Introduction. A variety of primary and secondary malignant tumours may present in the liver. In clinical practice the most commonly encountered hepatic tumours are primary hepatocellular carcinoma, metastatic carcinoma and primary cholangiocarcinoma, each with its separate prognostic and management implications. When these tumours are poorly differentiated and the biopsy size is limited to a needle core, the distinction can be extremely difficult.
Material and methods. All liver tumours reported between 1994 and 2004 were examined. Slides from each case were tested separately with each of nine antibodies (HepPar1, CD10, MOC31, Villin, pCEA, mCEA, CK7, CK19, and CK20).
Results. Liver biopsy tissue from 53 patients was examined in this retrospective study. The 53 liver biopsies were classified thus: hepatocellular carcinoma (n = 23); metastatic adenocarcinoma (n = 15); cholangiocarcinoma (n = 5); metastatic small cell carcinoma (n = 7); liver cell dysplasia (n = 1); carcinoid (n = 1); and unclassified (n = 1). Sensitivity and specificity values for different antibodies in relation to their positive staining of specific tumours was as follows: HepPar1 for HCC-81.8% and 100%; MOC31 for MA-73.3% and 92.1%; MOC31 for MA and CC as a combined group-65% and 100%; pCEA (canalicular) for HCC-82.6% and 83.3%; mCEA for MA-93.3% and 75.6%; CK7 for CC-100% and 68%; CK19 for MA and CC as a combined group-90% and 86.3%.
Conclusions. An antibody panel consisting of HepPar1, pCEA, CK19 and CK7 together with either MOC31 or mCEA is recommended for use in the differential diagnosis of HCC, MA and CC.
A broad variety of malignancies may be encountered in the liver and can be classified thus:
- •
Primary hepatic malignant tumors.
- •
Secondary hepatic malignant tumors.
Each of the above two categories can be divided hence:
- •
Malignant epithelial tumours (e.g. hepatocellular carcinoma, cholangiocarcinoma).
- •
Malignant mesenchymal tumours (e.g. angiosarcoma, haemangioendothelioma, other sarcomas).
- •
Others (e.g. lymphoma, germ cell tumors).
For pragmatic purposes and relevance to routine surgical pathology practice, the commonly encountered hepatic malignancies are essentially the following epithelial varieties:
- •
Primary hepatocellular carcinoma (HCC).
- •
Primary cholangiocarcinoma (CC).
- •
Metastatic carcinomas (MC).
Of the two major types of primary malignant epithelial tumours, HCC arises from hepatocytes, while CC arises from bile duct epithelium. CC and MC (usually adenocarcinoma, hence MA) can be designated “non-HCC”. When these tumours are poorly differentiated and the biopsy size is limited to a needle core, the distinction can be extremely difficult. The objective of this study was to identify a panel of antibodies that might be used on a routine basis to successfully differentiate these tumours.
Material And MethodsAll liver tumour specimens (HCC and non-HCC) reported between 1994 and 2004 were retrieved from the archives of the departments of Pathology at the Salmaniya Medical Complex and Bahrain Defense Forces Hospital. This consisted of tissue blocks and glass microscope slides for each case.
Clinical data relating to all the cases were abstracted from the medical charts, recorded and filed. All the cases were reviewed before inclusion in the study by two pathologists and diagnoses confirmed using histological features, available immunohistochemical stains and supporting clinical data. The most appropriate tissue blocks for each case were selected for further investigations in the study.
For particularly challenging biopsies full use was made of the extensive clinical data available in conjunction with histological appearances in order to arrive at a diagnosis (HCC or non-HCC). For example, evidence of chronic diffuse liver disease with either liver fibrosis or cirrhosis, raised serum AFP, and absence of an extrahepatic primary tumour strongly favoured a diagnosis of HCC. Tumours were therefore divided into HCC and non-HCC, and the non-HCC into cholangicarcinoma, metastatic adenocarcinoma, and others (as detailed in the Results section).
All selected tissue blocks were subjected to further processing which involved cutting of serial sections of 3 μm thickness and staining of sections with routine haematoxylin & eosin (H&E) and the nine separate antibodies (HepParl, CD10, MOC31, Villin, pCEA, mCEA, CK7, CK19, and CK2o) by immunoperoxidase techniques. Therefore from each tissue block (and there were one or more tissue blocks for each separate study case) at least ten test slides were prepared.
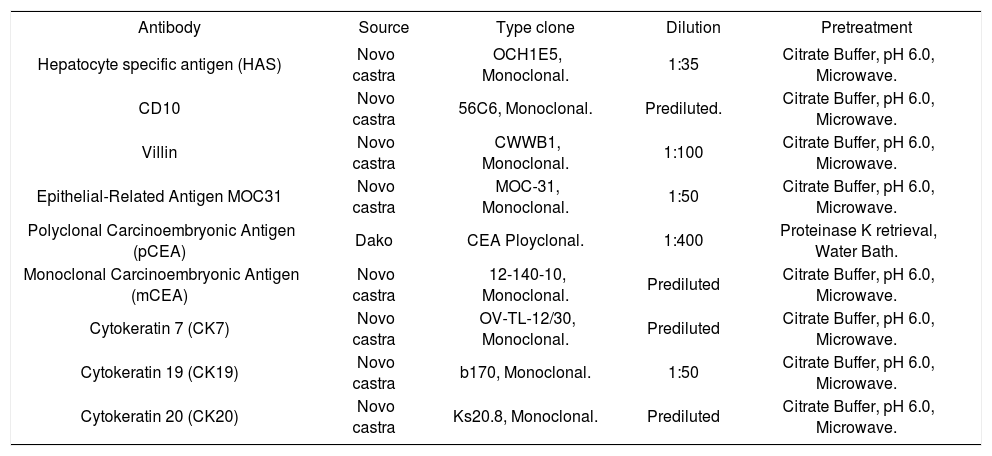
The technical aspects of the immunohistochemistry procedures included antigen retrieval by microwave heating, and the streptavidin-biotin-horse radish pe-roxidase-DAB technique for antibody application and signal detection. The suppliers and dilutions of antibodies used in the study are shown in table 1.
List of antibodies with suppliers and dilutions.
| Antibody | Source | Type clone | Dilution | Pretreatment |
|---|---|---|---|---|
| Hepatocyte specific antigen (HAS) | Novo castra | OCH1E5, Monoclonal. | 1:35 | Citrate Buffer, pH 6.0, Microwave. |
| CD10 | Novo castra | 56C6, Monoclonal. | Prediluted. | Citrate Buffer, pH 6.0, Microwave. |
| Villin | Novo castra | CWWB1, Monoclonal. | 1:100 | Citrate Buffer, pH 6.0, Microwave. |
| Epithelial-Related Antigen MOC31 | Novo castra | MOC-31, Monoclonal. | 1:50 | Citrate Buffer, pH 6.0, Microwave. |
| Polyclonal Carcinoembryonic Antigen (pCEA) | Dako | CEA Ployclonal. | 1:400 | Proteinase K retrieval, Water Bath. |
| Monoclonal Carcinoembryonic Antigen (mCEA) | Novo castra | 12-140-10, Monoclonal. | Prediluted | Citrate Buffer, pH 6.0, Microwave. |
| Cytokeratin 7 (CK7) | Novo castra | OV-TL-12/30, Monoclonal. | Prediluted | Citrate Buffer, pH 6.0, Microwave. |
| Cytokeratin 19 (CK19) | Novo castra | b170, Monoclonal. | 1:50 | Citrate Buffer, pH 6.0, Microwave. |
| Cytokeratin 20 (CK20) | Novo castra | Ks20.8, Monoclonal. | Prediluted | Citrate Buffer, pH 6.0, Microwave. |
A known positive and negative control was used for each batch of slides. The tissues used as positive controls for each antibody are shown in table 2.
Antibodies with their positive control tissues.
| No. | Antibody | Control type |
|---|---|---|
| 1 | Hepatocyte Specific Antigen (HSA) | Normal adult liver |
| 2 | CD10 | Normal kidney |
| 3 | Epithelial-related antigen (MOC31) | Normal large bowel |
| 4 | Villin | Normal small bowel |
| 5 | Polyclonal CEA | Normal large bowel |
| 6 | Monoclonal CEA | Colonic adenocarcinoma |
| 7 | Cytokeratin 7 (CK7) | Normal breast |
| 8 | Cytokeratin 19 (CK19) | Skin |
| 9 | Cytokeratin 20 (CK20) | Normal small intestine |
Archived liver biopsy tissue from 53 patients was examined in this retrospective study.
After careful examination of all diagnostic material and available clinical data the cases were classified as in table 3.
Classification of the 53 study cases.
| Histological classification | Total cases | HCC/non-HCC |
|---|---|---|
| Hepatocellular carcinoma | 23 | HCC |
| Metastatic adenocarcinoma | 15 | Non-HCC (MA) |
| Cholangiocarcinoma | 5 | Non-HCC (CC) |
| Metastatic small cell carcinoma | 7 | Non-HCC (MSCC) |
| Carcinoid | 1 | Non-HCC |
| Liver cell dysplasia | 1 | Non-HCC (LCD) |
| Unclassified | 1 | Unknown |
Of the HCC cases, one was a fibrolamellar variant, one was a clear cell variant and one was a small cell variant. The other 21 cases were HCC, not otherwise specified (NOS). The biopsy of case 4 (HCC) in addition contained well-formed granulomas with central necrosis. The possibility of concurrent TB was suggested but there was no further comment/supporting evidence for this view in the medical charts. The cause of the granuloma remains uncertain. One of the HCC cases was in fact a me-tastatic deposit of HCC in the gum. Figures 1, 2 and 3 shows histological features (H&E) from selected cases.
Figures 4, 5 and 6 show the staining characteristics of different tumour types with some antibodies used in the study. Tables 4 and 5 summarize the immunohistochemistry results in this study.
Summary of IHC results with respect to the different categories of tumours/lesions.
| Cases(n=53) | Immunohistochemistry antibodies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HepPar1, n(%) | CD10, n(%) | MOC31, n(%) | Villin, n(%) | pCEA, n(%) | mCEA, n(%) | CK7, n(%) | CK19, n(%) | CK20, n(%) | |
| HCC (n = 23) | 18/22 (81.8) | 12/22 (54.5) | 0/23 (0) | 2/23 (8.7) | 23/23 (100) | 2/22 (9) | 6/22 (27.3) | 3/22 (13.6) | 10/22 (45.5) |
| MA (n = 15) | 0/15 (0) | 0/15 (0) | 11/15 (73.3) | 0/15 (0) | 15/15 (100) | 14/15 (93.3) | 5/15 (33.3) | 13/15 (86.7) | 13/15 (86.7) |
| CC (n = 5) | 0/5 (0) | 1/5 (20) | 2/5 (40) | 1/5 (20) | 5/5 (100) | 3/5 (60) | 5/5 (100) | 5/5 (100) | 5/5 (100) |
| MSC Ca (n = 7) | 0/7 (0) | 0/7 (0) | 0/7 (0) | 0/7 (0) | 5/7 (71.4) | 3/7 (42.9) | 2/7 (28.6) | 1/7 (14.3) | 2/7 (28.6) |
| Carcinoid (n = 1) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 1/1 (100) |
| LCD (n = 1) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) |
| Unclassified (n = 1) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) |
HepParl: Hepatocyte Paraffin 1. pCEA: Polyclonal carcinoembryonic antigen. mCEA: Monoclonal carcinoembryonic antigen. CK: Cytokeratin. HCC: Hepatocellular carcinoma. MA: Metastatic adenocarcinoma. CC: Cholangiocarcinoma. LCD: Liver cell dysplasia.
IHC results for all cases of MA.
| Case No. | Diagnosis | Primary Site | Immunohistochemistry antibodies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HepPar1 | CD10 | MOC31 | Villin | AFP | pCEA | mCEA | CK7 | CK19 | CK20 | |||
| 24 | MA | UnK | - | - | - | - | - | + | + | - | + | + |
| 28 | MA | Small Bowel (Ileum) | - | - | - | - | - | + | + | - | - | - |
| 29 | MA | Stomach Ca | - | - | + | - | - | + | + | - | + | + |
| 30 | MA | Colon Ca | - | - | + | - | - | + | + | - | + | + |
| 31 | MA | Pancrease Ca | - | - | + | - | - | + | + | - | + | + |
| 32 | MA | UnK | - | - | - | - | - | + | - | - | + | - |
| 35 | MA | UnK | - | - | + | - | - | + | + | - | + | + |
| 36 | MA | Stomach Ca | - | - | + | - | - | + | + | + | + | + |
| 37 | MA | Colon Ca | - | - | + | - | - | + | + | + | + | + |
| 38 | MA | Esophagus Ca | - | - | + | - | - | + | + | + | + | + |
| 40 | MA | UnK | - | - | + | - | - | + | + | + | - | + |
| 45 | MA | UnK | ND | ND | - | - | - | + | + | ND | + | + |
| 48 | MA | UnK | - | - | + | - | - | + | + | - | + | + |
| 50 | MA | Ampullary Ca | - | - | + | - | - | + | + | - | + | + |
| 54 | MA | Colon Ca | - | - | + | - | - | + | + | + | + | + |
Unk: Unknown. MA: Metastatic adenocarcinoma. Ca: Carcinoma. ND: Not done.
HepPar1 antibody was positive in 18 of 22 (81.8%) HCC, and the pattern of staining was predominantly granular cytoplasmic. None of the MA and CC stained positive for HepPar1. Expression of the antigen did not appear to correspond to the degree of differentiation.
CD10 expression was present in 12 of 22 (54.5%) cases of HCC, eleven of which showed a canalicular staining pattern, whereas only one case (4.3%) of HCC showed cytoplasmic staining. In contrast, only I case of 5 (20%) CC was positive for CD10, and displayed cytoplasmic reactivity only. None of the 15 MAs were positive for CD10. The canalicular staining pattern for CD10 was not detected in any of the non-HCC specimens.
Immunoreactivity with MOC31 was observed in 11 of 15 MA (73.3%), and 2 of 5 (40%) CC. Of the 11 positive MA, two originated from stomach, three from colon, one from pancreas, one from esophagus, one from ampulla of Vater, and three were from unknown primary sites. The staining was strong and diffuse in most cases with a cytoplasmic pattern. There was no apparent correlation between the histologic grade and staining intensity. All 23 cases of HCC were negative for MOC31 expression.
Of the 23 HCC, two (9%) were positive for villin, and displayed a cytoplasmic staining pattern. Cytoplasmic and apical staining for villin was identified in only 1 case of 5 (20%) CCs. None of the 15 MA were immunoreactive with villin.
Polyclonal carcinoembryonic antigen (pCEA) expression was observed in 23 of 23 (100%) HCC. A predominant canalicular staining pattern for pCEA was identified in 19 of 23 (83%) HCC, and the remaining 4 of 23 (17%) HCC demonstrated a cyto-plasmic and/or membranous staining pattern. This distinct canalicular pattern of staining with pCEA was unique to hepatocellular carcinoma. Cytoplasmic pCEA positivity was present in 15 of 15 (100%) MA, and 5 of 5 (100%) CC studied.
Fourteen of 15 (93.3%) MA, and 3 of 5 (60%) CC reacted with monoclonal CEA antibody. In contrast, mCEA staining was present in 2 of 22 (9%) of HCC. Of the fourteen MA positive for mCEA, one originated from ileum, two from stomach, three from colon, one from pancreas, one from esophagus, one from ampulla of Vater, and five from unidentified primary sites. Staining in all of these was cytoplasmic.
CK7 stained 6 of 22 (27.3%) HCC, and 5 of 15 (33.3%) MA. CK7 immunoreactivity was noted in all 5 of 5 CC (100%). There was no significant difference in the CK7 expression among well, moderately, and poorly differentiated CC. Of the 5 cases of MA positive for CK 7 expression, one originated from stomach, two from colon, one from esophagus, and one from an unidentified primary site. Cytokeratin 7 (CK7) positivity was generally cytoplasmic.
CK19 was diffusely positive in 5 of 5 (100%) CC, whereas only 3 of 22 (13.6%) HCC were diffusely positive for CK19. CK19 expression was present in 13 of 15 (86.7%) MA. Of these 13 positive cases, three originated from colon, two from stomach, one from esophagus, one from ampulla of Vater, one from pancreas, while 5 were from unidentified primary sites. CK19 expression was absent in the single case of metastasis arising from small intestine. In addition, we found that CK19 was expressed diffusely in the bile duct epithelium in normal and neoplastic liver, the labeling of bile ducts producing a distinctive pattern which often helped to delineate normal and proliferated bile duct epithelium.
The staining of CK20 was always cytoplasmic, with occasional membranous positivity. CK20 was positive in 10 of 22 (45.4%) HCC, 13 of 15 (86.7%) MA, and in 5 of 5 (100%) CC. The staining was diffuse cytoplasmic in all cases of HCC, MA, and CC. No correlation was found between CK20 positivity and either the architecture or the grade of the tumor, and results were similar from one section to another in a case with heterogeneous morphology. Three cases of colon adenocarcinoma were positive for CK20 expression, and most unknown (5 cases) cases and metastatic adenocarcinoma from pancreas (single case) were strongly positive with CK20. MA from stomach (2 cases), esophagus (single case) and ampulla of Vater (single case) were diffusely positive for CK20.
DiscussionThe most common hepatic epithelial malignant neoplasms are metastatic. Of primary epithelial malignant neoplasms, 90% are hepatocellular in origin. Cholangiocarcinomas represent 8-25% of primary hepatic malignant neoplasms.1 Adenocarcinomas from many sites commonly metastasize to the liver and as a group are the most common malignant tumours in the adult liver.2
There have been several studies attempting to make a distinction between HCC and adenocarcinoma by immunohistochemistry. Often, the distinction between HCC and either primary CC or MA can be made on the basis of clinicoradiological studies, with H&E confirmation on histology. However, when limited material is available or when the tumour is poorly differentiated, the morphological features as demonstrated by H&E do not permit a firm diagnosis, and in these circumstances various immunohistochemical panels have been used to aid in this distinction.3-7
Unfortunately a relatively low number of cases were available to us for this study but this is largely due to the small population of the island nation of Bahrain. Despite this we feel the results are still significant and represent an important addition to the literature, especially as similar data from the region are limited.
Wennerberg, et al.8 reported expression in 37 of 38 HCC, the single exception being an example of sclerosing HCC. Similar results were obtained by Leong, et al.,9 who observed positive labeling in 30 of 32 HCC, while the 2 cases that failed to stain for HepPar1 included a poorly differentiated variant and a sclerosing variant of HCC. Our results are consistent with these and other previous reports.10-12 We found 18 of 22 (82%) HCC studied reacted with HepPar1, substantiating the high sensitivity of this marker. Similar to previous studies that have demonstrated a lack of expression in poorly differentiated HCC,9,12 the 4 cases that did not express HepPar1 were morphologically poorly differentiated tumours. HepPar1 immunoreactivity was not observed in any of the CC, MA or other non-HCC tumours evaluated in this study, and this is consistent with the lack of staining in normal bile ducts and nonparenchymal liver cells.13
In HCC CD10 shows a specific canalicular staining pattern3 similar to the one observed with pCEA, and the present study aimed to investigate the putative use of CD10 in differentiating HCC from non-HCC. In our present study, 12 of 22 (54.5%) HCC expressed CD10, 11 (47.8%) of which showed distinct canalicular staining, and 1 case (4.3%) demonstrated a cytoplasmic staining pattern.
In contrast, CD10 immunoreactivity was observed in 1 of 5 (20%) CC with a positive cytoplasmic pattern of staining, while none of the MA nor the other tumours studied expressed CD10. Our study showed that, although the specificity is 100%, the sensitivity of CD10 for HCC is only about 47.8%. Previous studies have also demonstrated the high specificity of CD10 (can) staining for HCC.12,14,15 Borscheri, et al.14 documented this pattern of CD10 reactivity in 68.3% of HCC and in none of the metastatic liver tumours examined. Interestingly, as in the present study, these investigators also observed a lack of CD10 expression in CC and MA with all 12 cases negative in their report. Based on these observations, the bile canalicular pattern of CD10 immunoreactivity appears to be specific for HCC, although poor sensitivity may limit its usefulness.
MOC31 has been proven useful in distinguishing between adenocarcinoma and mesothelioma,2 being a relatively sensitive and specific indicator of adenocarcinoma differentiation, both in tissue sections and in body cavity fluid.3,4,12
Porcell, et al.2 noted MOC31 expression in 13 of 14 CC and 27 of 27 MA, but 0 of 13 HCC. In keeping with such reports, we observed MOC31 expression was uniformly negative for all HCC. In the present study 11/15 (73.3%) MA (2 stomach, 3 colon, 1 pancreas, 1 esophagus, 1 ampulla of Vater, and 3 of unknown cases) stained diffusely and intensely with MOC31, yielding easy interpretation. We found im-munoreactivity for MOC31 in 2 of 5 (40%) of CC. In contrast all 23 cases of HCC were uniformly negative, with bile ducts in the adjacent non-neoplastic hepatic parenchyma served as an internal positive control. MOC31 negativity in HCC, and MOC31 positivity in CC and MA suggest that this marker is of potential value in the differential diagnosis.
We observed villin expression in 2 of 23 (8.7%) HCC, and in 1 of 5 (20%) CC studied, while none of the MA were positive. From our findings, villin would not appear to be a useful marker in this context.
The canalicular pattern of staining in HCC with pCEA has been described in 24-90% of cases.3,4,13,15 Tanaka, et al. found the canalicular pattern to be present predominantly in well-differentiated hepatocellular carcinoma.13 This was also borne out by our study in which the 4 non-canalicular- staining cases were poorly differentiated. The absence of bile canalicular staining does not entirely exclude HCC, as negative cases have been reported. Adenocarcinomas in contrast to HCC show a diffuse cytoplasmic pCEA staining pattern without canalicular accentuation.16 In our study, a bile canalicular pattern of pCEA expression was observed in 19 of 23 (82.6%) cases of HCC. The remaining 4 cases demonstrated cytoplasmic staining with pCEA, without a canalicular pattern. Several of the 19 cases showed cytoplasmic staining in addition to canalicular, and this sometimes caused difficulty in interpretation. All 5 CC (100%) showed positive cytoplasmic staining with pCEA. Three of these demonstrated a focal canalicular pattern also. The significance of this is uncertain, although it might indicate a mixed tumour differentiation, i.e. combined hepatocellular-cholangiocarcinoma. This canalicular pattern of staining was not observed in any MAs examined. The reported sensitivity of pCEA (can) for HCC has ranged from 60 to 95%.14 In this study, the sensitivity and specificity of pCEA (can) for HCC were 82.6 and 83.3%, respectively, indicating a potentially useful antibody for this particular problem.
In this context CD10 appeared to be useful in that the 4 cases which were pCEA (can) negative turned out to be CD10 (can) positive. CD10 would therefore appear to be of diagnostic value when used in conjunction with pCEA. The superior specificity of CD10 may be attributed to the fact that it specifically stains bile duct glycoprotein but does not react with other antigens in the CEA family. 17 A combination of pCEA (can) and CD10 (can) was diagnostic in 100% of our HCC cases. We highly recommend the use of pCEA as part of the IHC panel for the differential diagnosis of malignant hepatic tumours. CD10 used in conjunction with pCEA may improve specificity.
We found that only a small number (9%) of HCC were mCEA positive which is consistent with the findings of other investigators.5,13 In our study 14 of 15 (93.3%) cases of MA, and 3 of 5 (60%) cases of CC were positive for mCEA. These observations are in agreement with previous investigations, and suggest that this differential staining pattern of mCEA is of use in differentiating HCC from adenocarcinomas.
Maeda, et al.18 reported that the positivity of CK7 expression in CC was significantly higher than that in HCC or MA, and considered that could be useful in the differential diagnosis of CC from HCC and MA. CK7 is present in bile duct epithelium, but not in normal hepatocytes. As such, CK7 has been observed in most CC and occasionally in HCC.4,7 In keeping with these reports we found that all CC (100%) were CK7 positive, whereas CK7 expression was absent from most HCC (6/22 were positive, 27.3%), and MA (5/15 positive, 33.3%). CK7 would appear to be a useful member of the diagnostic panel.
CK19 expression appears relatively specific for CC, though HCC sometimes exhibit CK19 positivity.7,11 Similar to previous reports we documented only 3 of 22 (13.6%) HCC to be CK19 positive. Our observed sensitivity of CK19 for CC (100%) was similar to the experience of other investigators, who have reported CK19 positivity in 85% to 100% of CC.9 In our study, CK19 was demonstrated in 13 of 15 (86.7%) MA, which was much higher than that detected in HCC. CK19 is therefore considered to be useful in the differential diagnosis of HCC from CC and MA.
We found CK20 to be positive in most of the MA, but was also positive in all of the CC and almost 50% of the HCC cases. As such it would not appear to be a useful antibody for discriminating between these entities. Furthermore, the positive results with this antibody in a number of the MA of foregut origin –which was unexpected and contradicts the accepted literature– would suggest that our CK20 exhibited non-specific antigen binding and may not be entirely relied upon.
A panel consisting of [HepPar1 (+), pCEA (+), and MOC31 (-)] would be highly sensitive and specific for the detection of HCC. We observed that 17 of 23 (73.9%) cases of HCC were HepPar1+, pCEA+, MOC31-, while neither CC nor MA were detected in this combined panel.
A panel consisting of [HepParl (+), pCEA (+), and CD10 (+)] would identify 11 of 23 (47.8%) cases of HCC, but no cases of CC or MA. The latter combined panel has a lesser degree of sensitivity for HCC than the previous panel.
A combined panel of [CK19 (+), CK7 (+), and HepParl (-)] identified 100% of CC, 2 of 15 (13.3%) MA, but was absent in all HCC, indicating that this would be a highly effective diagnostic panel.
ConclusionBased on our study findings, some recommendations can be put forward in relation to optimal components of a diagnostic immunohistochemical panel for the differential diagnosis of common hepatic malignancies. The most appropriate panel should combine a good level of discriminatory power with cost-effectiveness; and so a panel consisting of 4 or 5 antibodies is probably justified. We recommend a panel consisting of HepPar1, pCEA, CK7, CK19 together with either MOC31 or mCEA. It is noteworthy that a recent larger study from Turkey4 yielded broadly similar conclusions.