associated with increased urine output more than the terlipressin group (MD = 257.48 [87.19, 427.77]) (P = 0.003). Pooled analysis was homogeneous (P = 0.77); I² = 0 %—Fig. 5B.
3.3.2.2. Mean arterial pressure (mmHg)Seven studies [9–11,24–27] reported the mean arterial pressure outcome. We conducted a time-based examination of the subgroups of this outcome assessment. Five studies [9–11,24,26] measured the outcome at day 15. The overall mean difference showed that there was no discernible distinction between the two groups. (MD = −2.05 [−5.37, 1.27]), (P = 0.23). Pooled analysis was heterogeneous (P = 0.006); I² = 72 % Fig. 6A. We solved the heterogeneity by excluding Singh et al. (26). There was no discernible difference between the two groups, according to the mean difference (MD = −0.01 [−1.59, 1.57]), (P = 0.99). Pooled analysis was homogeneous (P = 0.58); I² = 0 %. Two studies measured MAP at day 7 [25,27]. The mean difference showed that there was no significant difference between both groups (MD = 4.57 [−3.18, 12.33]), (P = 0.25). Pooled analysis was heterogenous (P = 0.001); I² = 94 %—Fig. 6B.
3.3.2.3. Creatinine clearance (ml/min)Three studies [9,11,27] reported the creatinine clearance outcome. The overall risk showed that the noradrenaline group was associated with increased creatinine clearance more than the terlipressin group (MD = 4.22 [0.40, 8.05]), (P = 0.03). Pooled analysis was homogeneous (P = 0.39); I² = 0 %, Fig. 7. We conducted a subgroup analysis based on the dose of terlipressin, and it showed that the noradrenaline group was more associated with increased creatinine clearance than the terlipressin.
3.3.2.4. Plasma renin activity (ng/ml/h)Three studies [9,24,26] reported the outcome of plasma renin activity. There was no discernible difference between the two groups, according to the total mean difference (MD = 0.31 [−1.93, 2.54]), (P = 0.79). Collective analysis was heterogeneous (P = 0.08); I² = 60 % Fig S1. We did a subgroup analysis according to the terlipressin dose, and the analysis showed the same result
3.3.2.5. Plasma aldosterone concentration (pg/ml)Three research reported the plasma aldosterone concentration result [9,24,26]. Between the two groups, there was no discernible change (MD=55.35 [−24.59, 135.29]; P = 0.17). Fig S2 shows that the pooled analysis was homogenous (P = 0.64; I2 = 0 %). Based on the terlipressin dosage, we performed a subgroup analysis, but there was no discernible difference between the two groups.
3.3.2.6. Reversal rate of HRSNine studies [2,9–11,24–28] reported the reversal rate of HRS outcome. The risk ratio showed that there was no significant difference between both groups (RR = 1.15 [0.96, 1.37]), (P = 0.12). pooled analysis was homogeneous (P = 0.66); I² = 0 % Fig S3. We conducted a subgroup analysis based on the terlipressin dose. We found that with the higher dose of terlipressin (3.5 mg/h), the noradrenaline group was associated with a higher reversal rate of HRS than the other group.
3.3.2.7. Mortality rateNine studies [2,9–11,24–28) reported the mortality rate outcome. The risk ratio showed that there was no significant difference between both groups (RR = 0.87 [0.74, 1.01]), (P = 0.08). pooled analysis was homogeneous (P = 0.19); I² = 28 % Fig S4., and we found that with the higher dose of terlipressin (3.5 mg/h), the noradrenaline group was associated with a lower mortality rate than the other group
4DiscussionCirrhosis-induced neurohormonal cascades release vasodilators and cytokines, leading to systemic and splanchnic vasodilation. It causes decreased systemic vascular resistance and renal injury (HRS) due to hypoperfusion [8,9]. Though Terlipressin is the first-line therapy for treating HRS, Norepinephrine, octreotide, and midodrine with albumin are still utilized due to Terlipressin's unavailability in many medical facilities.
Noradrenaline, a catecholamine with predominately alpha-adrenergic activity, leads to vasoconstriction of the splanchnic vessels, which improves the intravascular arterial volume. Hence, improving the glomerular filtration rate and renal blood flow leads to HRS reversal [10,30,31]. On the other hand, Terlipressin activates V1 receptors on the vascular smooth muscle cells [11] to constrict splanchnic and systemic vasculature, causing a reduction in intrahepatic resistance/portal pressure and improving intravascular volume and HRS [17].
Our analysis reports that Noradrenaline is associated with significantly higher creatinine clearance levels in HRS patients than Terlipressin. However, serum creatinine (mg/dl), urine output (ml/24 h), mean arterial pressure (mmHg), HRS reversal rate, mortality rate, urine sodium (mEq/l), plasma renin activity (ng/ml/h), and plasma aldosterone concentration (pg/ml) do not differ significantly between the two groups.
Several trials have assessed the effectiveness of the two drugs for HRS. Angelo et al., Arora et al., Indrabi et al., and Alessandria et al. [10,26,29,37] included patients with HRS-1 and HRS-2. Ghosh et al.[24] included patients with HRS-2 only. Other five studies [2,11,12,28,38] included only patients with HRS-1. According to a network meta-analysis of ten studies, Terlipressin was the most effective vasoconstrictor medication for HRS-1, but it also carried a higher risk of side effects. Terlipressin is associated with an increased risk of serious adverse events, including respiratory failure, fluid overload, and hyponatremia, especially with volume overload or ACLF Grade 3 [34]. Noradrenaline has been a popular substitute due to its fewer side effects and wide availability [19]. In 2019, Thomson et al. analyzed 14 clinical trials reporting that medical therapy has not increased the reversal rates of HRS since 2002 despite managing other complications of cirrhosis [32]. A Cochrane review compared the various vasoactive medications for treating HRS and found no concrete evidence to support or refute Terlipressin's efficacy and safety compared to other vasoactive medications [33]. Wang et al. reported Terlipressin plus albumin as a superior medical therapy for HRS but still considered Norepinephrine as a comparable alternative due to Terlipressin's higher cost and limited availability [35]. Facciorusso et al. found that Terlipressin combined with albumin significantly reduced mortality compared to placebo [36]. Also, HRS-1 responded more favorably to Terlipressin with albumin and Noradrenaline with albumin than to midodrine plus octreotide with albumin [36]. Angelo et al. report no clinically significant difference between Terlipressin and Noradrenaline except for the higher cost of Terlipressin [37].
Alessandria et al. reported no cardiac, intestinal, or distal ischemia with both drugs. However, the Terlipressin group had diarrhea and abdominal pain after the first injection [10]. Goyal et al. and Ghosh et al. [11,25] reported that Noradrenaline and Terlipressin cause non-severe cardiovascular adverse effects responsive to dose adjustment. Self-limiting diarrhea and abdominal pain were common with Terlipressin. Singh et al. and Arora et al. [26,27] added that Terlipressin had more frequent side effects than Noradrenaline. Arora et al. [26] concluded that Terlipressin with albumin was superior to Noradrenaline with albumin in treating HRS.
On the other hand, the remaining eight trials [2,10,25,27–29] concluded that Noradrenaline plus albumin had the same effect as Terlipressin plus albumin for treating HRS. Noradrenaline was cheaper and widely available, with fewer adverse effects than Terlipressin. Also, the American Association for the Study of Liver Diseases recommends using Noradrenaline with albumin in HRS for intensive care unit patients [39].
Strengths and LimitationsThe strengths of our study include analyzing results from all available published prospective clinical trials on HRS, including data points from 486 patients. Also, most included clinical outcomes showed low heterogeneity. The prevalence of bias across a number of the included studies is the primary drawback of our investigation. Five studies we had [2,10,11,28,29] reported inadequate data about randomization, and four studies [2,11,12,29] reported insufficient data about allocation concealment. Eight included studies [2,10–12,25–27,29] were not blinded to the participants, staff, or outcome evaluation. All of this may skew the findings of data obtained subjectively in favor of positive outcomes.
5ConclusionsWe conclude that Norepinephrine with albumin is an effective therapy for improving creatinine clearance in HRS patients. It is a safe alternative for Terlipressin in the intensive care setting, with the added advantages of wider availability and lower cost.