The impact of sleep on metabolic dysfunction-associated steatotic liver disease (MASLD) in American adults remains unclear. This study aimed to address the relationship of sleep patterns and disorders with MASLD and liver fibrosis comprehensively.
Materials and MethodsThis cross-sectional study included adult participants from the National Health and Nutrition Examination Survey 2017-2020. Multivariate adjusted regression analysis were used to examine the association of sleep with MASLD and liver fibrosis. We further addressed these associations using restricted cubic splines, mediation analysis, stratified analysis and multiple sensitivity analysis.
ResultsWe enrolled 5368 participants. Certain sleep disorders, sleep duration, high sleep debt and specific sleep-wake time were associated with MASLD. Late workday sleep was a shared risk factor for MASLD and liver fibrosis. Short sleep on workdays and free days favored MASLD, whereas average weekly long sleep protected against MASLD. Workday, free day and average weekly optimal sleep duration was 7.5 h, 8 h and 7.78 h, respectively. Mediation analysis suggested that fasting glucose and high-density lipoprotein cholesterol indirectly mediated the relationship between sleep duration and MASLD, whereas stratified analysis showed that sex influenced the relationship, and that the correlation was only observed in women and specific age groups.
ConclusionsSleep duration independently affected MASLD but only in women and specific age groups. Moreover, late sleep on workdays was a shared risk factor for MASLD and liver fibrosis. These results suggest targeting sleep behaviors for MASLD prevention and developing age- and sex-specific strategies.
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD), is defined as the presence of hepatic steatosis accompanied by at least one cardiometabolic risk factor (hypertension, obesity, type 2 diabetes and dyslipidemia) [1]. The course of MASLD ranges from simple steatosis to a more severe inflammatory form, metabolic dysfunction-associated steatohepatitis (MASH), which can progress to cirrhosis and even hepatocellular carcinoma (HCC) in some patients. The prevalence and incidence of MASLD have steadily increased over the decades. MASLD is the most common chronic liver disease globally, with one in three adults may develop MASLD. This, has now reached epidemic proportions, and its incidence may be higher in high-risk populations, such as people with type 2 diabetes mellitus and obesity [2]. MASH is currently the leading cause of liver transplantation and HCC, imposing a heavy burden on public health each year [3]. Recently, compelling evidence has demonstrated that MASLD is a multisystemic disease. In addition to increased liver-related adverse events, MASLD/MASH is strongly associated with an increased incidence of multiple extrahepatic outcomes, such as cardiovascular disease (CVD), chronic kidney disease, and multiple extrahepatic cancers, with CVD being the leading cause of death in MASLD/MASH patients [4-6].
Identifying modifiable risk factors for disease development and undertaking prevention measures are key factors for reducing disease prevalence. Sleep is a complex and highly regulated physiological activity necessary for the human body. Altered sleep patterns and sleep-related disorders play an important role in chronic liver disease [7]. However, the effect of sleep on MASLD remains controversial. A recent meta-analysis demonstrated that inadequate sleep duration was significantly associated with MASLD risk [8]. In contrast, a more recent cross-sectional study did not demonstrate an association between sleep duration and MASLD [9]. It is suggested that circadian misalignment, not sleep duration, was associated with MASLD [10]. Besides, multiple sleep disorders such as obstructive sleep apnea, and insomnia may also play a role in the pathogenesis of MASLD [11,12]. The current evidence is controversial and there are very few studies investigating the comprehensive impact of sleep patterns and disorders on MASLD and liver fibrosis. In this study, we employed a serial nationally representative population-based cross-sectional study, the National Health and Nutrition Examination Survey (NHANES), to address the effects of sleep patterns and disorders on MASLD and liver fibrosis in contemporary American adults.
2Methods2.1Study design and populationThe NHANES is a major program of the National Center for Health Statistics (NCHS) and is designed to develop a database of information about the health and nutrition of the American household population. In this study, we used data from the two most recent consecutive cycles of NHANES from 2017 to March 2020 (including 2017 to 2018 data and 2019 to March 2020 pre-epidemic data).
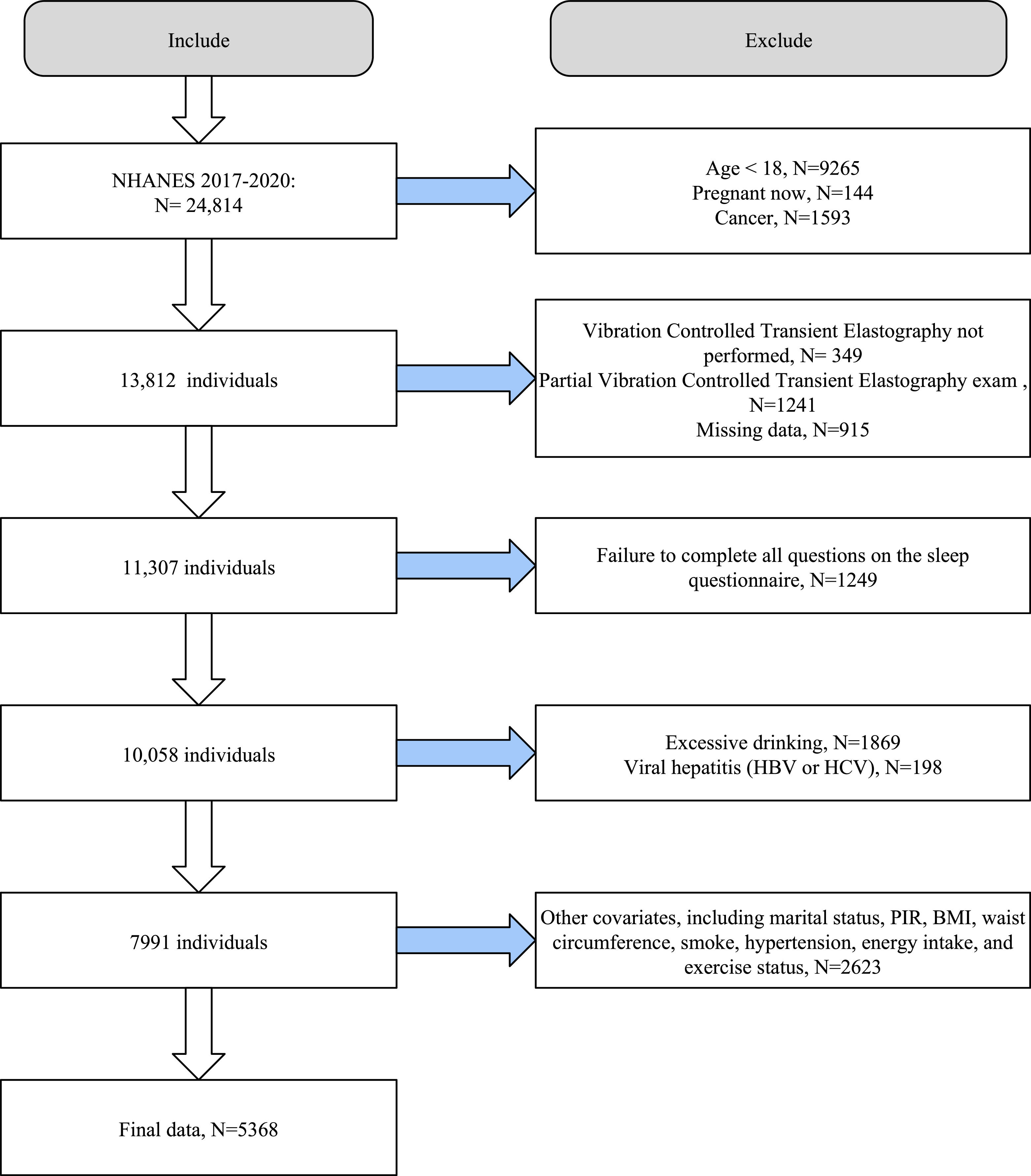
Of the 24,814 participants from the NHANES cycles from 2017 to 2020, we first excluded participants who were underage (n=9265), pregnant (n=144), and diagnosed with cancer (n=1593). Second, we excluded individuals who did not have vibration-controlled transient elastography (VCTE) data (n=349) and those with ineligible VCTE data (n=1241) and missing data (n=915). We also excluded participants who did not provide complete responses in the sleep questionnaire (n=1249). To avoid the interference of excessive alcohol consumption and viral hepatitis, we excluded participants with excessive alcohol intake (n=1869) and chronic viral hepatitis (n=198). Finally, we excluded participants with missing covariates (n=2623). Thus, we included 5,368 eligible participants (2,521 men [47 %] and 2,847 women [53 %]) for further analysis (Fig. 1).
2.2Assessment of sleep patterns and disordersWe assessed the sleep patterns and disorders of contemporary American adults based on the recently published survey (2017-2020) of sleep habits and disturbances among American adults from the NHANES by Di et al [13]. In the 2017-2020 cycle, the NHANES assessed the sleep and wake times of participants on workdays and free days (i.e., weekdays and weekends) using the Munich Chronotype Questionnaire. The sleep duration for workdays and free days was obtained by subtracting the wake time from the sleep time. The average weekly sleep duration of the included participants was calculated as (weekday sleep duration * 5 + free day sleep duration * 2) / 7. Sleep debt refers to the disparity between the sleep duration that a person needs and the actual sleep duration, which is calculated based on the absolute difference between the free days and the average weekly sleep duration. Social jet lag refers to a circadian rhythm disorder caused by a mismatch between the biological clock and the social clock, calculated from the absolute difference in the sleep midpoints (midpoint between the sleep time and the wake time) between workdays and free days. We categorized sleep duration as short sleep (<7 h), optimal sleep duration (7-9 h), and long sleep (≥9 h). Sleep time was categorized as early (before 10 p.m.), intermediate (10 p.m. to midnight), and late (midnight or later). Similarly, wake time was categorized as early (before 6 a.m.), intermediate (6 a.m. to 8 a.m.), and late (8 a.m. or later). We dichotomized sleep debt as low (<2 h) and high (≥2 h) and social jet lag as mild (<2 h) and heavy (≥2 h), based on the description by Di et al [13].
We obtained information on sleep disorders via questionnaire interviews in the NHANES. Sleep disorders included insomnia, snoring, sleep apnea symptoms, and daytime sleepiness [14]. Insomnia was determined based on a positive response to the question, “Have you ever told a doctor or other health professional that you have trouble sleeping?”. Snoring was categorized according to the questionnaire responses as never, rarely, occasionally, or frequently snoring during sleep in the past 12 months. Sleep apnea symptoms were categorized according to the questionnaire responses as never, rarely, occasionally, or frequently experiencing apnea symptoms in the past 12 months. Daytime sleepiness was categorized as never, rarely (1 per month), sometimes (2-4 per month), often (5-15 per month), and almost always (16-30 per month).
2.3CovariatesWe selected several important covariates that could potentially affect the relationship, including age, sex (men or women), ethnicity (Mexican American, non-Hispanic black, non-Hispanic white, other Hispanic, or other ethnicities), education (
In 2017-2018, the NHANES used the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) in VCTE for the first time to diagnose hepatic steatosis and liver fibrosis in mobile examination centers. Results were considered reliable if the participant had fasted for at least 3 h before the examination, had undergone ≥ 10 complete LSMs, and had an interquartile range/median of LSM < 30 %. In this study, we defined MASLD as CAP >248 dB/m (hepatic steatosis ≥ S1) while excluding significant alcohol consumption (>3 drinks/day for men and >2 drinks per day for women) and other chronic liver diseases. CAP of 248 dB/m was considered the optimal cutoff value for determining hepatic steatosis, with an area under the curve of 0.823. Accordingly, CAP <248 dB/m indicated no steatosis (S0), and CAP >248 dB/m indicated mild to severe steatosis (S1 to S3, respectively) [16]. In our study, median LSM ≥6.3 kPa and ≥8 kPa in MASLD patients indicated mild liver fibrosis (≥F1) and significant liver fibrosis (≥F2), respectively, based on previous widely accepted criteria [17,18].
2.5Mediation variablesTo explore the possible pathways through which sleep duration affects MASLD and liver fibrosis, we selected several possible mediating variables for mediation analysis based on previous literature. Abnormal sleep habits and sleep-related disturbances have been shown to be associated with dysregulated glucolipid metabolism, and these metabolic disturbances are central to the pathogenesis of MASLD [19,20]. Therefore, we explored whether glucolipid metabolism mediates the association of sleep patterns and disorders with MASLD and liver fibrosis. We utilized serum glucose and lipid metabolism-related indices from laboratory biochemical tests in the NHANES as mediating variables, such as fasting plasma glucose (FPG), HbA1c, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride-rich lipoprotein cholesterol (TRL-C, also known as remnant cholesterol, calculated by subtracting HDL-C and LDL-C from TC).
2.6Statistical analysisAll analysis were performed using R version 4.1.3 and EmpowerStats software. Due to the complex design of the NHANES, we properly weighted our data analysis according to the NHANES reporting guidelines for the survey. Continuous variables (mean and standard error [SE]) and categorical variables (percentages) were used to characterize the study population. The student's t-test for continuous variables and the chi-square test for categorical variables were applied for baseline analysis. We constructed three multivariate adjusted regression models to explore these relationships fully. Model 1 was a crude model that did not adjust for any covariates. Model 2 was a partially adjusted model that adjusted for age, sex, ethnicity, marital status, PIR, and education. Model 3 was a fully adjusted model that adjusted for variables based on Model 2 plus BMI, WC, smoking, diabetes, hypertension, CVD, total energy intake, and physical activity. We then performed a restricted cubic spline (RCS) analysis on the continuous independent variables to explore potential nonlinear relationships. The curve fitting term was defined by the RCS function from the rms package, and the degrees of freedom (or knots) were determined according to the magnitude of the “p” for nonlinear value.
We performed mediation analysis between the continuous independent variables and the outcomes (Fig. 2). Mediation analysis is used to study the mediating role of intermediate variables in the relationship between independent and dependent variables. It helps to understand the mechanisms or pathways through which the independent variable affects the dependent variable. By quantifying the indirect effects mediated by the intermediate variables, mediation analysis can reveal specific pathways and provide a more comprehensive understanding of the overall relationship between variables. In this study, the total effect of variables on MASLD comprised the direct effects of sleep and indirect effects through mediating variables. For the mediation variables included in this study, which included markers related to glucolipid metabolism, we performed individual mediation analysis based on previous studies and calculated the direct and indirect effects through these markers for the correlation between sleep-related variables and MASLD and liver fibrosis (if available). We calculated the proportion of the included mediating variables in the total effect. All mediation analysis were adjusted for all covariates. Additionally, we performed stratification and sensitivity analysis to verify the consistency and stability of the findings across subgroups. Since no standardized CAP reference value exists currently for MASLD diagnosis, sensitivity analysis were performed using other CAP criteria that define the presence of hepatic steatosis (≥ S1) in MASLD [21,22]. Moreover, to verify the robustness of the relationship between sleep duration and MASLD, we further established Model 4, which includes additional adjustment for sleep time based on Model 3. Since shift workers may have unique sleep habits compared to the general population and a different relationship with MASLD development, we conducted additional sensitivity analysis for this subgroup. A P value of <0.05 was considered statistically significant in all analysis.
Schematic diagram of the mediation analysis. Abbreviations: MASLD, metabolic dysfunction-associated steatotic liver disease; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TRL-C, triglyceride-rich lipoprotein cholesterol.
The data required for this survey are all from published NHANES data, without raw data collection, thus ethics committee approval is not necessary. The included data sources, which were approved by the local ethics committee, complied with local law, and all participants signed informed consent.
3Results3.1Baseline characteristicsWe included 5368 participants (47 % men; overall mean age, 47.41 years). In total, 2044 participants fulfilled the criterion of CAP >248 dB/m, resulting in an MASLD prevalence of 38 %. There were 473 patients with LSM ≥6.3 kPa and 183 patients with LSM ≥8 kPa in the MASLD population, resulting in a prevalence of 23 % and 9 % for mild (F1) and significant (F2) liver fibrosis, respectively. First, we analyzed the population at baseline according to the presence or absence of MASLD. Compared to individuals without MASLD, those with MASLD had lower PIR and education levels and were significantly older. Moreover, the MASLD patients had higher BMI and WC, higher proportions of women and non-singles, and higher tendency for diabetes, hypertension, and CVD than those without MASLD (Supplementary Table 1). Regarding the sleep-related characteristics, snoring, daytime sleepiness, weekday (continuous) and free-day (continuous and categorical) sleep duration, average weekly sleep duration (continuous), social jet lag (continuous and categorical), weekday sleep time and free-day wake time differed significantly between the MASLD and non-MASLD populations (Supplementary Table 2).
We then grouped the baseline population according to the presence or absence of liver fibrosis in the MASLD population. The liver fibrosis population (≥F1) was significantly older and had a lower PIR, higher BMI and WC, higher proportion of women, and higher prevalence of diabetes and hypertension compared to the participants without liver fibrosis (Supplementary table 3). Compared to participants without liver fibrosis, those with liver fibrosis snored more frequently and were more likely to be non-intermediate (i.e., more likely to be either too early or too late) in weekday sleep time, and free-day sleep and wake time (Supplementary table 4). Finally, we performed a baseline analysis of those with significant liver fibrosis (≥F2). A significant difference was observed in age, PIR, BMI, WC, total energy intake, education, diabetes, hypertension, and moderate physical activity between individuals with and without significant liver fibrosis (Supplementary Table 5). However, among all the sleep characteristics, only weekday sleep time was significantly different between the cohorts (Supplementary Table 6).
3.2Multivariate logistic regression analysisWe used multivariate logistic regression models to examine the relationship between sleep and MASLD and liver fibrosis. We constructed three multivariate regression models as follows: Model 1 did not adjust for any covariates; Model 2 adjusted for some of the covariates (age, sex, ethnicity, marital status, PIR, and education); and Model 3 was a fully adjusted model, adjusting for all included covariates. Regarding sleep disturbances, insomnia, occasional and frequent snoring, and daytime sleepiness were independently associated with the development of MASLD after adjusting for all potential confounders in Model 3. Moreover, the overall risk of MASLD increased with increasing frequency of snoring (p for trend = 0.006) (Table 1). Regarding the sleep habit characteristics, the workday, free-day, and average weekly sleep durations (continuous), workday and free-day sleep duration <7 h, average weekly sleep duration >9 h, sleep debt ≥2 h, workday sleep time after midnight, and free day wake time before 6 a.m. were independently associated with the development of MASLD. Specifically, workday, free day, and average weekly sleep durations were negatively associated with MASLD risk (odds ratio [OR] = 0.88, 0.90, and 0.86, respectively). However, we noted that both workday and free day sleep duration <7 h were positively associated with MASLD development (OR = 1.36 and 1.72, respectively), whereas average weekly sleep duration >9 h showed a negative association (OR = 0.60). These results suggest that short sleep on both workdays and free days independently increases the risk of MASLD, whereas long average weekly sleep duration reduces the risk of MASLD (Table 1). Sleep debt of ≥2 h was independently associated with an increased risk of MASLD (OR=1.38), suggesting that the accumulation of daily sleep deprivation may indeed increase the risk of MASLD (Table 1). Finally, workday sleep time after midnight and free day wake time before 6 a.m. were significantly associated with an increased risk of MASLD (OR = 1.86 and 1.48, respectively). This suggests that the chronotype of late sleep on workdays and early wake time on free days could be linked to MASLD development (Table 1).
Multivariate logistic regression analysis of the association between sleep characteristics and MASLD (CAP>248 dB/m).
| Variables | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| OR (95 %CI) | P | OR (95 %CI) | P | OR (95 %CI) | P | ||
| Insomnia | |||||||
| No | Ref. | Ref. | Ref. | ||||
| Yes | 1.11 (0.93, 1.34) | 0.252 | 0.98 (0.81, 1.20) | 0.871 | 0.79 (0.65, 0.96) | 0.028* | |
| Snoring | |||||||
| Never | Ref. | Ref. | Ref. | ||||
| Rarely | 1.18 (0.92, 1.50) | 0.197 | 1.22 (0.96, 1.55) | 0.122 | 1.10 (0.85, 1.44) | 0.481 | |
| Occasionally | 2.15 (1.57, 2.95) | <0.001⁎⁎⁎ | 1.92 (1.37, 2.71) | <0.001⁎⁎⁎ | 1.63 (1.12, 2.38) | 0.020* | |
| Frequently | 2.40 (1.78, 3.24) | <0.001⁎⁎⁎ | 2.19 (1.60, 3.00) | <0.001⁎⁎⁎ | 1.56 (1.11, 2.20) | 0.021* | |
| P for trend | <0.001⁎⁎⁎ | <0.001⁎⁎⁎ | 0.007⁎⁎ | ||||
| Sleep apnea | |||||||
| Never | Ref. | Ref. | Ref. | ||||
| Rarely | 1.24 (0.93, 1.64) | 0.151 | 1.23 (0.88, 1.71) | 0.235 | 1.06 (0.74, 1.52) | 0.763 | |
| Occasionally | 1.58 (1.05, 2.36) | 0.033 | 1.51 (0.92, 2.48) | 0.114 | 1.14 (0.70, 1.88) | 0.599 | |
| Frequently | 1.37 (0.91, 2.07) | 0.144 | 1.17 (0.80, 1.73) | 0.427 | 0.86 (0.62, 1.19) | 0.374 | |
| P for trend | 0.001⁎⁎ | 0.024* | 0.993 | ||||
| Daytime sleepiness | |||||||
| Never | Ref. | Ref. | Ref. | ||||
| Rarely | 1.20 (0.95, 1.51) | 0.136 | 1.26 (0.98, 1.60) | 0.082 | 1.17 (0.88, 1.54) | 0.290 | |
| Sometimes | 1.26 (1.01, 1.56) | 0.049* | 1.38 (1.15, 1.67) | 0.002⁎⁎ | 1.32 (1.06, 1.65) | 0.025* | |
| Often | 1.60 (1.16, 2.21) | 0.007⁎⁎ | 1.74 (1.29, 2.35) | 0.0013⁎⁎ | 1.30 (0.90, 1.86) | 0.175 | |
| Almost always | 1.14 (0.82, 1.58) | 0.454 | 1.18 (0.83, 1.67) | 0.370 | 0.95 (0.64, 1.43) | 0.826 | |
| P for trend | 0.039* | 0.015* | 0.464 | ||||
| Sleep duration workdays | 0.92 (0.87, 0.98) | 0.020* | 0.88 (0.83, 0.94) | <0.001⁎⁎⁎ | 0.88 (0.82, 0.93) | <0.001⁎⁎⁎ | |
| Sleep duration workdays | |||||||
| 7∼9 | Ref. | Ref. | Ref. | ||||
| < 7 | 1.28 (1.01, 1.62) | 0.048* | 1.35 (1.06, 1.72) | 0.020* | 1.36 (1.06, 1.74) | 0.025* | |
| > 9 | 1.19 (0.89, 1.59) | 0.238 | 1.00 (0.74, 1.35) | 0.999 | 1.00 (0.72, 1.38) | 0.998 | |
| Sleep duration freedays | 0.91 (0.86, 0.95) | <0.001⁎⁎⁎ | 0.92 (0.87, 0.97) | 0.005⁎⁎ | 0.90 (0.84, 0.96) | 0.004⁎⁎ | |
| Sleep duration freedays | |||||||
| 7∼9 | Ref. | Ref. | Ref. | ||||
| < 7 | 1.77 (1.38, 2.26) | <0.001⁎⁎⁎ | 1.59 (1.24, 2.03) | 0.001⁎⁎ | 1.72 (1.28, 2.30) | 0.002⁎⁎ | |
| > 9 | 1.00 (0.80, 1.23) | 0.966 | 0.98 (0.79, 1.22) | 0.869 | 0.89 (0.72, 1.10) | 0.286 | |
| Average weekly sleep duration | 0.90 (0.84, 0.96) | 0.003⁎⁎ | 0.87 (0.81, 0.93) | <0.001⁎⁎⁎ | 0.86 (0.80, 0.92) | <0.001⁎⁎⁎ | |
| Average weekly sleep duration | |||||||
| 7∼ 9 | Ref. | Ref. | Ref. | ||||
| < 7 | 1.14 (0.93, 1.41) | 0.204 | 1.13 (0.91, 1.40) | 0.271 | 1.11 (0.88, 1.39) | 0.395 | |
| > 9 | 0.83 (0.68, 1.01) | 0.067 | 0.64 (0.51, 0.80) | <0.001⁎⁎⁎ | 0.60 (0.49, 0.75) | <0.001⁎⁎⁎ | |
| Sleep debt | 0.95 (0.88, 1.03) | 0.252 | 1.10 (1.01, 1.19) | 0.030* | 1.07 (0.98, 1.16) | 0.136 | |
| Sleep debt | |||||||
| < 2 | Ref. | Ref. | Ref. | ||||
| ≥ 2 | 1.07 (0.79, 1.44) | 0.662 | 1.31 (0.99, 1.74) | 0.068 | 1.38 (1.05, 1.81) | 0.030* | |
| Social jet lag | 0.93 (0.89, 0.97) | 0.002⁎⁎ | 1.03 (0.99, 1.08) | 0.129 | 1.02 (0.98, 1.07) | 0.382 | |
| Social jet lag | |||||||
| < 2 | Ref. | Ref. | Ref. | ||||
| ≥ 2 | 0.71 (0.62, 0.82) | <0.001⁎⁎⁎ | 1.01 (0.88, 1.16) | 0.930 | 0.97 (0.83, 1.12) | 0.658 | |
| Sleep time workdays | |||||||
| 22∼ midnight | Ref. | Ref. | Ref. | ||||
| < 22 | 1.32 (0.96, 1.80) | 0.095 | 1.18 (0.87, 1.62) | 0.299 | 1.16 (0.86, 1.57) | 0.355 | |
| > midnight | 1.49 (1.16, 1.91) | 0.003⁎⁎ | 1.73 (1.33, 2.24) | <0.001⁎⁎⁎ | 1.86 (1.43, 2.42) | <0.001⁎⁎⁎ | |
| Wake time workdays | |||||||
| 6∼8 | Ref. | Ref. | Ref. | ||||
| < 6 | 1.13 (0.94, 1.37) | 0.208 | 1.13 (0.93, 1.37) | 0.232 | 1.18 (0.95, 1.48) | 0.146 | |
| > 8 | 0.93 (0.70, 1.22) | 0.592 | 1.00 (0.70, 1.42) | 0.979 | 1.07 (0.75, 1.54) | 0.703 | |
| Sleep time freedays | |||||||
| 22∼ midnight | Ref. | Ref. | Ref. | ||||
| < 22 | 1.37 (0.98, 1.91) | 0.070 | 1.16 (0.84, 1.59) | 0.374 | 1.13 (0.84, 1.52) | 0.418 | |
| > midnight | 0.86 (0.68, 1.10) | 0.244 | 1.13 (0.90, 1.42) | 0.306 | 1.16 (0.89, 1.53) | 0.286 | |
| Wake time freedays | |||||||
| 6∼8 | Ref. | Ref. | Ref. | ||||
| < 6 | 1.61 (1.33, 1.94) | <0.001⁎⁎⁎ | 1.35 (1.09, 1.67) | 0.010* | 1.48 (1.20, 1.83) | 0.002⁎⁎ | |
| > 8 | 0.75 (0.63, 0.88) | 0.001⁎⁎ | 0.91 (0.76, 1.09) | 0.312 | 0.91 (0.75, 1.09) | 0.317 | |
Abbreviations: OR, odds ratio; CAP, controlled attenuation parameter; MASLD, metabolic dysfunction-associated steatotic liver disease.
* P < 0.05, ** P < 0.01, *** P < 0.001.
We further explored the association between sleep and the progression to liver fibrosis in the MASLD population. Interestingly, most sleep characteristics were not associated with the development of liver fibrosis in MASLD patients. In Model 3, workday sleep time after midnight showed an independent association with the presence of hepatic fibrosis (≥F1) in MASLD individuals (OR = 1.55) (Supplementary Table 7). In Model 3, social jet lag (continuous), workday sleep time after midnight, and workday wake time after 8 a.m. showed a positive association with the presence of hepatic fibrosis (≥ F2) in MASLD individuals (OR = 1.10, 2.53, and 2.17, respectively) (Supplementary Table 8).
3.3Nonlinear relationship explorationWe applied the RCS model (adjusting for all covariates) to clarify whether sleep duration and MASLD risk have a nonlinear relationship. Workday sleep duration showed a nonlinear correlation with MASLD development (inflection point = 7.5; p nonlinear = 0.002) and exhibited a skewed 'Z' shape (flattening on both sides and steepening at approximately optimal sleep) (Fig. 3A). However, free day sleep duration was generally associated with MASLD risk in a negative but not a nonlinear manner (inflection point = 8; p nonlinear = 0.3871) (Fig. 3B). Notably, the average weekly sleep duration was also nonlinearly associated with MASLD risk (inflection point = 7.78, p nonlinear = 0.0422) and exhibited a similarly skewed 'Z' pattern (Fig. 3C). We further performed piecewise logistic regression analysis on both sides of the inflection point. The MASLD development was negatively associated with workday sleep duration <7.5 h (OR = 0.89, 95 % CI [0.84, 0.94], p < 0.001), but not with workday sleep duration ≥ 7.5 h. Similarly, we found that MASLD development was negatively associated with free day sleep duration < 8 h (OR = 0.94, 95 % CI [0.89, 0.98], p < 0.009), but not with free day sleep duration ≥ 8 h. Conversely, the average weekly sleep duration ≥ 7.78 h was negatively associated with MASLD risk (OR = 0.89, 95 % CI [0.85, 0.94], p < 0 .001) (Supplementary Table 9).
3.4Mediation analysisWe performed a mediation analysis of the relationship between sleep duration and MASLD development to determine whether glycolipid metabolism potentially contributes to this effect. Among the seven included markers, only fasting glucose and HDL-C may have mediated the association between workday sleep duration and MASLD (fasting glucose-mediated proportion: 4.7 %, p = 0.010; HDL-C mediated proportion: 5.2 %, p = 0.004) (Table 2). However, we did not find any marker mediating the association between free day sleep duration and MASLD (Supplementary Table 10). Nevertheless, in a similar direction, the average weekly sleep duration indirectly mediated the effect on MASLD through fasting glucose and HDL-C (fasting glucose-mediated proportion: 5 %, p = 0.008; HDL-C mediated proportion: 4.6 %, p = 0.010) (Supplementary Table 11).
Mediation analysis of the association between workday sleep duration and MASLD.
| Variables | Direct effect | Mediation effect | Proportion-mediated | ||
|---|---|---|---|---|---|
| β (95 % CI) | P | β (95 % CI) | P | ||
| FPG | −0.034 (−0.051, −0.018) | <0.001⁎⁎⁎ | −0.002 (−0.004, 0.000) | 0.010* | 0.047 |
| HbA1c | −0.038 (−0.055, −0.022) | <0.001⁎⁎⁎ | −0.001 (−0.003, 0.000) | 0.114 | 0.028 |
| TG | −0.038 (−0.056, −0.020) | <0.001⁎⁎⁎ | −0.0002 (−0.0014, 0.0009) | 0.690 | 0.006 |
| TC | −0.039 (−0.055, −0.023) | <0.001⁎⁎⁎ | −0.0002 (−0.0010, 0.0005) | 0.538 | 0.005 |
| HDL-C | −0.039 (−0.057, −0.024) | <0.001⁎⁎⁎ | −0.002 (−0.004, −0.001) | 0.004⁎⁎ | 0.052 |
| LDL-C | −0.038 (−0.055, −0.020) | <0.001⁎⁎⁎ | −0.0002 (−0.0010, 0.0003) | 0.410 | 0.006 |
| TRL-C | −0.037 (−0.055, −0.020) | <0.001⁎⁎⁎ | −0.0003 (−0.0012, 0.0002) | 0.418 | 0.008 |
Abbreviations: MASLD, metabolic dysfunction-associated steatotic liver disease; HbA1c, glycated hemoglobin; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TRL-C, triglyceride-rich lipoprotein cholesterol; FPG, fasting plasma glucose.
* P < 0.05, ** P < 0.01, *** P < 0.001.
We conducted stratified analysis to explore whether the effect of sleep duration on MASLD varied across different age and sex populations. Interestingly, sex significantly affected the association of workday and average weekly sleep durations with MASLD (p for interaction = 0.003 and 0.018, respectively), but not the association between free day sleep duration and MASLD. Moreover, an important finding was that sleep duration was negatively associated with MASLD development only in women (ORs for workday, free day, and average weekly sleep durations were 0.86, 0.94, and 0.86, respectively; p < 0.05 for all). The effect of sleep duration on MASLD differed significantly across age groups. For workday sleep duration, significantly negative associations were observed only in individuals aged > 30 years. For free day sleep duration, correlations with MASLD existed only in participants aged ≤ 30 years and > 60 years. Finally, for average weekly sleep duration, the effect was significant only among participants ≥ 45 years old(Fig. 4).
3.6Sensitivity analysisWe performed multiple sensitivity analysis to verify the stability of our results. First, we used CAP > 274 dB/m and 285 dB/m as additional diagnostic criteria for MASLD. Overall, most of the associations between sleep characteristics and MASLD remained significant, although the significance of some associations disappeared across the CAP criteria (Supplementary Tables 12 and 13). Next, as we considered that sleep time characteristics may influence the association between sleep duration and MASLD, we additionally adjusted the respective sleep time based on Model 3 (e.g., workday sleep duration adjusted for workday sleep time). In the new Model 4, workday and free day sleep durations maintained similar correlations with MASLD as shown in Model 3, demonstrating the stability of the findings (Supplementary Table 14). Finally, we considered the fact that the sleep routine of shift workers differs significantly from that of individuals with normal work schedules, and we conducted additional sensitivity analysis for this population. We found an overall agreement with the results obtained in the general population, albeit with discrepancies in several associations (Supplementary Tables 15-17).
4DiscussionIn this study, we systematically investigated the association of sleep patterns and disorders with MASLD and liver fibrosis in contemporary American adults. We derived several important findings. First, sleep duration (regardless of workday, free day, or average weekly sleep duration) was negatively associated with the risk of MASLD overall, with short sleep on workdays and free days associated with an increased risk of MASLD, and long average weekly sleep associated with a reduced risk of MASLD. Other sleep habits, including sleep debt ≥2h, workday sleep time after midnight, and free-day wake-up time before 6 a.m., were positively associated with MASLD risk. Furthermore, workday sleep duration and average weekly sleep duration showed a nonlinear relationship with MASLD. Second, sleep duration may indirectly influence MASLD development by affecting fasting glucose and HDL-C. Finally, stratified analysis revealed that sex influenced these effects, and they seemed to be present only in women and varied across different age groups. Although most sleep characteristics were not associated with liver fibrosis progression in MASLD, it is noteworthy that workday sleep time after midnight was positively associated with liver fibrosis (≥F1 and ≥F2).
The importance of sleep to health can hardly be overstated. In the human body, almost all physiological processes are affected by sleep. However, the accelerated pace of life and increased screen time have significantly affected the sleep habits of modern individuals and increased the incidence of sleep disorders. The blue light emitted by cell phones and tablets can alter the secretion of melatonin, disrupt sleep patterns and lead to mutiple metabolic changes [23]. It is worth noting that disrupted sleep duration and decreased sleep quality have been linked to decreased quality of life and the development of mutiple diseases, such as depression, Type 2 diabetes and CVD [24].
Recently, mounting evidence suggests a bidirectional link between sleep and liver health and disease, including MASLD [7,25]. However, inconsistencies between the findings have prevented further clarification of this relationship. Early meta-analysis showed either no clear or a marginally small association between abnormal sleep duration and MASLD [26]. However, more recent meta-analysis have shown a significantly increased risk of MASLD with abnomal sleep duration [8]. In addition, the significance of concepts such as sleep-wake time, sleep debt, and social jet lag (characterized as sleep-related circadian rhythm disorders) in the development of MASLD and liver fibrosis remains unknown. Finally, the role of sleep disturbances in the development of MASLD has been increasingly recognized over the past decade. Symptoms of sleep apnea obstruction/intermittent hypoxia have shown significant correlations with the development and severity of MASLD but lack description in the contemporary American adult population [11]. The role of other less-described symptoms including snoring, insomnia, and daytime sleepiness, in the development of MASLD remains understudied.
Therefore, we comprehensively addressed the potential impact of sleep habits and disorders on the development of MASLD and liver fibrosis in contemporary American adults using a nationally representative population-based cross-sectional study. By adjusting for all potential confounding factors, we demonstrated that insomnia and snoring, but not other factors, were independently associated with MASLD development in the general population. Insomnia or trouble sleeping showed an association with an increased risk of MASLD in a mendelian randomization study, implying causality in this relationship [27]. One of the possible explications of the found data can be related to the excess of cortisol production due to insomnia, and subsequently changes in respiratory acts [28]. Also, the type of alimentary regimen rich in “junk food” can influence circadian rhythm and hormon delivery in the blood, independently to the day/night circle. However, surprisingly, in the fully adjusted model, the presence of insomnia was associated with a reduced risk of MASLD, whereas in the crude model, no association was found. This scenario suggests the presence of strongly influencing components of MASLD in the adjusted variables. On examining the multivariate regression model, we found that BMI and WC were significant influential factors, i.e., there was no association between insomnia and MASLD without adjusting for either of these two factors, and when either BMI or WC was included, we immediately found a negative correlation. In fact, both BMI and WC were strongly associated with MASLD, and after adjusting for both, we found true associations independent of BMI and WC. This suggests that previous studies with positive findings may have exaggerated the relationship with BMI and WC. Notably, the diagnosis of MASLD was made using accurate noninvasive VCTE, distinguishing it from the previous widespread use of serological indicators as diagnostic markers, and therefore more likely to represent reliability [17]. Furthermore, the population included in our study was a contemporary cohort of American adults, and differences among the participants could have contributed to the contrasting results. Nevertheless, reverse causality and retrospective bias cannot be ruled out, considering the nature of cross-sectional studies and insomnia diagnosis by self-reported questionnaires. Future prospective cohort studies are warranted to uncover the trueness of this relationship. Consistent with the existing literature, we found a positive correlation between snoring frequency and MASLD [29]. This association could be attributed to disturbed energy metabolism due to intermittent hypoxia, although no relevant mechanistic studies have confirmed this.
The main finding of our study was that any sleep duration (continuous) was negatively associated with MASLD development, and when used as a categorical variable, short sleep (<7 h) on both workdays and free days was positively associated with MASLD. In contrast, average weekly long sleep (>9 h) was negatively associated with MASLD. Growing evidence suggests that short sleep is associated with an increased risk of MASLD, but the findings remain inconsistent [9,30,31]. Furthermore, we found that long average weekly sleep duration was associated with a reduced risk of MASLD. No previous study has explored the relationship between average weekly sleep duration and MASLD, and the effect of long sleep duration on MASLD remains controversial. Several studies have suggested that long sleep duration is associated with a reduced risk of MASLD [9,32], while others suggest the opposite [33,34]. It is worth stating that these results were from largely different ethnicities or countries, and the thresholds for defining short/long sleep varied, thus affecting the conclusions. Although no mechanistic studies have implicated how sleep duration affects the development of MASLD, short sleep duration has been linked to depression, metabolic disorders, and chronic inflammation [24,35,36], all of which are closely associated with MASLD development. Similarly, we focused on the disorders of glucolipid metabolism through mediation analysis, which suggested that fasting glucose and HDL-C indirectly mediated this relationship.
In addition to sleep duration, we identified that sleep debt ≥2h, workday sleep time after midnight, and free day wake time before 6 a.m. were independently associated with increased risk of MASLD. Sleep debt is the cumulative result of chronic buildup of sleep deprivation, which is associated with an increased incidence of obesity, diabetes, CVD, and other complications [37], [38]. No other study has examined the relationship between sleep debt and MASLD development, and this study is the first to show that high sleep debt is independently associated with MASLD. An in vivo study in mice demonstrated that sleep deprivation increases hepatic lipogenic enzyme synthesis, which affects hepatic glycolipid metabolism and hepatic steatosis [39]. A metabolomic and proteomic analysis revealed significant alterations in glutathione metabolism, fructose and mannose metabolism, and pyruvate metabolism pathways in sleep-deprived mice, potentially contributing to metabolic dysregulation and metabolic diseases such as obesity [40]. Moreover, we demonstrated for the first time that the chronotype of workday late sleep and free-day early wake time are associated with an increased risk of MASLD. These sleep habits are associated with circadian rhythm misalignment. Circadian dysregulation is an important causative mechanism for the development of MASLD, which contributes to metabolic homeostasis imbalances and adverse metabolic outcomes, including MASLD, through multiple factors such as impaired appetite, energy expenditure, and metabolically involved hormone production [41], [42].
Notably, most sleep characteristics did not seem to affect the progression of liver fibrosis in the MASLD population. Although previous studies have suggested that sleep disorders are associated with the development of liver fibrosis in the general population, they rarely addressed the relationship between sleep and the progression of liver fibrosis in MASLD and rarely used LSM for the accurate diagnosis of liver fibrosis [10,43]. However, we have suggested a possible detrimental effect of workday sleep time after midnight on the progression of both mild liver fibrosis (≥F1) and significant liver fibrosis (≥F2). Circadian rhythm disruption has also been implicated in liver fibrosis onset and progression. However, it remains to be elucidated in future studies why only a few chronotypes are associated with the progression of liver fibrosis in MASLD.
We found that workday sleep duration was nonlinearly associated with MASLD development, with sleep duration <7.5h negatively associated with MASLD development. This negative correlation is in contrast with the correlation in the multivariate regression analysis of workday sleep duration <7 h, which may be attributed to the inflection point being within the optimal sleep duration. Alternatively, it suggests that being closer to the inflection point (7.5 h) is associated with a reduced risk of MASLD. However, free-day sleep duration, although similarly negatively associated with MASLD on the left side of the inflection point (<8 h), was not associated nonlinearly overall. On the other hand, average weekly sleep duration was negatively associated with MASLD on the right side of the inflection point (≥7.78 h), suggesting that a longer average weekly sleep duration is beneficial in preventing MASLD. These results may have public health applications, i.e., sleeping enough for 7.5 h on workdays, sleeping enough for 8 h on free-days, and an average weekly sleep duration of ≥7.78 h can help prevent MASLD development in the general population.
Interestingly, stratified analysis suggested that sex cloud influence the effect of sleep duration on MASLD, particularly for workdays and average weekly sleep duration. All types of sleep duration were associated with MASLD only in women. This is consistent with the findings of a large observational study on middle-aged workers and their spouses and a single-center study on Korean men and women [44]. However, other studies have suggested the complete opposite conclusion [31,45]. Sexual dimorphism has also been documented in circadian rhythms and sleep physiology. Women generally sleep earlier and have longer sleep durations as well as more slow-wave sleep as compared to men [46]. In contrast, men are more likely to be of the later chronotype [46]. Our results suggest that sex is central to the nexus between sleep and MASLD in contemporary American adults and that sex-specific recommendations for sleep should be considered. Given the inconsistencies between studies, further high-quality large-sample studies are warranted to clarify this picture. Moreover, we found that the effect of sleep duration on MASLD differs across age groups. Workday sleep duration in individuals aged >30 years, free-day sleep duration in those aged ≤30 and >60 years, and average weekly sleep duration in those aged >45 years showed significant MASLD-protective effects at specific ages. The specific mechanisms remain unclear but may indicate that the benefits of sleep duration are related to age-related factors such as lifestyle and physiologic status of the body.
Finally, we demonstrated the stability of the main findings of our multiple sensitivity analysis, though there were disagreements in some of the associations. Notably, some relationships disappeared or emerged among shift workers, suggesting a unique sleep routine schedule. However, workday sleep time after midnight remained an independent risk factor for the development of MASLD and progression of hepatic fibrosis (≥F1 and ≥ F2), demonstrating that this circadian rhythm disturbance is robustly associated with the development of MASLD and liver fibrosis. Furthermore, we found that late workday wake time was an emerging predictor of the development of mild and significant liver fibrosis in shift workers, reaffirming the significant alteration of circadian rhythms in this unique population compared to the general population.
Our study has certain strengths. This is the first time that the potential effects of sleep on MASLD and liver fibrosis have been addressed in a large nationally representative cross-sectional survey. Second, we employed accurate imaging tools for the diagnosis of MASLD and liver fibrosis to ensure the reliability of our results. We used various rigorous statistical tools including multivariate adjusted regression analysis, RCS models, mediation analysis, stratified analysis, and multiple sensitivity analysis, to explore these relationships fully in a reproducible and robust manner. Our results have important public health implications and may enlighten the general population on the prevention of MASLD and liver fibrosis by adjusting sleep as a modifiable risk factor. We are aware that our study has certain limitations. It is a cross-sectional study, and there are inevitably unadjusted residual confounders and reverse causality. However, cross-sectional studies usually have the highest level of evidence available in large, nationally representative surveys. Second, our sleep variables were obtained via self-reported questionnaires, which may be subject to some bias. However, extensive research has demonstrated that the questionnaire results of the NHANES have good accuracy and can therefore be largely representative of the actual conditions.
5ConclusionsIn this nationally representative cross-sectional study of contemporary American adults,sleep duration showed a negative association with MASLD risk in women but not in men. Short sleep independently increased the MASLD risk, whereas long average weekly sleep duration protected against MASLD. Late sleep on workdays was associated with both MASLD and liver fibrosis. The optimal workday, free-day, and average weekly sleep durations were 7.5 h, 8 h, and 7.78 h, respectively. Adjusting for sleep required age- and sex-specific strategies. Future prospective cohort studies are warranted to validate these findings.
Author contributionsStudy conception: Guannan Zong, Guanghui Liu; data acquisition: Guannan Zong, Wangjia Mao and Ming Wen; statistical analysis: Guannan Zong, Wangjia Mao, Ming Wen and Xiaoyun Cheng; drafting the initial manuscript: Guannan Zong, Ming Wen; critical review of the manuscript: Xiaoyun Cheng and Guanghui Liu. All authors reviewed and approved the final version of the manuscript.
FundingThis study is funded by Project supported by Clinical Research Project of Tongji Hospital of Tongji University (Grant No. ITJ(QN)2002).
We thank all the participants in the NHANES for providing data for this study. We also thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.