Background. Apurinic/apyrimidinic endonuclease1/ redox factor-1 (APE1/Ref-1) is a multifunctional protein involved in DNA base excision repair and redox regulation of many transcription factors. It is an important pro-survival protein activated in response to oxidative stress. Increased level of this essential redox sensi¬tive protein correlates closely with cellular survival against oxidative insults. Curcumin (diferuloylmethane) a naturally occurring compound derived from turmeric has attracted interest because of its anti-inflamma¬tory, anti-oxidative, and chemopreventive activities.
Material and methods. The current study evaluates the in vivo role of curcumin in protecting and treating liver injury and fibrogenesis caused by carbon tetrachloride (CCl4) in rats. It also addresses the possible involvement of the multifunctional protein APE1 in hepatoprotection. Analysis of APE1 expression was performed at mRNA and protein levels by reverse trans¬criptase (RT)-PCR and western blotting respectively. Profile of HSCs-activation related genes were assayed by RT-PCR and pro-inflammatory cytokines levels were determined by enzyme-linked immune assays.
Results. Here we show that oral administration of curcumin was accompanied by a robust increase in APE1 protein and mRNA levels, and improved the histological architecture of rat liver. In addition, curcumin attenuated oxidative stress by increasing the content of hepatic glutathione within normal values, leading to the re¬duction in the level of lipid hydroperoxide. Curcumin remarkably suppressed inflammation by reducing le¬vels of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), nuclear factor-kappa B (NF-kB) and interleukin-6 (IL-6). It also inhibited hepatic stellate cells (HSCs) activation by elevating the level of PPARγ and reducing the abundance of transforming growth factor-β (TGF-β). We found that oral adminis¬tration of curcumin at 200 mg/kg dose not only protected against CCl4-induced hepatic injury, but also re¬sulted in more than two-fold induction of APE1 protein expression in CCl4-induced rat group.
Conclusions. It can be concluded that curcumin reduced markers of liver damage in rats treated with CCl4, with conco¬mitant elevation in APE1 protein level indicating a possible protective effect with unknown mechanism. The induction of DNA repair enzymes may be an important and novel strategy for hepatic protection against oxidative injury.
The pathogenesis of liver fibrosis is not yet com¬pletely understood. Hepatic fibrosis is due to an im¬balance in extracellular matrix (ECM) breakdown and deposition.1–3 Research over the past two deca¬des has established that hepatic stellate cells (HSCs) are the primary ECM-producing cell type during hepatic fibrogenesis.1,2 HSCs activation, characterized by enhanced cell proliferation, overproduction of ECM and de novo synthesis of α-smooth muscle actin, is triggered by the release of mitogenic plateletderived growth factor (PDGF) and epidermal growth factor (EGF) from activated HSCs and fibrogenic transforming growth factor (TGF-β1), mostly from Kupffer cells1,2 as well as the dramatic down-expression of peroxisome proliferator-activated receptor-γ (PPARγ).4–6 The alteration in PPARγ activity plays an important role in HSCs activation.7,8
Oxidative stress has been recently recognized as a fundamental factor in the pathogenic changes obser¬ved in liver fibrosis. It plays an important role in pathophysiological changes that progress to liver ci¬rrhosis.
Cellular response to oxidative stress is a highly regulated and complex biological process.9 APE1 is a ubiquitous and remarkably multifunctional protein. It plays a central role in the base excision repair (BER) pathway for damaged bases and DNA singlestrand breaks induced by reactive oxygen species (ROS) and alkylating agents, and hence is an attrac¬tive target for possible hepatoprotection, as it is a critical component of the oxidative stress up-regula¬ted proteins. To prevent ROS accumulation, cells have several antioxidant systems including enzymes and redox-sensitive molecules (Trx, APE1/Ref-1) that protect the cells from oxidative stress.10 Activa¬tion of APE1/Ref-1 increases the binding of oxidative stress regulating transcription factors (Hif-1, p53, Ap-1). In aged rats, APE1/Ref-1 is decreased and res¬ponses to stress in the brain are defective.11,12 There are many studies on the relationship between p53 and APE1/Ref-1.13,14
Discoveries during the past 2 decades have begun to clarify genetic regulation of fibrogenesis and have thereby indicated areas of potential therapeutic intervention. Therefore, the development of inter¬ventions designed to impede or reverse hepatic fibrosis is of high priority and is urgently needed. Most evolving antifibrogenic therapies will be aimed at inhibiting HSCs activation. The antioxidant curcumin’ a polyphenolic antioxidant purified from turmeric, is an important and widely used spice of the diet in several countries. It has received attention as a promising dietary supplement for the treatment of hepatic fibrogenesis.15–19
In vitro, curcumin induces apoptosis and inhibits activation and proliferation of HSCs. In addition’ it prevents formation and development of the extrace¬llular matrix by inhibiting collagen α1(I), fibronectin and a-smooth muscle actin gene expression, by enhancing matrix metalloproteinase-2 and -9 expres¬sion and suppressing CTGF expression.19–21 In addi¬tion to several intracellular signaling pathways are modulated by curcumin in HSCs.19,20
The current study was designed to investigate the prophylactic effects and the mechanisms of curcu-min on liver fibrosis in rats, and the concomitant modular expression of APE1 and thus to explore the possible relationship between them.
Materials And MethodsAnimalsSeventy male Sprague-Dawley rats’ weighing 90¬120 g were used in this study. The animals were maintained under controlled temp (25 ± 2°C) and constant periodic conditions (12 h light, 12 h dark). The dams had free access to water and to standard food (20% protein, 54% carbohydrates, 4% lipid, 7% ash, 10% moisture). The principles of laboratory animal’s care were followed in all experimental pro¬tocols and were approved by ethics committees for animal’s research at Faculty of Medicine, Alexan¬dria University.
Induction of hepatic injury and fibrogenesis using CCl4Rats were divided into three groups: control group (group 1) received subcutaneous injection of empty vehicle), CCl4-induced group (group 2) recei¬ved subcutaneous injection of CCl4 (0.3 mL/kg body weight) once per week for 4 weeks followed by twice injections weekly for 12 weeks) and pre-cur & CCl4-induced (group 3) where rats were administered cur-cumin (200 mg/kg) by gavages tube twice weekly for 4 weeks before CCl4 administration as in group 2.
At the end of fibrosis-induction period, 10 rats from groups 2 and 3 were treated by curcumin admi¬nistration (200 mg/kg; suspended in sterile PBS) for 8 weeks.
The study aimed to investigate the molecular changes caused by CCl4-induced fibrosis and curcumin treatment in a dose- and time-dependent man¬ner; therefore three rats from each group (groups 1, 2 and 3) were sacrificed by cervical dislocation at time intervals of 6, 10 and16 weeks following CCl4 injection and at weeks 20 and 24 following curcumin treatment (i.e. after 4 and 8 weeks of curcu-min treatment respectively). Blood sample from each sacrificed rat was collected by heart puncture. Sera were collected and stored at -80°C for liver function biochemical analyses (ALT, AST, albumin, total protein, and bilirubin).
Liver tissues were removed from each sacrificed rat; small portion was fixed in 10% neutral-buffered formalin (NBF) for histological analysis and the remaining tissue was immediately frozen in liquid nitrogen then stored at -80°C.
Histopathological analysisThe fixed liver tissues in formalin were dehydra¬ted in ascending grades of alcohol, and then cleaned by immersing the tissues in xylene for 1 h (three ti¬mes), followed by impregnation in melted paraffin, in wax, then dried at oven for 1 h. Then the speci¬mens were embedded in paraffin and were left to so¬lidify at room temperature. Using a rotatory micro¬tome, multiple sections of 5 μm thick were cut and were mounted on clean glass slides. All sections were stained with hematoxylin and eosin (H&E) and examined for any histopathological changes.22 Pathological diagnosis of each liver specimen was assessed and graded from 0 to VI by two pathologists in a blinded manner according to the criteria described by Wang, et al.23
Analyses of hepatocytic death and hepatic injury parametersBlood was collected from each rat by heart punc¬ture when sacrificed. After coagulation, sera were collected and stored at -20°C for further analyses. Activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total pro¬tein and albumin levels in sera were analyzed using routine laboratory assay kits.
Determination of lipid peroxidationPart liver tissue (10% w/v) was washed with sali¬ne solution, minced and homogenized in ice cooled buffer 50 mm potassium phosphate pH 7.5, the homogenate was centrifuged at 10,000 rpm for 20 min at 4°C, and the supernatant was used for the as¬say. Level of lipid per oxidation was determined ba¬sed on Ohkawa, et al.,24 colorimetric method. Thiobarbiuitic acid (TAB) reacts with malondialdehyde (MDA) in acidic medium at temp 95°C for 30 min to form thiobarbiuitic acid reactive product the absorbance of the resultant pink product can be measured at 534 nm and is expressed in nmol/g.tissue.
Determination of reduced glutathione (GSH) and glutathione peroxidase activity (GPX)Part liver tissue (10% w/v) was washed with sali¬ne solution, minced and homogenized in ice cooled buffer1.15% kcl, 0.01 M sodium phosphate buffer pH 7.4 the homogenate was centrifuged at 10,000 rpm for 20 min at 4°C and the supernatant was used for determination of GSH, GPX and protein content. The concentration of GSH was determined as descri¬bed by Eliman;25 Eliman’s reagent [5,5’, dithiobis (2-nitrobenzoic acid), DTNB] was reacted with GSH giving 2-nitro-5-thiobenzoic acid, a yellow colored product with a maximum absorbance at 412 nm, GSH level is expressed as mg/g liver.
Total glutathione peroxidase (Gpx) was determi¬ned spectrophotometrically using cumene hydroperoxide as substrate. The excess amount of reduced glutathione was determined using Ellman method. Gpx activity were calculated as (μg/min/mL), the consumed GSH (μg/mL)/time of incubation (min).
Total RNA isolation and reverse transcriptase polymerase reaction (RT-PCR)Total RNA was extracted from frozen liver tissues according to Chomczynski and Sacchi procedure26 using GStractTM RNA Isolation Kit II Guanidinium Thiocyanate method. The extracted dried pellets were resuspended in 50-100 μl RNAase free and stored at -80°C. Alterations in the steady-state mRNA levels of genes relevant to hepatic stellate ce¬lls (HSCs) activation and fibrosis development in CCl4 induced rats is determined using reverse-transcriptase PCR analysis. Using one-step RT-PCR (RT/PCR Master Mix. Gold Beads, BIORON) reaction, the cDNA was synthesized and used for amplifica¬tion of target gene(s) using specific primer sets. Briefly, total RNA (1-3 μg) and random primer (3 μM) mixture were denatured at 70°C for 5 min and placed on ice. The incubated mixture was added to the RT/PCR Gold mix that contains all the compo¬nents necessary for cDNA synthesis and amplifica¬tion in one tube. The cDNA synthesis reaction was performed at 42°C for 60 min then 5 min at 94°C for RTase inactivation. The primers then subjected to PCR cycles. Annealing temperature and time need to be optimized for each primer/template combina¬tion. We investigated the expression of pro-fibrotic genes that is induced by activated hepatic stellate ce¬lls (HSCs) (TGF-β, CTGF, TIMP-1, STAP and α-SMA) vs. anti-fibrotic genes (MMP-2 and PPARγ) as well as the expression of rat albumin, APE1 and p53.
The sequences of the primers set for
- •
TTMP: F-GCCCCAACCCACCCACAGA- R-TTT-GCAAGGGATGGCTGAACAG (405 bp).
- •
MMP-2: F-GAGAAAAGCGCAGCGGAGTGACG-R- TTCCCCCGCAAGCCCAAGTG (145 bp).
- •
CTGF: F-ATCCCTGCGACCCACACAAG- R-CAACTGCTTTGGAAGGACTCGC (450 bp).
- •
TGFβ-1: F-CGGGAAGCAGTGCCAGAA- R-TGC-TCCACAGTTGACTTGAATCTC (470 bp).
- •
APE-1: F-CTGCCTGGACTCTCTCATCAATAC-R-CCTCATCGCCTATGCCGTAAG (200 bp).
- •
p53: F-CACAGTCGGATATGAGCATC- R-GTCG-TCCAGATACTCAGCAT (600 bp).
- •
α-SMA: F-ACTGGGACGACATGGAAA AG- R-CATCTCCAGAGTCCAGCACA (280 bp).
- •
STAP: F-ATGGAGAAAGTGCCGGGCGAC- R-TGGCCCTGAAGAGGGCAGTGT (340 bp).
- •
Rat-albumin: F-GCTCAGAGACTGCCCTGTGT-R-ACAAGGTTTGGCCCCTCAGT (400 bp).
- •
GAPDH: F-TGCTGGTGCTGAGTATGTCG- R-ATTGAGAGCAATGCCAGCC (646 bp).
Nuclear extract was prepared as described by Schreiber et al.27 500 mg of frozen tissue was homoge¬nized in 5 mL of ice-cold extraction buffer A (0.6% NP-40, 150 mM NaCl, 10 mM HEPES pH 6.9, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride). The homogenate was centrifuged at 2,000 rpm for 30 s at 0°C. After incubation for 5 min on ice, the superna¬tant was centrifuged at 5,000 rpm for 5 min at 0°C. The supernatant is the cytoplasmic fraction was co¬llected and stored at -80°C. The precipitate was resus-pended in extraction buffer B (25% glycerol, 420 mMNaCl, and 20 mM HEPES pH7.9, 1.2 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5mM phenylmethylsulfonyl fluoride, 5 mg/mL each of aprotinin, leupeptin, and pepstatin). After being kept on ice for 20 min, it was transferred to a centrifuge tube and centrifuged at 12,000 rpm for 25 min at 0°C. The supernatant was transferred to a new tube and stored at -80°C.
Cytokines determinationIL-6, TNF-α, and TGF-β levels were assayed in total cell extracts prepared form liver tissues and sera samples. Cytokines levels were determined using commercially available kits. The analyses were performed according to the manufactures’ guidelines. IL-6 level assayed using BioLegend ELISA MAX™ Set Standard Rat IL-6 Kit (Cat.430502). TNF-α was determined using Rat TNF-α Prprotech kit (cat# 900-K25) and TGF-β using Quantikine kit (Cat# MB100B). Nuclear factor-κB p50/65 DNA-binding activity in nuclear extracts of hepatic tissue samples was evaluated. Analysis was performed according to manufacturer’s protocol guidelines using commer¬cial Kit (NF-κB p50/65 Transcription Factor As¬say Colorimetric. Cat. No. SGT510 Themicon International Inc. Temecula, CA, USA).
Western blottingAccording to the method described by Burnette,28 APE1 and p53 immunoblots were performed on pre¬pared cytosolic and total cell extracts respectively. Briefly, 50 μg protein lysates from each sample were separated on 12% SDS-PAGE then transferred to a nitrocellouse membrane. Primary antibody to APE1 (rabbit Polyclonal anti-APE1) and p53 (sc-DO-1, Santa Cruz Biotechnology) was used. Then incu¬bated with HRP-conjugated secondary antibodies (Santa Cruz; diluted).
Hydroxyproline (hyp) assay in rat liverA colorimetric assay was performed as described by Nakamura, et al.29 Briefly, lyopholized liver sec¬tions (0.5 g) were hydrolyzed for 20 h in 6 mol/L HCl at 100°C, redissolved in water, and centrifuged to remove any impurities. Samples were incubated for 10 min in 0.05 mol/L chloramine-T (Fisher, Fair Lawn, NJ, USA) at room temperature, followed by 15-min incubation in Ehrlich’s-perchloric acid solu¬tion at 65°C. Sample absorbencies were assessed at 560 nm and resulting values compared to a hyp standard curve. Each sample was assayed in dupli¬cate. The hyp content was expressed as micrograms per gram of wet liver.
Statistical analysisAll experiment was repeated two or more times independently and typically graph are represen¬ted. In some cases data are presented as mean ± SD. Data were analyzed by student T-test and di¬fferent considered significant at p <0.05 or at p < 0.01.
ResultsPre-treatment with curcumin ameliorated CCl4-induced liver injury through apoptosisRepresentative microscopic photographs of liver sections stained with Hematoxylin-eosin (H&E) staining of CCl4-induced and pre-cur & CCl4-in-duced groups then treated are shown in figure 1. We assessed the fibrosis degree (score) according to the pathological index described by Wang, et al.23 Stained and examined liver sections of CCl4-induced rats were graded from score 0 (at week zero, control), II (at week 6), III (at week 10) and from IV to V, (at week 16). At score IV-V the CCl4-injection was stopped and curcumin treat¬ment was started.
Representative microscopic photograph of liver of CCl4-induced or curcumin-treated rats by hematoxylin-eosin (H&E) staining. A-0 to A-16 shows the histological findings of the experimental rats of group 2 that was fibrosis-induced with CCl4 in¬jection for 16 weeks. The results are represented at time intervals of 0, 6, 10, and 16 of CCl4 injection and at week 24 which re¬presents the result after 8 weeks of curcumin treatment of fibrotic liver. (0) Represents rat’ liver section of normal control, A-24 show fibrosis-induced liver that has been treated with curcumin for 8 weeks. B-0 toB-16 shows results of group 3 that was pre-cur & CCl4-induced for 16 weeks. Treatment with curcumin before CCl4 injection has protective effect on rat liver as represented in figure B during the follow up weeks. B-24: Sections of curcumin pre-treated, fibrosis-induced then curcumin treated rats for 8 weeks (week 24) showing thick wall of portal track collapsed with congested hepatocytes cell forming pseudo nodules, rest of hepatocytes was vacuolated.
Pre-treatment with curcumin for 4 weeks has pro¬tective effect on rat liver as represented in the stai¬ned sections in figure 1-B where the fibrosis grade assessment was I at week 6, II at week 10, III to IV at week 16 of CCl4-injection compared to advanced progression that has been observed in CCl4-induced rats of group 2. Appearance of area of apoptotic cells in different stages of pre-cur & CCl4-induced indu¬ced group was observed as well. Sections of fibrotic rats (group 2) that has been treated with curcumin for 8 weeks indicated portal track collapsed with congested hepatocytes cell forming pseudo nodules, rest of hepatocytes was vacuolated.
The general conditions of rats improved after cur-cumin treatment within 14 days as assessed by change in the body weight and hyp content. During fibrogenesis induction, the body weight of group 2 rats dramatically reduced within the first 6 weeks (the mean loss body mass was 35.40 ± 8.34 g) and then slowly increased within 6-16 week (increased by 19.44 ± 6.78 g). From week 20, the body weight of rats slightly increased in treatment group with no obvious changes in control group, showing that rats become adapted to toxicity. In addition, hyp content significantly (p< 0.05) attenuated in wet liver of pre-cur & CCl4-induced group to 396.8 ± 116.3 mg/g liver compared to 566.0 ± 237.4 mg/g liver in CCl4-induced.
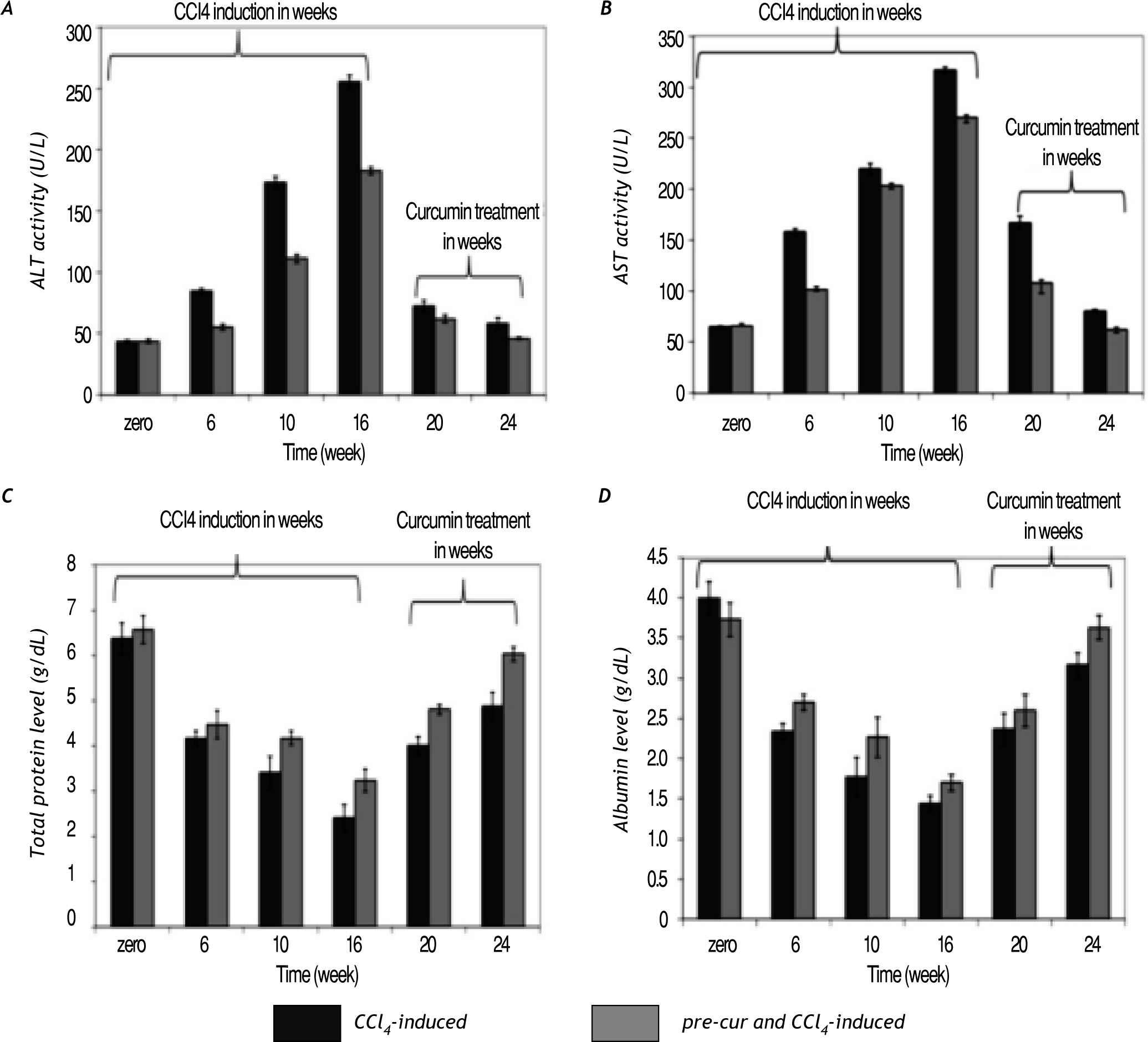
Curcumin reduced CCl4-induced hepatocytes toxicity and injuryCurcumin pre-treatment ameliorated the signifi¬cant elevation in sera ALT and AST activities com¬pared to CCl4 injected rats without curcumin pre-treatment (Figure 2A and 2B). At weeks 20 and 24 (curcumin treatment for 4 and 8 weeks) the re¬sults indicated significant (p< 0.05) reduction in the ALT and AST serum activities in both groups. Also pre-treatment with curcumin induces less re¬duction in total serum protein and albumin levels compared to CCl4-induced rats (Figure 2C and 2D). Alterations in albumin mRNA level as a marker of hepatocytes synthetic function was found to be hig¬hly reduced in liver tissues of CCl4-induced rats compared to pre-cur & CCl4-induced rats (Figure 7). Curcumin treatment for 4 and 8 weeks of fibrotic liver, restored hepatocytes function as indicated by significant elevation (p< 0.05) in serum tissue expression of at albumin mRNA level in groups 2 and 3.
Changes in liver function indices. The activities of serum AST and ALT as well as the total protein and albumin le¬vels were analyzed during CCl4-induced fibrosis and curcumin. A significant elevation in AST and ALT activities were observed in all groups. In pre-cur & CCl4-induced rat curcumin protected rat liver from CCl4-induced in serum elevated ALT and AST activities as well as reduction in total protein and albumin levels compared to the CCl4-induced group without curcumin pre-treatment (group 2; CCl4-induced). Curcumin administration for 4 and 8 weeks (at weeks 20 and 24), restored hepatocytes function as indi¬cated by significant elevation (p < 0.05) in sera total proteins and albumin levels as well.
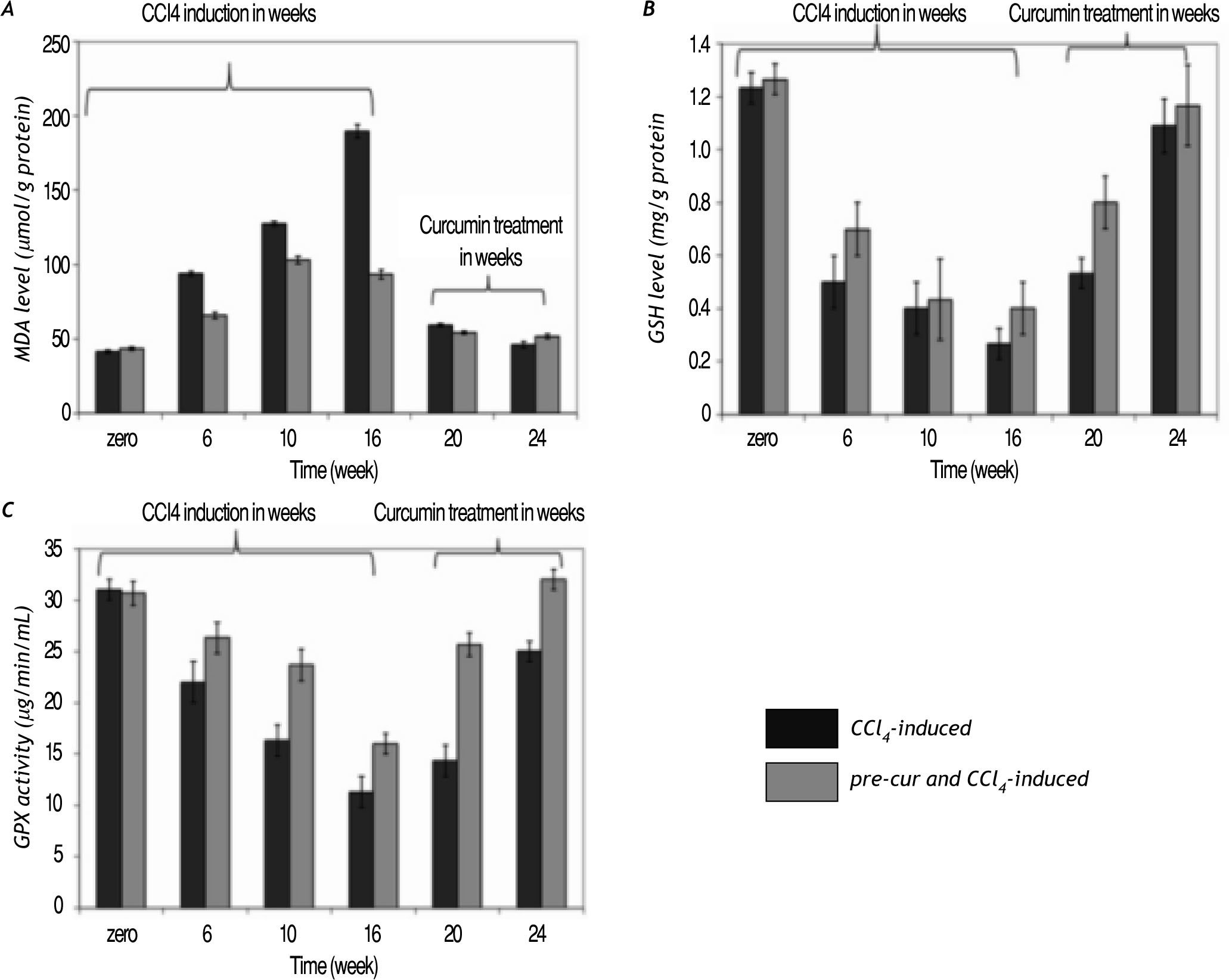
Curcumin treatment reduced lipid hydroperoxide (calculated as MDA level) production in the liver of CCl4-induced rats. A significant increase in MDA le¬vel during CCl4-injection vs. the pre-cur & CCl4-induced experimental rats was observed. Treatment of fibrotic rats with curcumin was shown to significan¬tly reduce the lipid peroxidation level (p< 0.05) in both groups at weeks 20 and 24 of the follow up pe¬riod (Figure 3A).
Curcumin pre- and post-fibrosis induction treat¬ment reduced oxidative stress in fibrotic rat liver. A. Lipid hydroperoxide (calculated as MDA level) in the liver of CCl4-induced rats was increased significantly during the period of CCl4-induced group vs. the pre-cur & CCl4-induced experimental rats. Treatment with curcumin after fibrosis induction was shown to significantly reduce the MDA level (p < 0.05) in both groups at weeks 20 and 24 of the follow up period. B and C. GSH level and GPX activity were reduced significantly in CCl4-treated rats (CCl4-induced), while pretreatment with curcumin (pre-cur & CCl4-induced) reduced the CCl4-mediated oxidative stress. Treatment with curcumin for 4 and 8 weeks significantly elevated (p <0.05) the GSH level and GPX activities in both groups.
GSH level and GPX activity were reduced signifi¬cantly in CCl4-treated rats, while pretreatment with curcumin (pre-cur & CCl4-induced) reduced the CCl4-induced oxidative stress (Figure 3B and 3C). Treatment with curcumin for 4 and 8 weeks after CCl4 administration for 16 weeks significantly eleva¬ted (p< 0.05) the GSH level and GPX activity in both experimental groups.
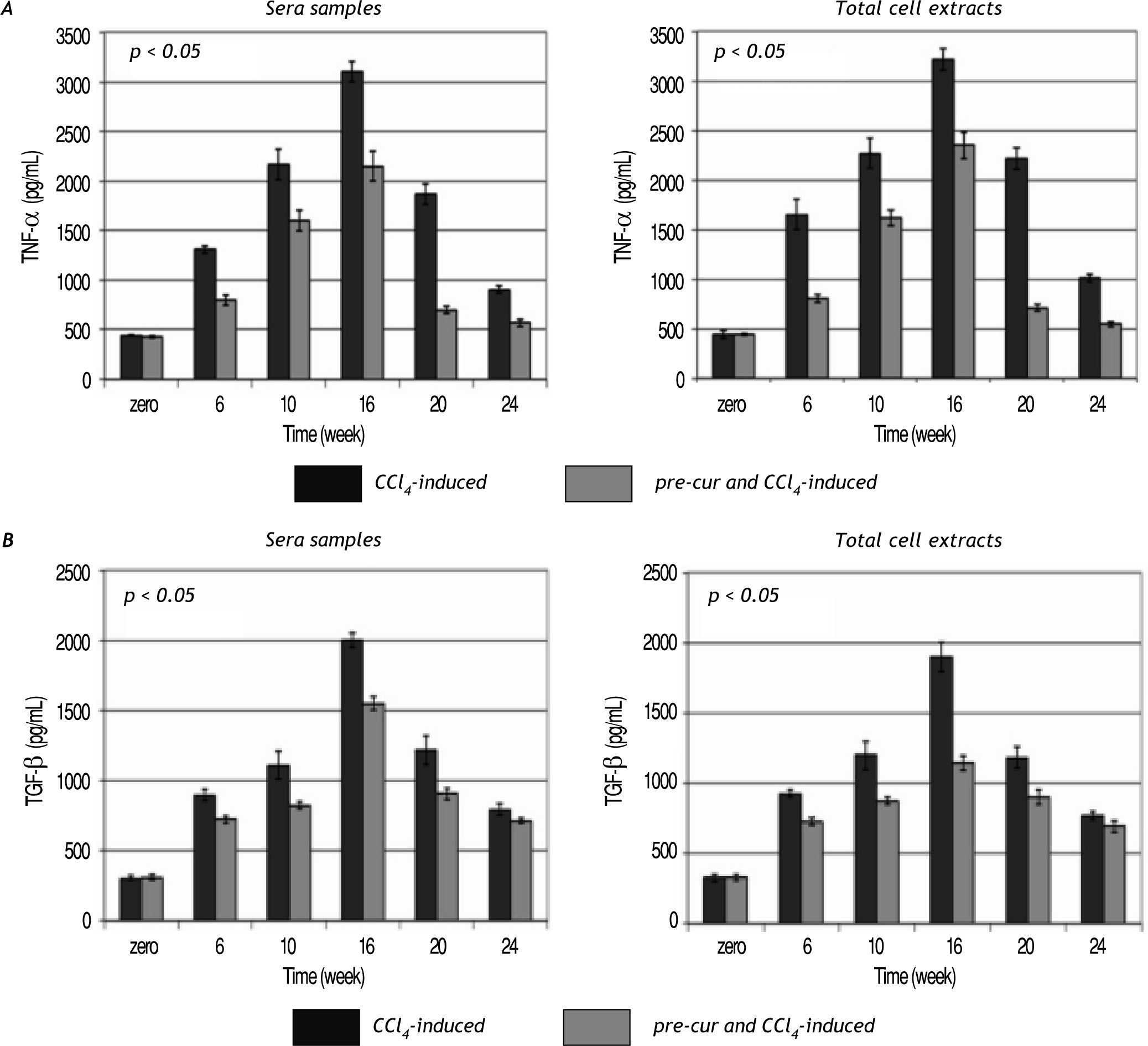
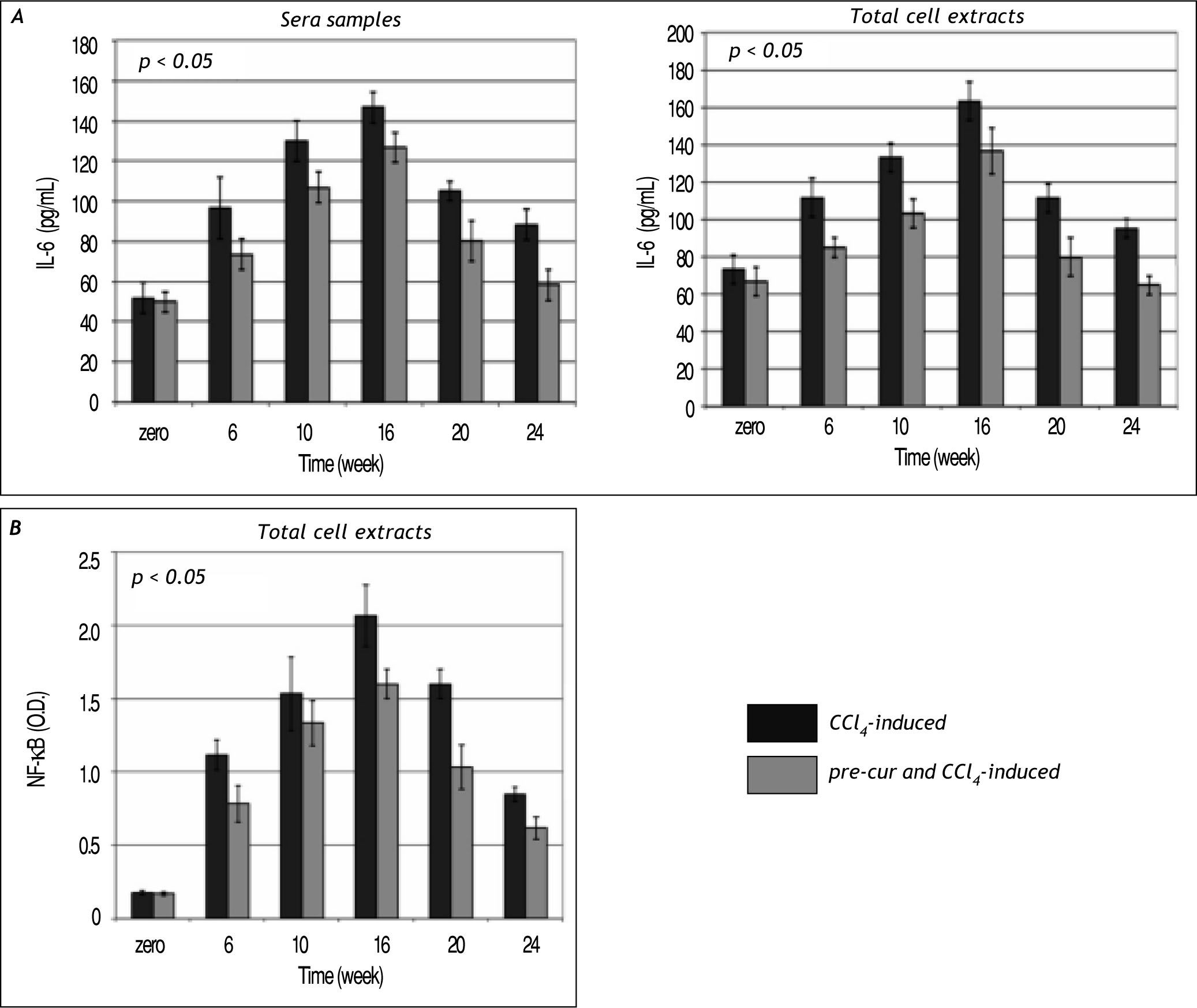
Modulation of sera and liver inflammatory cytokines levels during the course of fibrosis induction and curcumin treatmentHepatic fibrogenesis is commonly associated with inflammation. Levels of inflammatory cytokines; TNF-α, TGF-β, and IL-6 were determined in serum and liver homogenates. TNF-α, TGF-β, and IL-6 le¬vels were elevated significantly (p< 0.05) during the 16 weeks of fibrosis induction compared to the con¬trol group (Figures 4 and 5). Pre-treatment with curcumin reduced the CCl4-induced HSCs activation and inflammation as indicated by marked reduc¬tion in TNF-α, TGF-β, and IL-6 levels during the course of fibrosis induction compared to CCl4-induced rats. Curcumin treatment of both groups (group 2 and 3) induced-hepatocytes regeneration and inhibi¬tion of HSCs activation and inflammation as indica¬ted by significant reduction in sera and total cell extracts TNF-α, TGF-β, and IL-6 levels (p< 0.05) at weeks 20 and 24. In addition, NF-κB level in nu¬clear extracts of CCl4-induced and curcumin pre-and post-fibrosis treated rats showed a significant elevation (p < 0.05) during fibrosis development in the rat liver vs. control group. Treatment by curcumin reduced significantly (p< 0.05) NF-κB level at weeks 20 and 24 (Figure 5).
Time course study of the change in TNF-α and TGF-β levels in sera and total cell extracts of CCl4-induced and curcu-min-treated groups. Data indicate significant elevation (p < 0.05) in both TNF-α (A) TGF-β (B) levels sera samples and cell extracts during the follow up weeks fibrotic rat liver versus control group. Pre-cur administration ameliorated the CCl4-induced hepato-toxicity as indicated in group 3 compared to group 2. A significant reduction (p < 0.05) in both cytokines levels was observed at weeks 20 and 24.
Time course study of IL-6 (A) and NF-κB (B) in the CCl4-induced liver fibrosis and treated rats. Data indicate sig¬nificant elevation (p < 0.05) in IL-6 during the follow up weeks 6, 10 and 16 in the total cell extracts vs. control. The pretreatment with curcumin before CCl4 injection protected the hepatocytes as indicated by change in IL-6 level when compared to samples of group 2. Curcumin injection attenuated hepatic stellate cells activation and reduced IL-6 significantly (p < 0.05) in total cell extracts and sera samples at weeks 20 and 24. Also a significant elevation (p < 0.05) in NF-κB during the follow up period of fibrosis development in the rat liver versus control was observed. Pre-treatment and treatment by curcumin showed significant reduction (p < 0.05) in NF-κB at weeks 20 and 24.
We investigated the changes in the mRNA levels of pro-fibrotic genes (TGF-β, CTGF, TIMP-1, α-SMA, and STAP) and anti-fibrotic genes (MMP-2 and PPARγ) by reverse transcriptase-PCR.
Previous reports showed that curcumin inhibited in vitro HSCs activation by suppressing expression of genes relevant to cell growth and fibrogenesis.19,30 Curcumin pre- and post-fibrosis induction treatment suppressed pro-fibrotic genes expression including TGF-β, CTGF, TIMP-1, α-SMA, and STAP and indu¬ced MMP-2. A marked elevation of TGF-β, CTGF, MMP-2, α-SMA, and STAP mRNA levels were obser¬ved as well (Figure 6). Pre-cur treatment ameliora¬ted the CCl4-induced HSCs activation and pro-fibrotic genes expression compared to rats in group 2 (Figure 6); 8 weeks of curcumin treatment for groups 2 and 3 reduced pro-fibrotic genes expres sion (Figure 6) and induced increase in MMP-2 le¬vel. Moreover PPARγ, which plays critical role du¬ring in vivo fibrogenesis and in HSCs during liver injury, was markedly reduced during CCl4-induced fibrogenesis in group 2 (Figure 7). Pre-treatment with curcumin reduced the CCl4-induced HSCs acti¬vation by maintain higher expression of PPARγ up to week 16 compared to group 2. PPARγ mRNA was re-elevated in both groups of fibrotic rats after curcumin treatment for 8 weeks (at week 24).
Reverse transcriptase (RT)-PCR analyses of fibrosis vs. antifibrotic genes profile. The alterations in the mRNA levels were analyzed by RT-PCR. Curcumin modulates the expression of genes related to HSCs growth and fibrogenesis. mRNA levels of genes related to fibrogenic transformation, including, TGF-β, CTGF, TIMP-1, α-SMA, and STAP were significantly elevated during the CCL4-injection weeks. On the contrary, marked reduction in MMP-2 was observed. Curcumin treatment for 8 weeks reduced pro-fibrotic genes expression (TGF-β, CTGF, TIMp-1, α-SMA, and STAP) in both groups and induced increase in MMP-2 level. GAPDH was used as an invariant internal control.
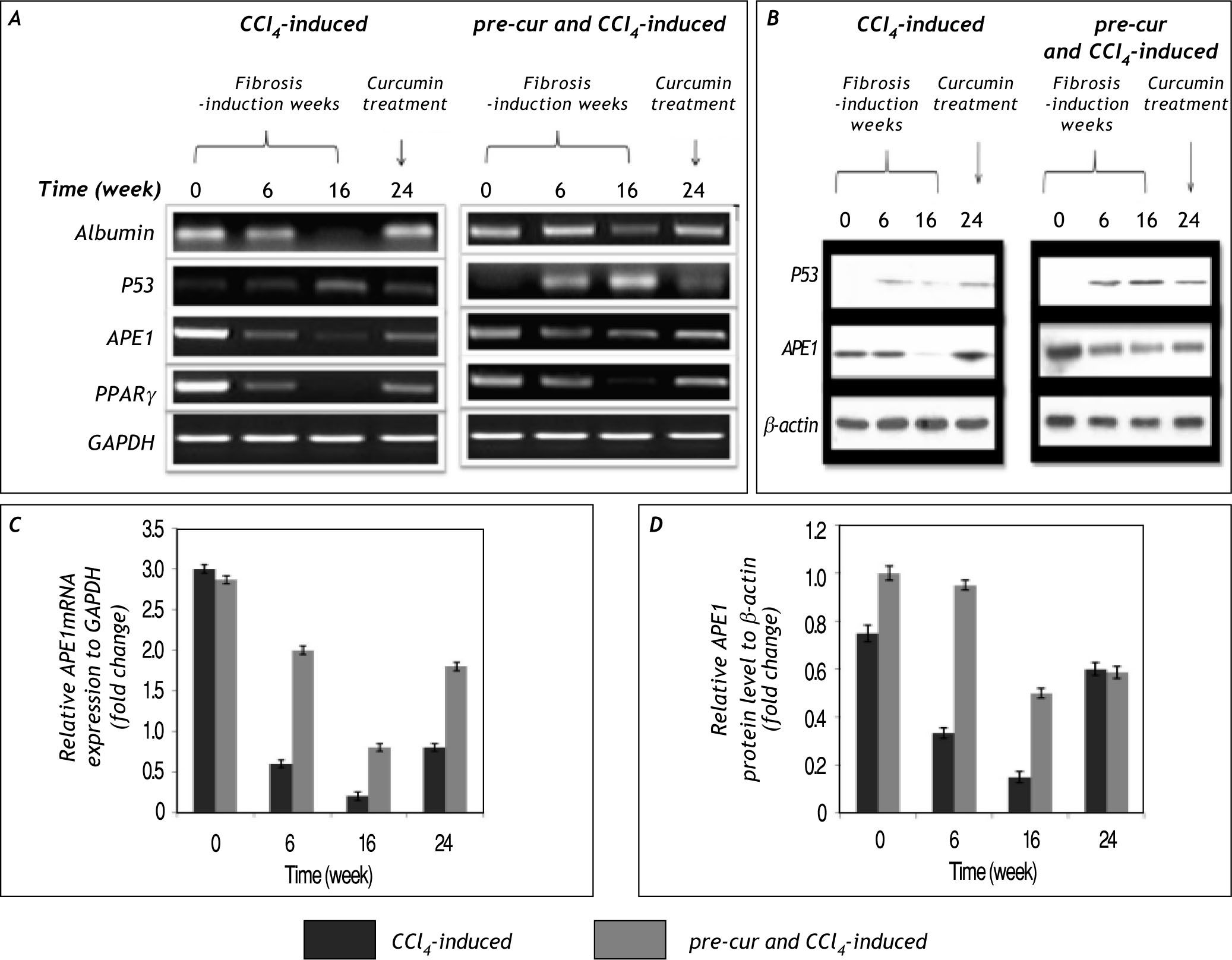
The change in p53 and APE1 mRNA and protein levels in CCl4-induced fibrogenesis. A and B. Show the results of group 2 (CCl4-induced) and group 3 (pre-cur treated & CCl4-induced) of mRNA and protein levels respectively. Elevation in p53 mRNA level at weeks 6 to 16 of Cur- pre-treated & CCl4-induced was detected. In addition, APE1 level is elevated at week 6 due to CCl4-induced oxidative stress, its level is reduced significantly at week 16 and upon curcumin treatment for 8 weeks (week 24) a significantly induced APE1 mRNA and protein level (A and B) was observed. Moreover PPARγ, which plays crucial role during in vivo liver injury mRNA is reduced markedly during CCl4-induced fibrogenesis in group 2 compared to less reduction in pre-cur treated & CCl4-induced rats. Curcumin treatment attenuated the HSCs and possibly mediated an increase in APE1 and PPARγ mRNA levels at week 24. C and D. Represent the fold change in APE1 mRNA and protein levels in relative to GAPDH and β-actin levels respectively. Curcumin treatment may correlates with the significant (p< 0.05) expression of APE1 mRNA and pro¬teins levels in cur- pre-treated & CCl4-induced vs. CCl4-fibrosis group. Representatives of three independent experiments are shown.
In the present study we report for the first time that APE1 protein and mRNA levels are reduced sig¬nificantly (p < 0.05) during the course of CCl4-induced liver fibrosis (Figure 7 and 8). In addition to, in the pre-cur & CCl4-induced group maintaining high level of APE1 expression was observed. Upon curcumin treatment of fibrotic livers for 8 weeks (week 24) APE1 mRNA and protein levels were re-elevated in both groups. The significant alteration in APE1 mRNA and protein levels of groups 2 and 3 (calcula¬ted as alterations of the controls; GAPDH and β-actin for mRNA and protein respectively) is represented in figure 7C and 7D. Also the percenta¬ge of fold change in APE1mRNA and protein levels of the control is shown (Figure 8). A significant re¬duction (p < 0.01) in APE1 both protein and mRNA levels from weeks 6 to 16 in CCl4-induced (20 to 6% and 44 to 20% respectively) and pre-cur & CCl4-induced groups (70 to 28% and 95 to 50%, respectively) was observed. Upon curcumin treatment a signifi¬cant (p< 0.01) re-elevation in APE1 mRNA and pro¬tein levels was observed (30 and 95% in CCl4-induced and 66 and 70% in pre-cur & CCl4-induced for mRNA and protein levels respectively).
Fold change of APE1 mRNA and protein levels calculated as per¬centage of the control. Significant al¬terations (p < 0.01) in APE1 mRNA and protein levels calculated as fold chan¬ge of the control values at zero week was observed in both groups. The signi¬ficant reduction (p < 0.01) in APE1 mRNA and protein levels occurred during fibrosis induction by CCl4 injection (at weeks 6 and 16; the % of fold change in mRNA le¬vel was 20 and 6% respectively and in protein level was 44 and 20% respectively) was significantly inhibited in pre-cur and CCl4-induced group (at weeks 6 to 16; the percentage of fold change in mRNA level was 70 to 28 respectively and in protein level was 95 to 50, respectively). While at week 24 where rats were treated with curcumin for 8 weeks the APE1 mRNA and protein levels markedly elevated in both groups as indicated.
Reduction in p53 mRNA and protein levels were observed during the course of fibrosis induction as¬sociated with elevated TGF-b mRNA and protein ex¬pression (Figure 7). Curcumin pre-treatment has protected rat liver during the first 6 weeks possibly by inducing apoptosis as confirmed by the presence of apoptotic bodies in histological examination and by detection of p53 expression at both mRNA and protein levels.
DiscussionCCl4 is one of the most widely used hepatic toxins for experimental induction of hepatic fibrosis and ci¬rrhosis in laboratory animals. CCl4-induced fibrosis or cirrhosis in experimental animals resembled hu¬man cirrhosis in some aspects of morphology and pathophysiology.31 Thus, CCl4-induced hepatic fibrosis due to stimulation of lipid peroxidation and the production of free radicals32 has also been used to assess the efficacy of anti-fibrotic reagents and to verify correlation between pathophysiologic fea¬ture of the liver and serum marker of fibrosis.33
In the present work, we have performed a study on a chronic model of liver fibrosis obtained by CCl4 treatment combined with alcohol in drinking water during the last 4 weeks. We investigated therapeutic effects of curcumin on hepatic fibrosis and the varia¬tion of correlated cytokines. On this basis, oral ad¬ministration of curcumin at 200 mg/kg dose signifi¬cantly reduced elevated indices of liver, serum tran-saminases, and hyp contents in liver tissue. Histological examination showed curcumin evident¬ly alleviated the progression of hepatic fibrosis. The¬refore, intra-gavage administration of curcumin had apparently inhibitory effect on hepatic fibrosis indu¬ced by CCl4 in rats.
Herein we present the relationship between the oxidative stress induced HSCs activation, hepatic in¬jury and fibrosis development and APE1/Ref-1. In addition, we examined p53 activity during CCl4-induced liver fibrosis. We predicted APE1/Ref-1 would have an anti-fibrotic effect via effects on fun¬damental cellular antioxidant system. Our results indicated a significant elevation in the total antioxidant defense system activity with concomitant ele¬vation in APE1 mRNA and protein levels at very early follow up time of fibrosis induction (weeks 2-4; data not shown). A significant reduction in APE1 mRNA and protein levels was observed during wee¬ks 6, 10 and 16 of follow up period. The degree of re¬duction was milder in the group of rat pre-treated with curcumin before fibrosis induction (group 3). Due to chronic liver injury and chronic HSCs acti¬vation, p53 level was elevated only in group 3, rats pre-treated with curcumin followed by CCl4 injec¬tion, consistently with appearance of apoptotic bo¬dies in the microscopic examination.
Regulation of critical DNA repair enzymes such as APE1 in hepatic fibrosis presents a logical appro¬ach to therapeutic intervention against injury and fibrogenesis caused by carbon tetrachloride (CCl4); however, the functional significance of the DNA re¬pair pathways in CCl4-induced hepatic fibrosis has not been directly tested. Although the mechanism was not completely clear, the present study suggests the unique finding that curcumin may act via induc¬tion of APE1 expression by unknown mechanism to afford protection to hepatic fibrosis.
APE1 is a critical cellular protein that performs multiple functions. In addition to its repair activity, APE1 acts as a transcriptional cofactor, as well as a suppressor of reactive oxygen species via a redox site.34 Many of these functions could lead to hepato-protection by curcumin.
Results from histopathological analyses of rats pre-treated with curcumin before CCl4 injection (group 3) demonstrated area of apoptotic cells. In addition, results from determination of the level of hepatic injury-related biochemical markers indica¬ted that the CCl4-elevated partially the levels of hepatocytes biochemical markers. Curcumin treat¬ment of experimental rats of groups 2 and 3 for 8 weeks induced hepatocytes regeneration and im¬proved histological and biochemical results signi¬ficantly.
Lipid peroxidation and necrosis are significantly suppressed in the liver of animals supplemented with antioxidants.35 It was further demonstrated that de novo synthesis of GSH was a prerequisite for curcumin to inhibit HSC activation.36 In this study, we hypothesized that curcumin might protect the liver against CCl4-induced injury and fibrosis by attenua¬ting oxidative stress. Curcumin attenuated oxidative stress as demonstrated by the reduction in the levels of lipid hydroperoxide in the CCl4 rat model. These observations are supported by other studies. Our re¬sults in this study indicated that oral administration of curcumin not only increased the level of total he¬patic GSH but also significantly improve activities of GST and catalase in the liver.
Pro-inflammatory TNF-α, IFN-γ, and IL-6 are major players in hepatic inflammation. TNF-α sti¬mulates the development of hepatic fibrosis.37 IL-6 produced by activated HSCs facilitates the produc¬tion of ECM, including type I collagen, leading to hepatic fibrosis.38 We reported in the present study that curcumin significantly reduced the levels of TNF-α, TGF-β and IL-6 in sera samples and the to¬tal cell extracts prepared form liver homogenates. As represented, sera and total cell extracts levels of TNF-α, TGF-β and IL-6 were significantly increased in the rats injected with CCl4, and to a milder extent in the group of rats pre-treated with curcumin before CCl4 injection vs. mock-treated group. Curcumin treatment for 8 weeks at the end of time course of fibrosis-induction, inhibited hepatic stellate cells acti¬vation and reduced the levels of TNF-α, TGF-β and IL-6 in sera samples and total cell extracts signifi¬cantly (p < 0.05).
Further experiments revealed that curcumin en¬hanced the activity of antioxidant enzymes and mo¬dulated cytokines level by inducing expression of the genes. Activation of HSCs is triggered by various cytokines and chemokines, including the potent mitogens PDGF and EGF39 released from Kupffer cells and activated HSCs and oxidative stress.40,41 Results in this report further confirmed the in vivo effect of curcumin on the suppression of expression of these genes in the CCl4 rat model demonstrated by semiquantitative RT-PCR. In addition, HSCs activation coincides with a dramatic reduction in the abundan¬ce of PPARγ,42,43 which might play an important role in hepatic fibrogenesis.
It has been previously demonstrated that curcumin dramatically induced gene expression of PPARγ in activated HSCs.30 It was further reported that the interruption of the signaling pathways for TGF-β, PDGF, and EGF by curcumin was required for the curcumin induction of gene expression of PPARγ in vitro.20 It was of interest to verify that the treatment with curcumin diminished the role of CCl4 in the reduction of the number of PPARγ-positive HSC in the rat model. Our results demonstrated that curcumin protected the rat liver from CCl4-caused injury and fibrogenesis in vivo by suppressing hepatic inflammation, attenuating hepatic oxidative stress and inhibiting HSCs activation. The mRNA level of PPARγ was significantly reduced in the ex¬perimental rats of group 2 during the CCl4-induced time course. Pre-treatment with curcumin prior to CCl4 injection did not inhibit completely the expres¬sion of PPARγ compared to group 2. In addition treatment with curcumin after fibrosis induction in both groups, significantly elevated PPARγ mRNA le¬vel. These results confirm and extend our prior in vitro observations, and they provide novel insights into the mechanisms of curcumin in the protection of the liver.
In agreement with our work, Chen, et al. confir¬med the previous work suggesting that curcumin activation of PPAR-γ results in the inhibition of NF-kB activation in HSCs.8 In addition, they re¬port that both NF-κB and the ERK MAP kinase pathway are required for the expression of CTGF, a key fibrogenic growth factor produced by HSCs. Furthermore, our results suggest and agree with this study that curcumin may suppress CTGF ex¬pression in HSCs by inhibiting the activation of NF-κB.
Collectively, our findings have important impli¬cations for understanding the pathophysiology of liver fibrosis and suggest that combined targeting of portal myofibroblasts activation could represent a central strategy to treat or delay the progres¬sion of chronic liver fibrosis. Curcumin can heal rat hepatic fibrosis. Effects of reducing the expres¬sion of correlated cytokines may be mechanisms of therapeutic effects of curcumin on hepatic fibrosis. This is in full agreement with recent studies.44
Our results show that curcumin may have multiple targets in liver, including inhibition of HSCs activation and inhibition of fibrotic signaling in portal myofibroblasts, thereby modulating several central cellular events in a rat model of liver disease. Further studies will be devoted to understanding the causal role of APE1 activation in curcumin hepatoprotective function.
AcknowledgementsAuthors would like to thank Dr. Marie Moftah, lecturer of Zoology, Faculty of Science, for her va¬luable cooperation. We extend our thanks to the De¬partment of Biochemistry-Faculty of Science, Alexandria University, Egypt, for supporting part of the present work. Also we would like to thank our group assistants for their efforts.