Adrenal insufficiency (AI) is common in patients with cirrhosis. We aimed to assess the presence of AI in stable patients with cirrhosis using the gold-standard insulin tolerance test (ITT) and to propose an algorithm for screening AI in these patients.
Material and methodsWe studied 40 stable patients with cirrhosis. We determined the basal total (BTC) and peak cortisol (PTC) levels. Using the ITT, we defined AI as a serum PTC < 18 ng/dL at 30 min after insulin-induced hypoglycemia. We assessed the diagnostic accuracy of BTC in different stages of liver disease to discriminate between those with NAF and AI.
ResultsOf the 40 patients, 24 (60%) presented with AI. Child-Pugh and MELD scores differed between the NAF and AI groups (Child-Pugh: NAF 7.2 ± 1.7 vs. AI 8.8 ± 2.4, p = 0.024 and MELD: NAF 9.9 ± 2.5 vs. AI 14.9 ± 6.3, p = 0.001). The BTC level was lower in patients with AI than in those with NAF (7.2 ± 2.4 vs. 12.5 ± 5.2, p < 0.001). A BTC value <10.0 |ig/dL had a 96% sensitivity (negative predictive value: 90%) for identifying AI. This cutoff value (BTC <10.0 |ig/dL) provided 100% specificity (positive predictive value: 100%) in patients with advanced liver disease (Child-Pugh >9 or MELD >12).
ConclusionAn algorithm including the use of BTC and the severity of liver disease may be a useful and simple method for assessing adrenal function in stable patients with cirrhosis.
Currently adrenal insufficiency (AI) is widely recognized as an important condition occurring in some patients with liver cirrhosis.1-3 Cortisol plays an essential role in the response to stress4 and contributes to the maintenance of normal immune function, vascular tone, cardiac output, and cellular homeostasis.5 In cirrhosis, AI has been recognized both in the critically ill1,2 and in the stable pa-tient3,6 and is associated with an increased risk of mortali-ty.1,2 This inadequate adrenal response to stress in cirrhosis is defined as the hepatoadrenal syndrome.7
The diagnosis of AI in patients with cirrhosis is based on the total cortisol levels after direct stimulation with 1 μg of corticotropin.3 Salivary cortisol has also been proposed as a reliable and practical surrogate for free cortisol levels in the diagnosis of AI.8,9 Even though the controversy of which test is the most appropriate for the assessment of adrenal function, the indirect stimulation with the insulin tolerance test (ITT) is considered the gold standard for the diagnosis of AI mainly because of the work by Plump-ton and Besser.10 To the best of our knowledge there are no previous studies using this test to assess the presence of AI in stable patients with cirrhosis. Unlike the tetracos-actin test, the ITT employs a real stress stimulus that allows the evaluation of the integrity of the entire HPA axis and its ability to respond to physiologic stress by increasing corticotropin-releasing hormone (CRH) release and subsequently, increasing ACTH and cortisol secretion.11 This is relevant since ACTH stimulation does not directly measure the HPA axis responsiveness and then it carries the risk of diagnostic error in patients with pituitary or hypothalamic disease. This is especially important in the context of liver dysfunction, where the response to the ITT determined by the cortisol levels is diminished,12 supporting the idea that the functional defect in the HPA axis in patients with liver cirrhosis could be located centrally (hypothalamic-pituitary), rather than in the adrenal gland itself.
The prompt and accurate recognition of AI in stable patients with cirrhosis is valuable, since AI can lead to cirrhosis decompensation and higher short-term mortality.13,14 Currently, no practical algorithm exists in day-to-day clinical practice to screen for and diagnose AI in stable patients with cirrhosis.
To help address this, we carried out a cross-sectional study in patients with cirrhosis attending our gastrointestinal outpatient clinic. The aims of the study were:
- •
To determine the prevalence of AI in stable patients with compensated cirrhosis assessed by ITT.
- •
To correlate the prevalence of AI with the patient's functional hepatic reserve as determined by Child-Pugh (CP) score and the Model For End-Stage Liver Disease (MELD).
- •
To determine the safety of the ITT.
- •
To propose an algorithm for screening and diagnosis of AI in stable patients with cirrhosis.
This cross-sectional study was performed from September 2011 through August 2012 at the Gastroenterology Service and Endocrinology Service of the “Dr. José E. González” University Hospital, Universidad Autónoma de Nuevo León, Monterrey, México. Consecutive patients with cirrhosis attending the gastroenterology clinic as outpatients were included in the study. Diagnosis of cirrhosis was based either on histology or on clinical, laboratory, and ultrasonographic findings.
Patients were excluded from the study if they had signs of infection or sepsis, acute gastrointestinal bleeding, he-modynamic instability (mean arterial pressure less than 60 mmHg), a concomitant diagnosis of cancer, a previous history of AI or use of steroids, or grade III or IV encepha-lopathy (assessed by the West Haven scale).15 Adrenal function was assessed by performing the ITT,16 which causes a major stress response with subsequent expected increases in plasma corticotropin (ACTH) and cortisol levels. The severity of liver disease was graded by the CP score17 and MELD.18
This study was reviewed and approved by the Ethics Committee of the School of Medicine and University Hospital, which certified that it adhered to the guidelines of the General Health Law on Health Research in Human Beings of Mexico and the Helsinki Declaration. Informed consent was obtained from all patients.
ProcedureAll procedures were performed between 8:00 to 9:00 a.m. The patients fasted for at least eight hours before the test and remained seated during the procedure. Intravenous access was achieved 30 min before the procedure and maintained with an infusion of 0.9% normal saline. An IV injection of regular human insulin, 0.15 U/kg, was administered at the 0 min mark. Serum glucose levels were determined immediately before insulin administration and every 15 min thereafter for 90 min. Hypoglycemia was defined as adequate if the subject's serum glucose levels reached 45 mg/dL or lower11,19 or if there was a decrease of ≥ 50% from the basal glucose levels. If 30 min after the initial administration of insulin, hypoglycemia was not achieved, a second dose of IV insulin (0.20 U/kg) was injected. Hypoglycemia was determined by using a standard capillary glucose meter. Blood samples obtained before and at 30, 60 and 90 min after insulin administration were sent for determination of plasma glucose and cortisol levels to the endocrinology clinical laboratory. Subjects with symptomatic hypoglycemia were allowed to drink a glucose-containing liquid (cola) to relieve the symptoms; if needed, a 10% glucose solution was administered intravenously. Throughout the procedure, an internal medicine resident and a nurse monitored vital signs and watched for adverse effects.
Measurements of serum cortisol levels were performed using the Roche Chemiluminescence Immu-noassay Analyzer Reagent (ELECSYS 2010, Rotkreuz, Switzerland). Serum cortisol levels were reported in ng/ mL and converted to μg/dL. Serum glucose levels were determined by an enzymatic-colorimetric (GOD PAP) method (Spinreact, Catania, Italy).
DefinitionsBasal total cortisol (BTC) was defined as the morning total cortisol concentration (between 8:00 and 9:00 a.m.), before ITT. Peak total cortisol (PTC) was defined as the total cortisol concentration at 30 min after hypoglycemia.
Normal adrenal function (NAF) was defined as an increase in PTC value of at least 18 μg/dl (≥ 497 nmol/L) in response to ITT. AI was defined as an inadequate adrenal response to ITT as indicated by a PTC < 18 μg/dL (< 501 nmol/L).20
Statistical analysisStatistical analysis was performed with SPSS Statistics version 20.0 (Armonk, NY: IBM Corp). Data are expressed as frequencies (percentages), means with standard deviation (SD) or medians with interquartile range (IQR), as appropriate. Comparisons between groups were performed depending on the distribution of variables; continuous variables were compared by Student's t-test or the Mann-Whitney test. Categorical variables were compared using Fisher's exact test. To compare means between more than two independent groups, we used one-way analysis of variance. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to measure the contributions of variables to the risk of developing AI. Continuous variable correlation was assessed by the Pearson coefficient.
ROC analysis was used to evaluate the serum basal corti-sol, CP score, and MELD score as surrogates to identify patients with NAF and those with AI. We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of different combination of BTC values and stages of liver disease severity. A p value < 0.05 was considered statistically significant.
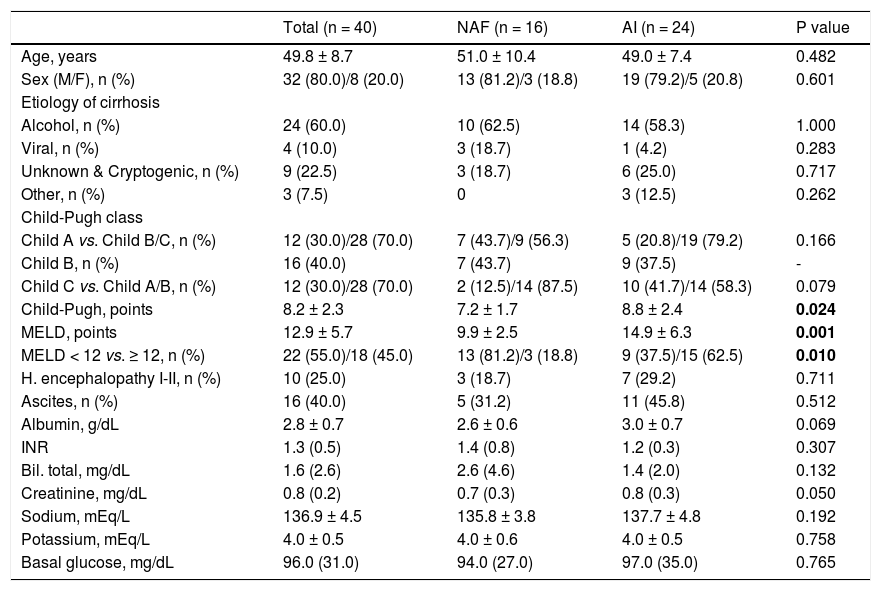
ResultsPatients’ characteristicsA total of 40 patients with liver cirrhosis were enrolled during the one-year period study. There were 32 men (80%) and 8 women (20%); mean patient age was 49.8 ± 8.7 years; and alcohol was the most prevalent cause of chronic liver disease (60%) (Table 1). Of the 40 patients evaluated, 24 (60%) had AI and 16 (40%) presented with NAF. The CP score differed significantly between groups, NAF 7.2 ± 1.7 vs. AI 8.8 ± 2.4, p = 0.024. The MELD score also showed a statistically significant difference between those with NAF 9.9 ± 2.5 and AI 14.9 ± 6.3, p = 0.001 (Table 1). There were no missing data for the variables of interest.
Baseline characteristics of patients with cirrhosis according to adrenal function status.
| Total (n = 40) | NAF (n = 16) | AI (n = 24) | P value | |
|---|---|---|---|---|
| Age, years | 49.8 ± 8.7 | 51.0 ± 10.4 | 49.0 ± 7.4 | 0.482 |
| Sex (M/F), n (%) | 32 (80.0)/8 (20.0) | 13 (81.2)/3 (18.8) | 19 (79.2)/5 (20.8) | 0.601 |
| Etiology of cirrhosis | ||||
| Alcohol, n (%) | 24 (60.0) | 10 (62.5) | 14 (58.3) | 1.000 |
| Viral, n (%) | 4 (10.0) | 3 (18.7) | 1 (4.2) | 0.283 |
| Unknown & Cryptogenic, n (%) | 9 (22.5) | 3 (18.7) | 6 (25.0) | 0.717 |
| Other, n (%) | 3 (7.5) | 0 | 3 (12.5) | 0.262 |
| Child-Pugh class | ||||
| Child A vs. Child B/C, n (%) | 12 (30.0)/28 (70.0) | 7 (43.7)/9 (56.3) | 5 (20.8)/19 (79.2) | 0.166 |
| Child B, n (%) | 16 (40.0) | 7 (43.7) | 9 (37.5) | - |
| Child C vs. Child A/B, n (%) | 12 (30.0)/28 (70.0) | 2 (12.5)/14 (87.5) | 10 (41.7)/14 (58.3) | 0.079 |
| Child-Pugh, points | 8.2 ± 2.3 | 7.2 ± 1.7 | 8.8 ± 2.4 | 0.024 |
| MELD, points | 12.9 ± 5.7 | 9.9 ± 2.5 | 14.9 ± 6.3 | 0.001 |
| MELD < 12 vs. ≥ 12, n (%) | 22 (55.0)/18 (45.0) | 13 (81.2)/3 (18.8) | 9 (37.5)/15 (62.5) | 0.010 |
| H. encephalopathy I-II, n (%) | 10 (25.0) | 3 (18.7) | 7 (29.2) | 0.711 |
| Ascites, n (%) | 16 (40.0) | 5 (31.2) | 11 (45.8) | 0.512 |
| Albumin, g/dL | 2.8 ± 0.7 | 2.6 ± 0.6 | 3.0 ± 0.7 | 0.069 |
| INR | 1.3 (0.5) | 1.4 (0.8) | 1.2 (0.3) | 0.307 |
| Bil. total, mg/dL | 1.6 (2.6) | 2.6 (4.6) | 1.4 (2.0) | 0.132 |
| Creatinine, mg/dL | 0.8 (0.2) | 0.7 (0.3) | 0.8 (0.3) | 0.050 |
| Sodium, mEq/L | 136.9 ± 4.5 | 135.8 ± 3.8 | 137.7 ± 4.8 | 0.192 |
| Potassium, mEq/L | 4.0 ± 0.5 | 4.0 ± 0.6 | 4.0 ± 0.5 | 0.758 |
| Basal glucose, mg/dL | 96.0 (31.0) | 94.0 (27.0) | 97.0 (35.0) | 0.765 |
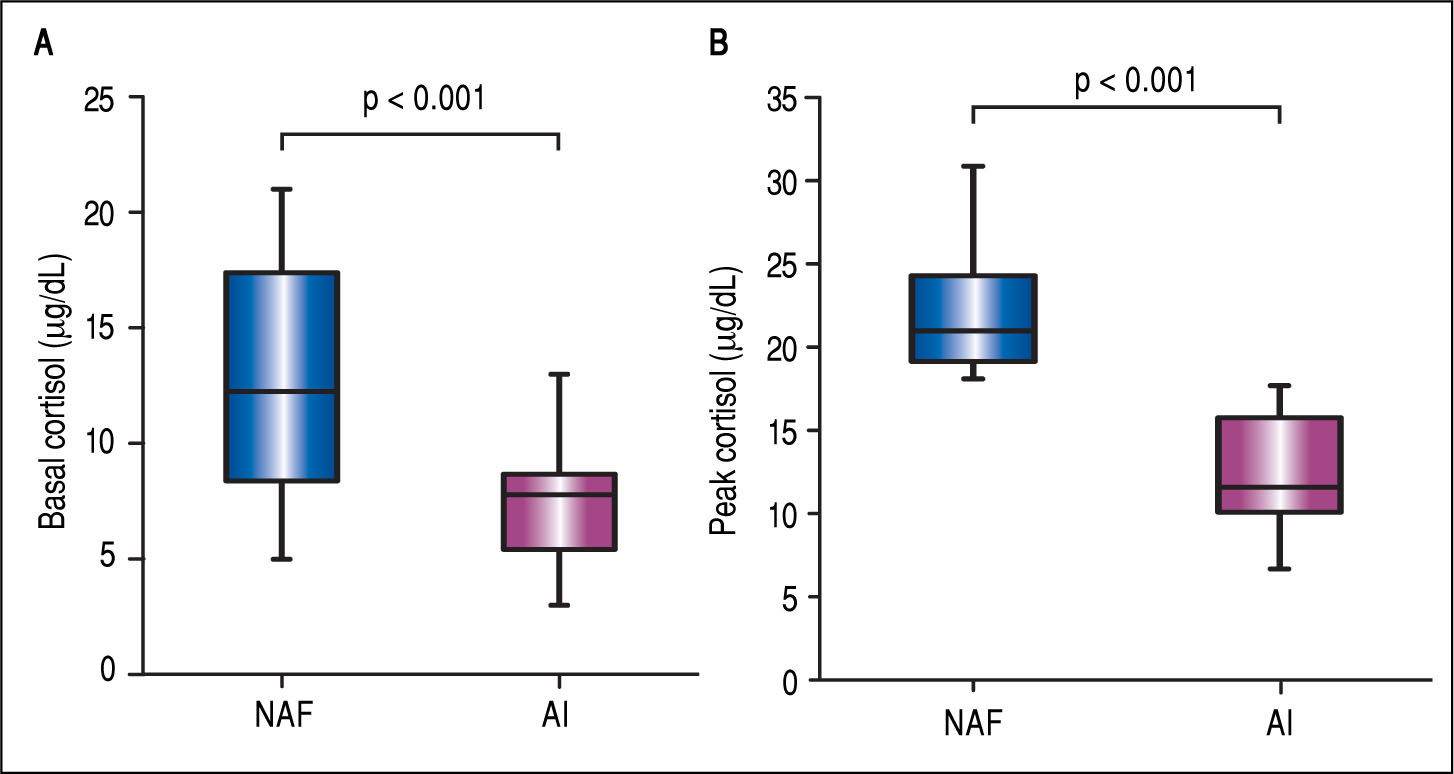
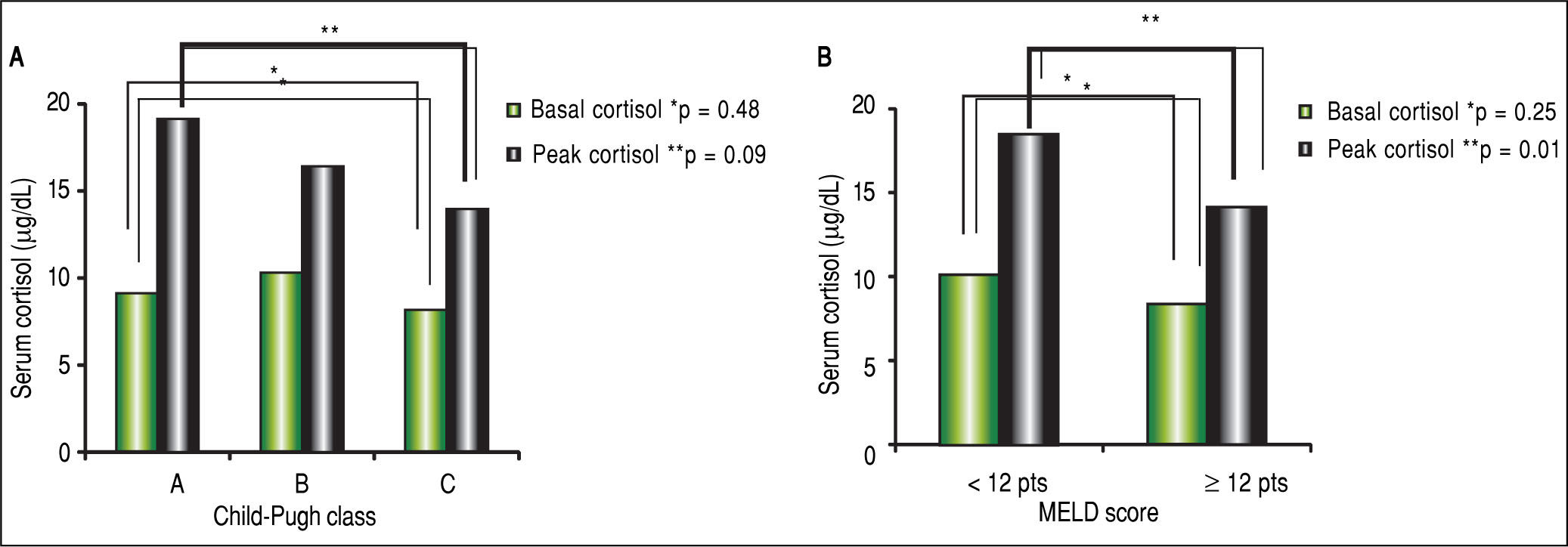
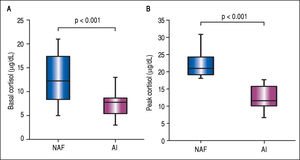
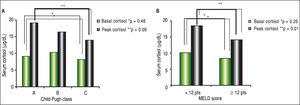
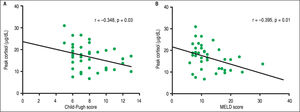
The mean BTC and PTC were significantly lower in the AI group than in the NAF group (Figure 1). No differences were observed in adrenal function measured by BTC levels through any Child-Pugh strata (Figure 2A). However, PTC progressively decreased with worsening severity of liver disease through the different CP classes, although this was not statistically significant, p = 0.09 (Figure 2A). Patients in CP class C had a higher prevalence of AI (83.3%) than did those in lower CP classes (CP A/B, 50%), with a tendency toward statistical significance, p = 0.08. Patients with MELD scores ≥ 12 points had significantly decreased peak cortisol levels (p = 0.01) compared with those with MELD scores < 12 pts (Figure 2B). Patients with MELD scores ≥ 12 had a high AI prevalence (83.3%) and a significantly increased risk of AI (OR 7.22, 95% CI 1.60-32.46).
Box-plots for comparison of basal and peak cortisol levels in patients with liver cirrhosis. A. Basal cortisol levels in patients with normal adrenal function (NAF) and adrenal insufficiency (AI), 12.5 ± 5.2 vs. 7.2 ± 2.4, p < 0.001. B. Peak cortisol levels in patients with NAF and AI, 22.1 ± 3.6 vs. 12.7 ± 3.3, p < 0.001.
We also stratified the patients into two groups based on serum albumin level; the mean values of BTC (8.9 ± 5.0 vs. 10.0 ± 3.5, p = 0.439) and PTC (16.3 ± 5.5 vs. 16.7 ± 6.3, p = 0.824) and the prevalence of AI (65.4 vs. 50%, p = 0.500) did not differ between those with albumin ≥ 2.5 g/ dL and those with albumin < 2.5 g/dL.
Prevalence of AI did not differ between patients with cirrhosis of alcoholic and non-alcoholic etiology (58.3 vs. 62.5%, p = 1.000).
Insulin-hypoglycemia stimulationAll patients achieved hypoglycemia. Twenty-six patients (65%) did so with a single dose of IV insulin, and 14 (35%) presented a blunted hypoglycemia response and so required a second dose. There was no statistically significant difference in the rate of AI between those requiring one or two doses of insulin, 57.7 vs. 64.3%, p = 0.746.
The capillary glucose levels (mg/dL) at 30 min after ITT among patients with NAF and those with AI did not differ significantly (60.5 ± 21.7 vs. 59.1 ± 32.5, p = 0.881). Among patients requiring only one dose of intravenous insulin, the capillary glucose levels at 30 min after ITT were lower among patients with AI than those with NAF (41.4 ± 11.7 vs. 50.3 ± 13.4; p = 0.09), although no statistically significant difference was reached. In those requiring a second dose of IV insulin, we found basal glucose level to be significantly higher than in those requiring a single dose (143 ± 65.4 vs. 92.9 ± 15.9, p = 0.001). Body weight (kg) did not differ between those requiring a single insulin and those requiring a second dose (82.1 ± 19.2 vs. 85.4 ± 17.8, p = 0.611). Additionally, patients with alcohol-related liver disease required a second insulin dose less frequently (35.7 %) than those with non-alcohol-related cirrhosis (64.3%), p = 0.04. No difference in basal plasma glucose concentration was found between patients with cirrhosis of alcoholic and non-alcoholic etiology (111.3 ± 54.7 vs. 107.4 ± 34.8, p = 0.71).
The symptoms most frequently associated with hy-poglycemia after the administration of one or two IV insulin doses were heart palpitations, headache and diaphoresis; all symptoms were successfully relieved by a drink of a glucose-containing liquid. No patient required the IV administration of glucose solution because of hy-poglycemia. Neither significant hemodynamic changes nor major adverse effects related to the ITT were observed.
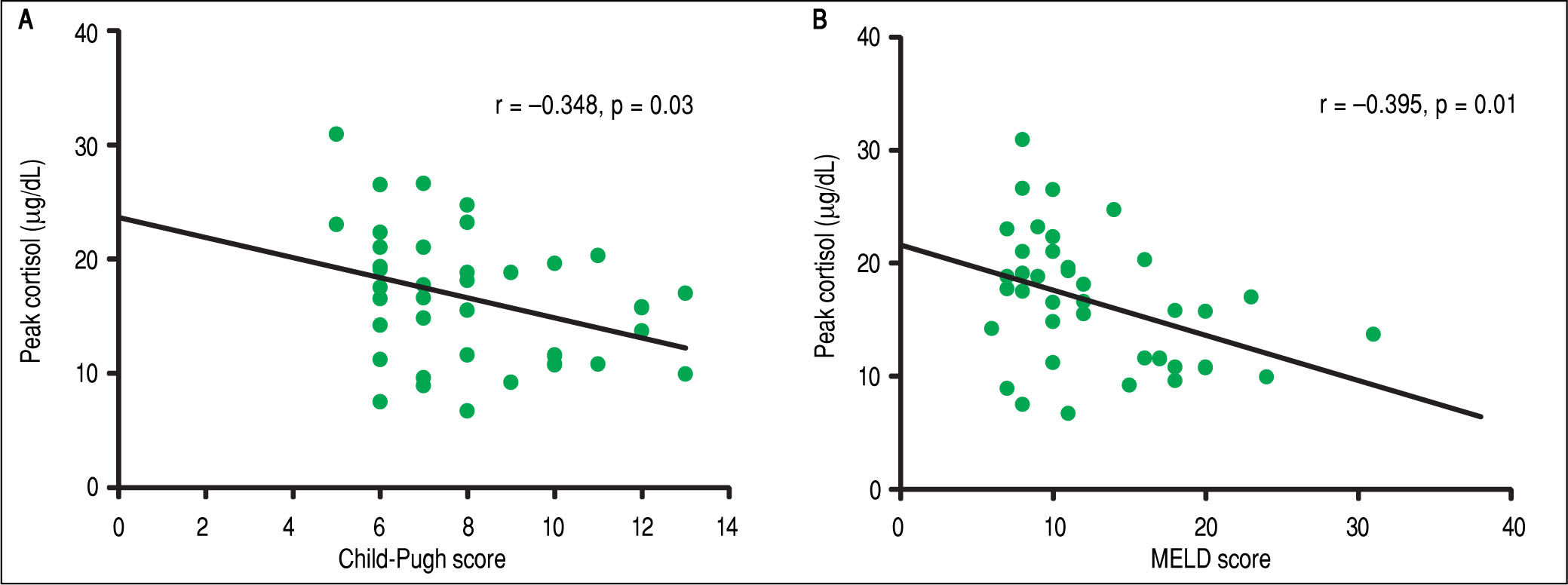
Correlation analysisIn the overall analysis, a statistically significant positive correlation was found among BTC and PTC levels (r = 0.561, p < 0.001). Correlation analysis between PTC and severity of liver disease by CP and MELD was statistically significant (Figure 3). BTC did not show a significant negative correlation with severity of liver disease by CP (r = -0.093, P= 0.57) and MELD (r = - 0.257, p = 0.11) scores.
ROC analysisWe used a ROC analysis to evaluate the performance of serum BTC, CP and MELD scores to distinguish patients with AI from those with NAF. BTC showed a higher area under the curve (0.809) than CP (0.684) and MELD (0.740), p < 0.001. A BTC of ≤ 10 μg/dL (sensitivity = 96%, specificity = 56%, accuracy = 80%) was the best cutoff value for identifying AI.
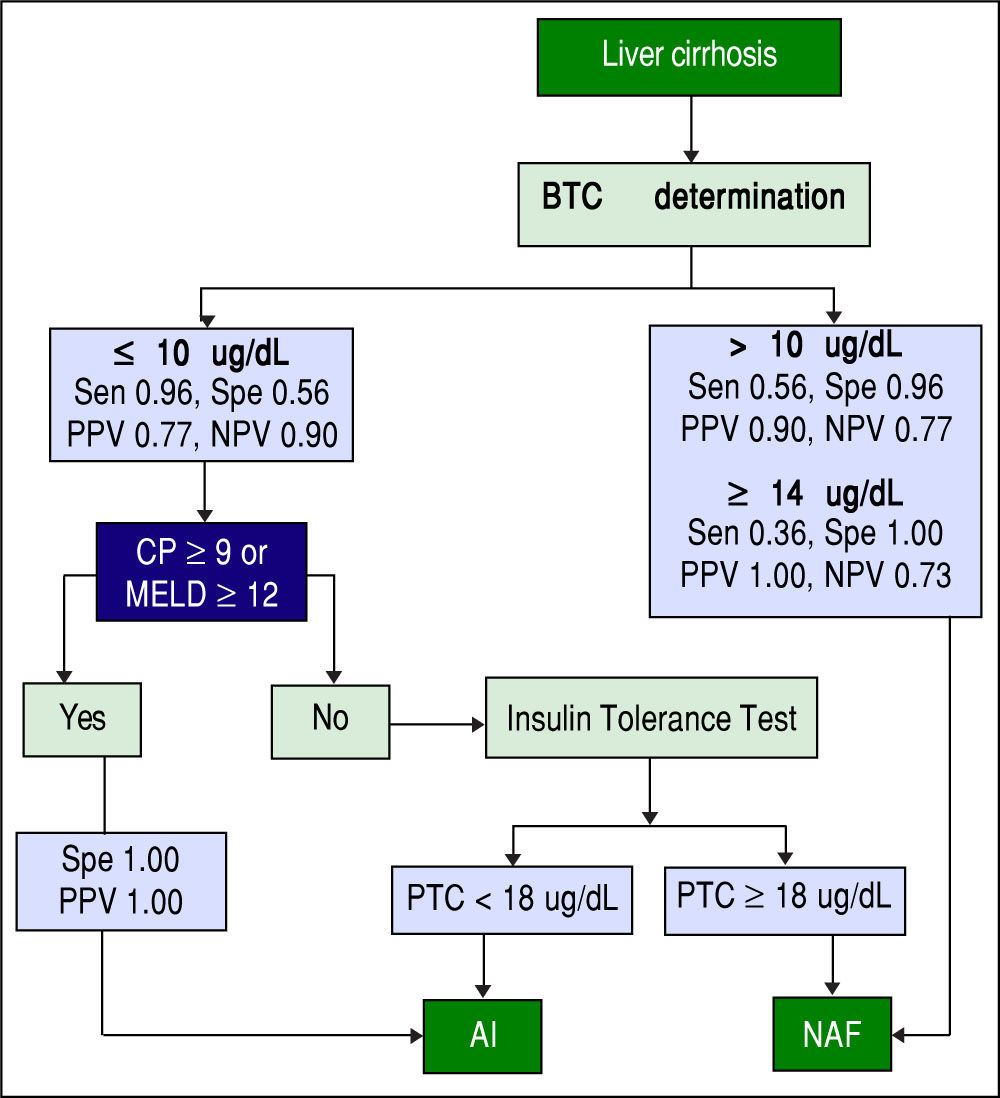
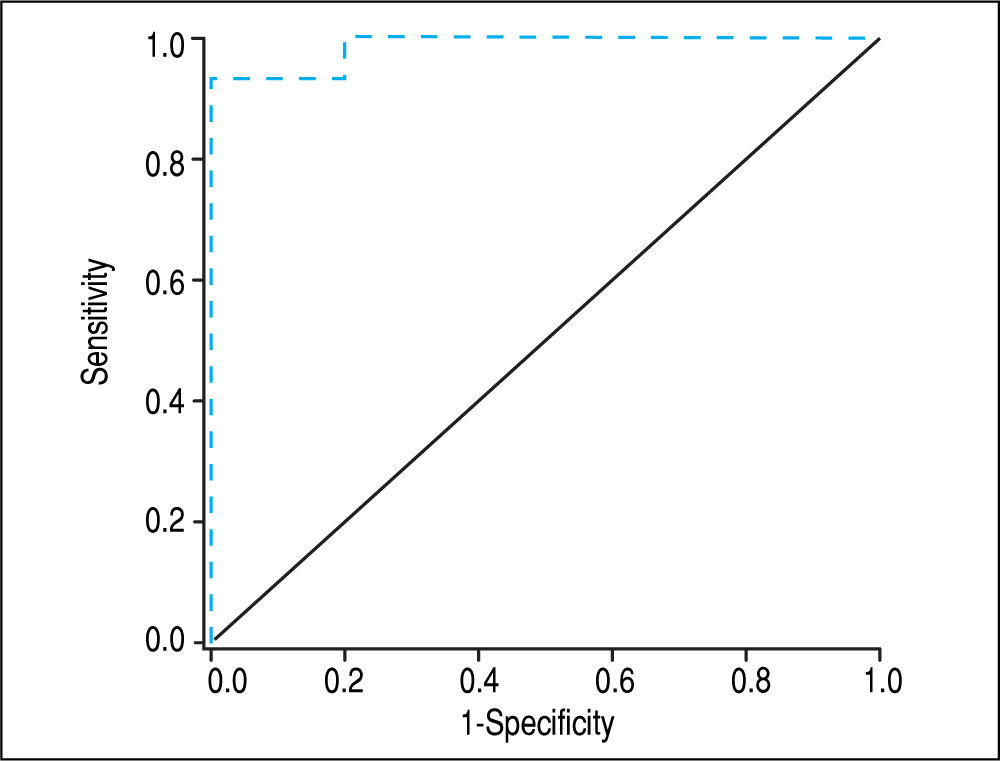
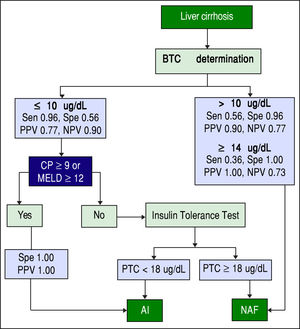
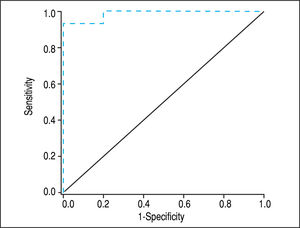
Screening algorithm for assessment of adrenal functionAmong patients with AI, > 95% had a BTC <10 ug/dL. Among those with BTC >10 ug/dL, > 95% of patients had NAF. Among those with a BTC <10 ug/dL and a CP score >9 or a MELD score >12, all had AI. Based on these results, we developed an algorithm for identifying AI in stable patients with cirrhosis based on the value of BTC and the severity of chronic liver disease as determined by CP or MELD scores (Figure 4). This is a simple and practical algorithm that can help clinicians to assess adrenal function in patients with cirrhosis. The diagnostic performance of BTC in patients with severe liver disease (CP score >9 or MELD >12) was excellent with an AUC of 0.947, p < 0.001 (Figure 5). A BTC of <10^g/dL (sensitivity = 93%, specificity = 100%, accuracy = 95%) was the best cutoff value for identifying AI in the subgroup of patients with severe liver disease.
Proposed algorithm for the assessment of adrenal function in stable patients with cirrhosis. BTC: basal total cortisol. Sen: sensitivity. Spe: Specificity. PPV: positive predictive value. NPV: negative predictive value. CP: Child-Pugh. MELD: Model for End-stage Liver Disease. PTC: peak total cortisol. AI: adrenal insufficiency. NAF: normal adrenal function.
Our study had several important results. First, stable patients with cirrhosis had a high prevalence of AI (60%), demonstrated by a significant reduction in cortisol response to the ITT. Our study appears to be the first using the ITT to assess adrenal function in stable patients with cirrhosis. A study by Fede, et al.3 using direct adrenal stimulation by injection of 1 (Xg of tetracosactrin in stable patients with cirrhosis found a lower prevalence of AI (38%). Second, no significant adverse effects related to the ITT were observed in this study, indicating that the ITT is a safe and well-tolerated test in stable patients with cirrhosis. The ITT has been demonstrated to be safe when performed in an experienced endocrine unit under adequate supervision and excluding patients with cardiovascular disease and seizures disorders.21 Third, the frequency of AI was related to the severity of liver disease; multiple other studies also have shown this association.3,22 Finally, our study appears to be the first to propose an algorithm using BTC levels and the severity of liver disease as indicated by Child-Pugh and MELD scores, allowing for accurate discrimination between patients with NAF and AI. Previously, others proposed using morning basal cortisol to assess the integrity of the HPA axis; however, doing so has a moderate overall diagnostic accuracy.23,24
Currently, there is not a consensus about which is most appropriate method for evaluation and diagnosis of AI. In spite of this, the ITT has been traditionally regarded as the gold standard for evaluating patients with suspected primary or secondary AI.10,11 Unlike the tetracosactin test, it employs a real stress stimulus that allows the evaluation of the integrity of the entire HPA axis and its ability to respond to physiologic stress by increasing corticotropin-releasing hormone (CRH) release and subsequently, increasing ACTH and cortisol secretion.11 Previously, McDonald, et al.12 evaluated adrenal function in patients with liver disease by indirect ITT; however, unlike in our study, they included patients with advanced end-stage liver disease waiting for liver transplantation. They found that patients with liver cirrhosis have about 60% lower mean peak cortisol levels than controls after the indirect adrenal stimulation by the ITT.12 This finding, as well as our results, supports the hypothesis that the functional defect in the HPA axis is located in the central (hypothalamic-pitui-tary) regulation of HPA axis function, rather than intrinsic to the adrenal gland itself. This emphasizes the importance of using indirect adrenal stimulation in this group of patients with liver disease.
We found that cirrhotic patients with AI have significantly lower BTC and PTC levels than those with adequate adrenal response, which is in accordance with previously reported data.3 Additionally, the inverse correlation between PTC and severity scores (CP and MELD) suggests that AI is a consequence of chronic liver disease itself as previously described.3,12 Furthermore, we found that the prevalence of AI in stable subjects with liver cirrhosis resembled that of critically ill patients,1,2,25 which suggests that a distinct mechanism leading to AI exists in cirrhosis.
Among stable patients with cirrhosis, alcohol was the most frequently reported cause of chronic liver disease. Several studies have shown that alcohol itself can cause HPA axis injury, probably due to chronic ethanol exposure and consequently reduced pituitary responsiveness to CRH.26-28 However, we did not find a significant difference in the prevalence of AI among stable patients with cirrhosis of alcoholic and non-alcoholic etiology. This may negate a possible selection bias in the occurrence of AI depending on the alcoholic or non-alcoholic etiology in our study.
In our study, basal glucose levels did not differ between those with NAF and AI. However, among patients with hypoglycemia 30 min after the administration of the first dose of IV insulin, those with AI had a lower level of capillary glucose. It is possible that defective hepatic (glu-coneogenesis) compensation is responsible for the severe hypoglycemia in some AI patients. Importantly, 35% of patients in our study required a second IV insulin dose to attain hypoglycemia. More than 60% of patients with non-alcoholic liver cirrhosis presented a blunted response to insulin. These results are consistent with those obtained by McDonald, et al.12 They reported a blunted response to IV insulin to occur in approximately 50% of patients with non-alcoholic liver cirrhosis and about 6% of healthy controls, OR 14.3, p = 0.004.12
The higher mean basal glucose level (in the diabetic range) in the group of patients requiring a second dose in our study is not surprising, given the well-recognized increased frequency (80%) of glucose metabolism disorders in liver cirrosis.29
In accordance with the aforementioned, we found that non-alcoholic etiology was associated with the need for a second dose of insulin. This finding, even though we lacked of histologic confirmation in the group of patients with nonalcoholic liver cirrhosis, may be mediated by an increase in insulin resistance, since we speculated steatohepatitis was the cause of liver injury in most of our patients.
Although there is a clear relationship between the prevalence of AI and severity of liver disease, individual variables (encephalopathy, ascites, albumin, total bilirubin, international normalized ratio [INR] and creatinine) did not differ significantly between groups, as reported before.3 In addition, no significant differences were demonstrated regarding serum sodium, potassium and basal fasting glucose between patients with and without adrenal dysfunction. These results, as well as the significant AI prevalence among patients with mild liver disease (37.5% in patients with MELD score < 12 pts and 41.7% in CP class A), suggest that a simple method entailing more than combination of single clinical and laboratory variables is needed to identify AI in stable patients with cirrhosis.
We found a positive correlation between BTC and PTC. The latter showed a significant negative correlation with severity of liver disease measured by CP score and MELD. Even though BTC showed no significant negative correlation with severity, it proved to be an important surrogate for the identification of cirrhotic patients with AI. In this study, almost all cirrhotic patients with AI (> 95%) had BTCs <10 fig/dL. ROC analysis showed that a BTC cutoff value of 10 fig/dL had the best overall performance. Although a BTC cutoff value of <10 fig/dL has a low specificity (56%) for the identification of AI, it rules out AI with high certainty (NPV 90%). Therefore, in clinical practice BTC could become a screening test for accurate identification (BTC <10 (Xg/dL: Sensitivity 96%, NPV 90%) and exclusion of AI (BTC >14 ug/dL: Specificity 100%, PPV 100% for NAF) in patients with cirrhosis.
Previously, morning cortisol levels (in non-cirrhotic patients) have been proposed for assessing the integrity of HPA axis, in order to obviate the need for dynamic tests. Hagg, et al.24 found that BTC levels > 10.9 μg/dL (300 mmol/L) identified patients with normal responses to hy-poglycemia with a sensitivity of 67% and specificity of 94%. Similarly, de Lange, et al.23 found that a BTC < 9.4 μg/ dL had a sensitivity of 96% and specificity of 64% for identifying an abnormal cortisol response to ITT. In addition to using different BTC level cutoffs, we added to our algorithm the use of common scores for measuring liver disease severity (CP and MELD scores), improving substantially the accuracy of identification of AI. Based on this algorithm, we can define three groups (Figure 4):
- •
Patients with BTC ≤ 10 μg/dL + CP ≥ 9 or MELD ≥ 12 (100% PPV for AI).
- •
Patients with BTC ≥ 14 μg/dL (100% PPV for NAF).
- •
Patients with BTC between 11 and 13 μg/dL or BTC ≤ 10 μg/dL + CP < 9 and MELD < 12 (further testing needed).
The main limitations of the study were the small sample size, and also the use of total cortisol levels for determining the occurrence of AI. Even though the small sample size in this study, we believe this information is valuable because currently there is not previous data showing the performance and safety of the ITT in stable patients with liver cirrhosis. It has been extensively noted that BTC may overestimate the prevalence of adrenal dysfunction in patients with cirrhosis.8,22,30-32 The overestima-tion of AI is given by the fact that more than 90% of the circulating cortisol in serum is bound to proteins (transcortin and albumin).33,34 Because the levels of protein and albumin in patients with liver cirrhosis is related to the stage of severity of liver disease (lower the levels in more advanced stages of liver disease), the assumption that the prevalence of adrenal insufficiency is overestimated by measurement of the basal total cortisol levels may be not entirely true, especially in those with less severe stage of disease.3,31 This is relevant since the majority of our patients (70%) presented a mild-moderate [Child-Pugh A or B] severity of liver disease.
Even though salivary cortisol determination is a promising surrogate for determination of free cortisol in critically ill patients with or without cirrhosis,22,30,31,35 we found only one study using salivary cortisol determination for diagnosis of AI in stable patients with cirrhosis.9 The researchers found a significant correlation between salivary cortisol and pre- and post-stimulation (cortico-trophin injection) levels of free cortisol.9 So the utilization of salivary cortisol together with liver disease severity scores could become an accurate and useful tool in the assessment of adrenal function in stable patients with cirrhosis.
Additionally, assessment of adrenal function may aid in elucidating the not-entirely-explained clinical entity termed acute-on-chronic liver failure.36,37 The resulting knowledge may help permit prompt institution of therapeutic and possibly preventive actions, such as cortisol replacement or the performance of liver transplantation.
In conclusion, an algorithm including the use of BTC and the severity of liver disease was a useful and simple method for assessing adrenal function in stable patients with cirrhosis. However, there is still a need for more studies in order to define the most appropriate test and the optimum cortisol cut-off value in this context.
AcknowledgmentsWe thank Barbara Gastel, MD, professor, and Colin Young, PhD, adjunct professor, at Texas A&M University for their help in editing the manuscript.
Abbreviations- •
ACTH: plasma corticotropin
- •
AI: adrenal insufficiency
- •
BTC: basal total cortisol
- •
CP: Child-Pugh
- •
HPA: hypothalamus-pituitary-adrenal
- •
ITT: insulin tolerance test
- •
MELD: Model for End-stage Liver Disease
- •
NAF: normal adrenal function
- •
PTC: peak total cortisol
The authors declare that they have no conflict of interest or financial disclosures.
Financial SupportWe received no funding for the research reported in this paper.