Our objective was to measure and compare the intake of macro and micronutrients in a cohort of individuals with Metabolic Syndrome Associated Steatotic Liver Disease (MASLD) compared with matched controls to identify areas of further research in this area; we identified nutrition-associated associations with MASLD in the United States general population.
Materials and MethodsWe used the 2017 – 2018 NHANES dataset. Elastography Controlled Attenuation Parameter (CAP score>280) in the absence of other liver disease was defined as MASLD in adults (>18). Advanced fibrosis was defined by transient elastography >10 kPa. Controls were adults without liver disease.
Results1648 MASLD cases (11.4 % advanced fibrosis) and 2527 controls were identified. MASLD cases were older (P<0.001), more likely males (P = 0.01), less likely to have a college education (P = 0.04) and more likely married (P = 0.002). MASLD cases were more likely to be of Mexican American or Hispanic ethnicity (P = 0.002), have higher BMI, and have higher prevalence of diabetes, hyperlipidemia and hypertension (P<0.001 for all). MASLD cases had higher hs-CRP (P = 0.02) and ferritin (P = 0.02). MASLD cases had lower total (P = 0.004) and added vitamin E in their diet (P = 0.002), lower vitamin K intake (P = 0.005), and higher selenium intake (P = 0.03). Caloric intake (P = 0.04), carbohydrate intake (P = 0.02), cholesterol intake (P = 0.03) and saturated fatty acid intake (P = 0.05) were higher in MASLD. Individuals with MASLD were more likely to be on a diet (P<0.001), sedentary (P = 0.008) and less likely to participate in moderate or vigorous recreational activities (P<0.001).
ConclusionsThe deficiencies of micronutrients and excess of macronutrients point to oxidative stress, pro-inflammatory state, and lipotoxicity as pathways linking the US diet to MASLD. MASLD patients are more often on special diets, which may reflect prior provider counseling on diet changes to improve health.
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) is a common cause of chronic liver disease worldwide, closely associated with metabolic syndrome and approximately 25–33 % of the adult United States population is affected by MASLD [1–4]. Disease progression can lead to non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis, leading to an increased risk of liver-related mortality, hepatocellular carcinoma (HCC) [5] and the need for liver transplantation [6].
While the pathophysiology remains complex, MASLD reflects an accumulation of fat, mainly in the form of triglycerides, in the hepatocyte, with further progression leading to hepatocyte cell damage and liver fibrosis. Factors associated with MASLD pathogenesis include obesity and insulin resistance [7,8].
Dietary factors are thought to play an essential role in the development of MASLD. Excess caloric intake has been shown to increase intrahepatic triglyceride deposition [9]. Furthermore, specific types of carbohydrates, such as fructose, have been linked to increased incidence of MASLD [10]. Excess intake of saturated fatty acids promotes fatty liver and impairs insulin signaling, while dietary monounsaturated fatty acids and polyunsaturated fatty acids have been shown to reduce intrahepatic triglyceride accumulation and inflammation [11,12]. Micronutrients have been investigated in their role in MASLD pathogenesis, and antioxidants, such as vitamin E, are now being used in the treatment of MASLD. However, despite the high prevalence and public health burden, targeted therapeutic approaches for MASLD remain limited, with no FDA approved medications for this condition and lifestyle interventions of diet and exercise remain the mainstay of treatment [13–16].
The aim of this study was to analyze nutritional, demographic, physical activity and socioeconomic correlates of MASLD in a nationwide sample of US adults (NHANES 2017–2018 cohort). Our objective was to measure and compare the intake of macro and micronutrients in a cohort of individuals with MASLD compared with matched controls to identify areas of further research in this area.
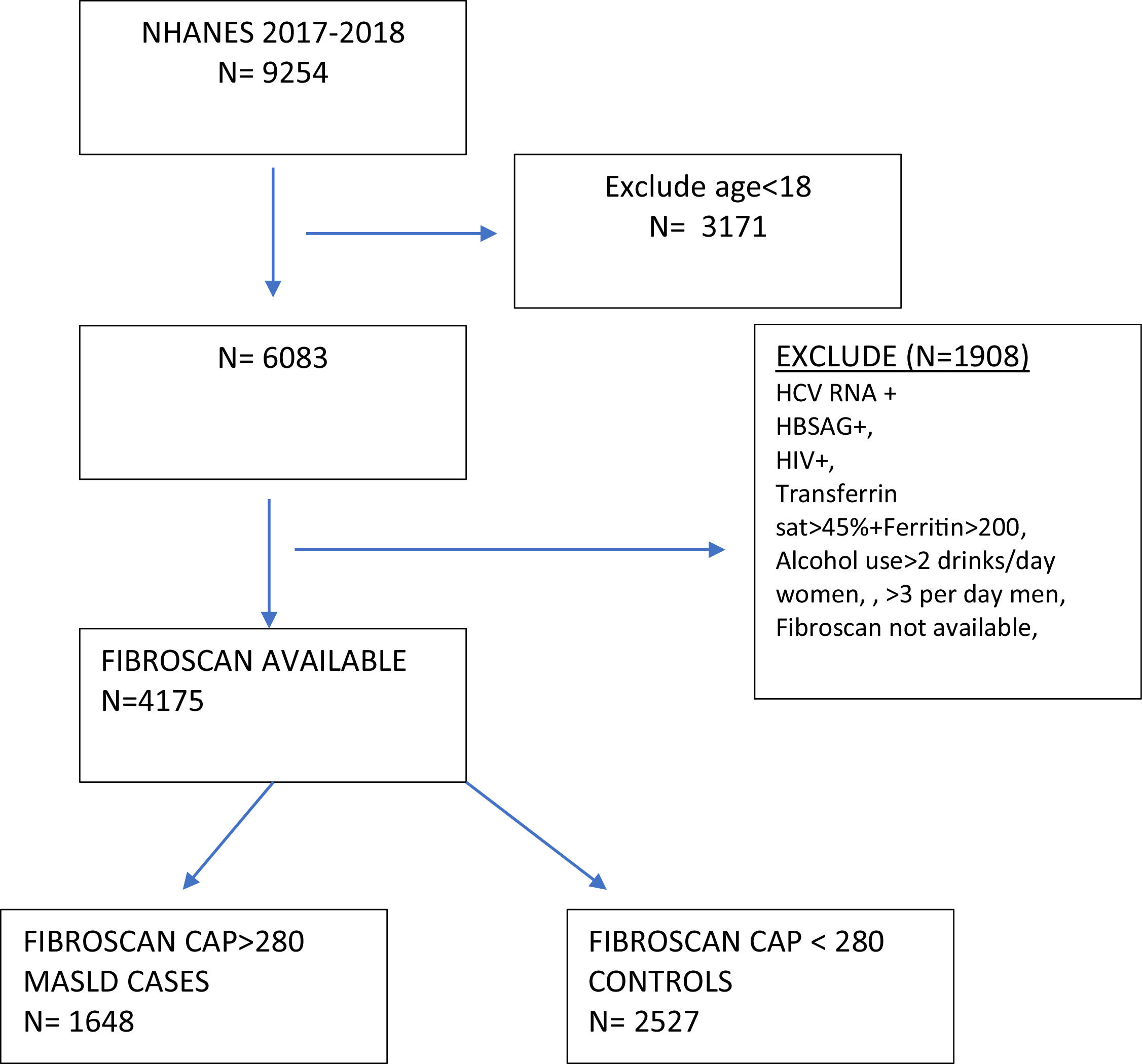
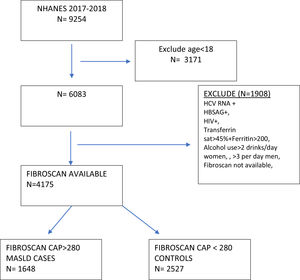
2Materials and methods2.1Study design and populationThis was a cross-sectional nationwide study utilizing the 2017–2018 National Health and Nutrition Examination Study (NHANES), which is an ongoing program that annually assesses the health and nutritional status of a nationally representative sample of more than 9000 individuals in the United States [17]. We restricted our analysis to adults in the survey (age≥18 years.) The MASLD group was defined by a median controlled attenuated parameter (CAP) ≥ 280 dB/m on the FibroScan® examination performed in the survey [4,18]. The control group had a CAP <280 dB/m. Exclusion criteria in both groups included a positive hepatitis B surface antigen, positive hepatitis C RNA, positive HIV-1, 2 combo test, transferrin saturation > 45 % with ferritin >200 ng/mL, and dietary intake averaging more than 2 alcoholic drinks daily for women and 3 for men to minimize contributing etiologies of liver disease (Fig. 1). In the MASLD cohort, individuals with a liver stiffness score of ≥ 10 kPa on FibroScan® were categorized as MASLD with advanced fibrosis [4].
2.2Data collectionInterview content included demographic, socioeconomic, dietary, and health-related questions. Detailed dietary intake for a 24-hour recall period was recorded utilizing the USDA's Automated Multiple-Pass Method. Interviews were conducted on two separate occasions, with the first interview occurring in a medically equipped mobile center staffed by trained interviewers. The second dietary intake interview occurred within 3–10 days after the first and was conducted via telephone. The data from each dietary recall was linked to a nutritional database that was used to estimate the macronutrients and micronutrients consumed [19,20].
Health measurements were performed in medically equipped mobile centers that were staffed with a physician, medical and health technicians, and trained interviewers [17]. The 2017–2018 dataset was used as liver transient elastography was performed during this cycle. FibroScan® was utilized to assess liver stiffness and was completed by trained technicians. Based on body habitus, either the M+ or XL+ probes were used and at least ten valid measurements were obtained with an interquartile range/median (IQR/M) less than 30 % to reduce sampling error [19,21]. The elastography was performed in a mobile examination center on the same day as the dietary interviews. For Physical activity, sedentarism, as well as light, moderate and heavy physical activity, was defined using the Global Physical Activity Questionnaire and asked in the home by trained interviewers.
2.3Statistical analysisWe applied all sample weights using the svyset command in STATA [20,22]. This command allows for a single-stage survey design with clustering and stratification without replacement. We applied the full sample weights for the 2017 – 2018 survey. Subsequently, each dietary data was weighted using either dietary day one or day two sample weights together with masked variance PSU (clusters) and masked variance strata. Categorical outcomes were summarized as proportions and percentages using a survey-based Pearson corrected Chi-square design. Continuous outcomes were summarized as mean plus standard deviation and compared using a linearized survey regression to obtain p-values. Variables with greater than 5 % missing data were corrected using multiple imputations with regression estimates. Due to the number of comparisons in this analysis, we adjusted the significance level using a Sidak p-value adjustment method ((1 – (1 – a)1/n), where n represents the number of independent tests and a represents the significance level. We conducted all analyses using STATA version 17 SE (StataCorp). 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC) [19,22].
2.4Ethical statementsWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of National Center for Health Statistics (NCHS) (Protocol #2018–01).
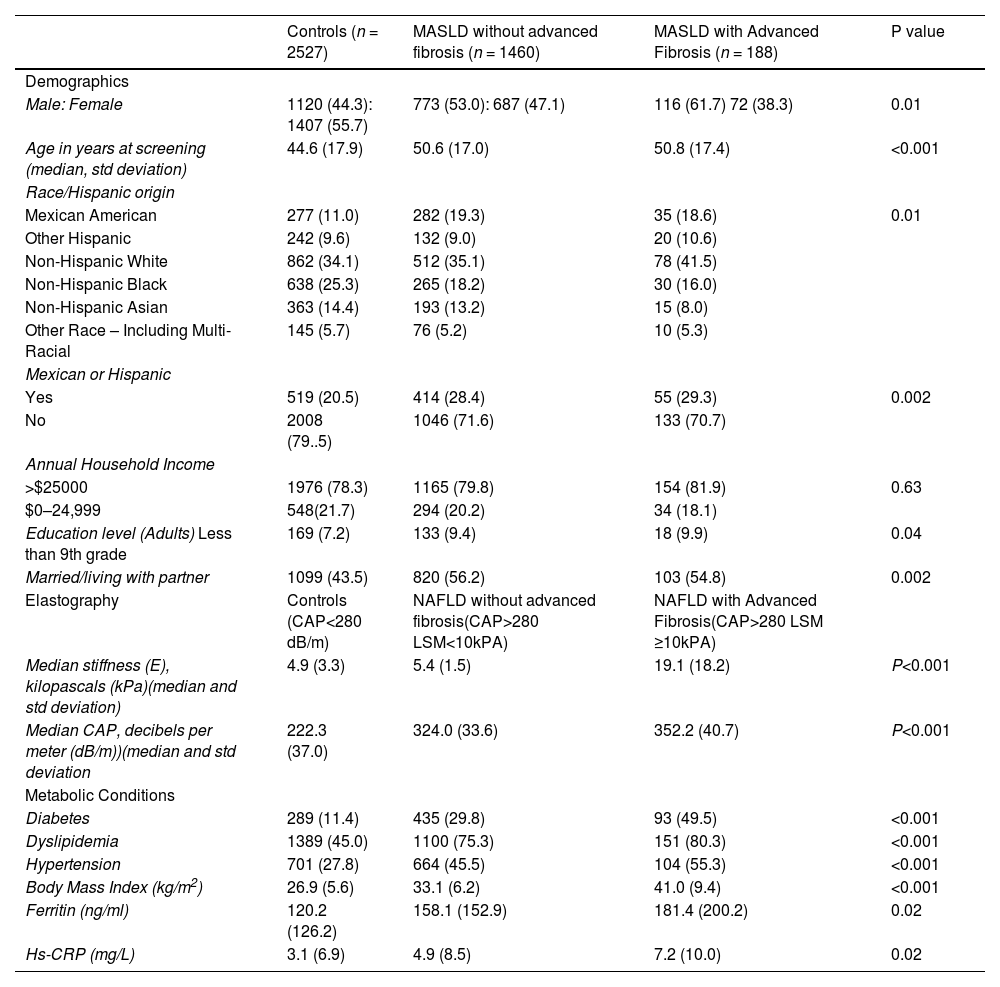
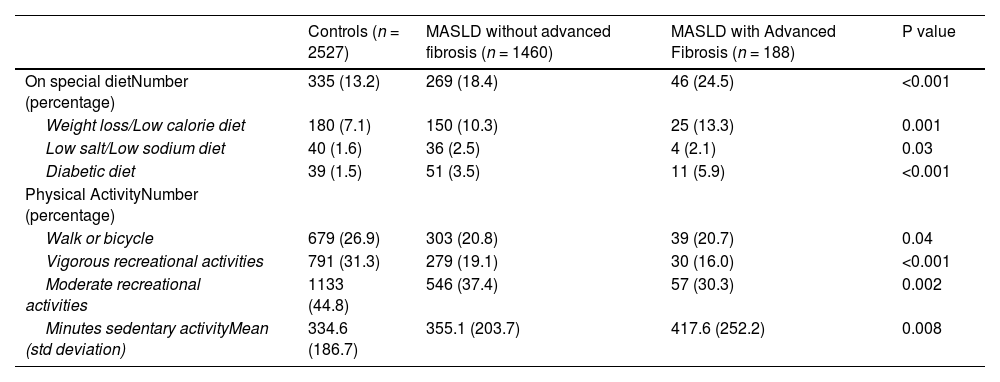
3Results3.1Study populationAfter applying inclusion and exclusion criteria, we identified a MASLD cohort (n = 1648) and a control group (n = 2527) (Fig. 1). The majority of individuals in the MASLD cohort did not have advanced fibrosis (n = 1460) (Table 1), Individuals with MASLD were more likely to be older (P<0.0001), male (P = 0.01), married or living with partner (P = 0.002), of Mexican or Hispanic ethnicity (P = 0.002), and have an educational level less than college (P = 0.04) compared to controls but with similar income levels (P = 0.63) (Table 1). Individuals in the MASLD cohort were also less likely to participate in vigorous recreational activities (P<0.001), moderate recreational activities (P = 0.002), and less likely to walk or bicycle (P = 0.04), but more likely to spend time sedentary (P = 0.008) (Table 2). Individuals with MASLD were more likely to be on a special diet (P<0.001), especially a diabetic diet (P<0.001) or low sodium diet (P = 0.03) compared to controls (Table 2).
MASLD Demographics, Elastography and Medical Conditions.
| Controls (n = 2527) | MASLD without advanced fibrosis (n = 1460) | MASLD with Advanced Fibrosis (n = 188) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Male: Female | 1120 (44.3): 1407 (55.7) | 773 (53.0): 687 (47.1) | 116 (61.7) 72 (38.3) | 0.01 |
| Age in years at screening (median, std deviation) | 44.6 (17.9) | 50.6 (17.0) | 50.8 (17.4) | <0.001 |
| Race/Hispanic origin | ||||
| Mexican American | 277 (11.0) | 282 (19.3) | 35 (18.6) | 0.01 |
| Other Hispanic | 242 (9.6) | 132 (9.0) | 20 (10.6) | |
| Non-Hispanic White | 862 (34.1) | 512 (35.1) | 78 (41.5) | |
| Non-Hispanic Black | 638 (25.3) | 265 (18.2) | 30 (16.0) | |
| Non-Hispanic Asian | 363 (14.4) | 193 (13.2) | 15 (8.0) | |
| Other Race – Including Multi-Racial | 145 (5.7) | 76 (5.2) | 10 (5.3) | |
| Mexican or Hispanic | ||||
| Yes | 519 (20.5) | 414 (28.4) | 55 (29.3) | 0.002 |
| No | 2008 (79..5) | 1046 (71.6) | 133 (70.7) | |
| Annual Household Income | ||||
| >$25000 | 1976 (78.3) | 1165 (79.8) | 154 (81.9) | 0.63 |
| $0–24,999 | 548(21.7) | 294 (20.2) | 34 (18.1) | |
| Education level (Adults) Less than 9th grade | 169 (7.2) | 133 (9.4) | 18 (9.9) | 0.04 |
| Married/living with partner | 1099 (43.5) | 820 (56.2) | 103 (54.8) | 0.002 |
| Elastography | Controls (CAP<280 dB/m) | NAFLD without advanced fibrosis(CAP>280 LSM<10kPA) | NAFLD with Advanced Fibrosis(CAP>280 LSM ≥10kPA) | |
| Median stiffness (E), kilopascals (kPa)(median and std deviation) | 4.9 (3.3) | 5.4 (1.5) | 19.1 (18.2) | P<0.001 |
| Median CAP, decibels per meter (dB/m))(median and std deviation | 222.3 (37.0) | 324.0 (33.6) | 352.2 (40.7) | P<0.001 |
| Metabolic Conditions | ||||
| Diabetes | 289 (11.4) | 435 (29.8) | 93 (49.5) | <0.001 |
| Dyslipidemia | 1389 (45.0) | 1100 (75.3) | 151 (80.3) | <0.001 |
| Hypertension | 701 (27.8) | 664 (45.5) | 104 (55.3) | <0.001 |
| Body Mass Index (kg/m2) | 26.9 (5.6) | 33.1 (6.2) | 41.0 (9.4) | <0.001 |
| Ferritin (ng/ml) | 120.2 (126.2) | 158.1 (152.9) | 181.4 (200.2) | 0.02 |
| Hs-CRP (mg/L) | 3.1 (6.9) | 4.9 (8.5) | 7.2 (10.0) | 0.02 |
CAP+ Controlled Attenuation Parameter, hs-CRP- High sensitivity C-reactive protein.
Reference ranges in NHANES: CAP (100–400 dB/m) Elastrography, Median Stiffness (1.6 – 75 kPa), Ferritin (1–5190 ng/ml), Hs-CRP (0.1 to 182).
MASLD diets and physical activity.
| Controls (n = 2527) | MASLD without advanced fibrosis (n = 1460) | MASLD with Advanced Fibrosis (n = 188) | P value | |
|---|---|---|---|---|
| On special dietNumber (percentage) | 335 (13.2) | 269 (18.4) | 46 (24.5) | <0.001 |
| Weight loss/Low calorie diet | 180 (7.1) | 150 (10.3) | 25 (13.3) | 0.001 |
| Low salt/Low sodium diet | 40 (1.6) | 36 (2.5) | 4 (2.1) | 0.03 |
| Diabetic diet | 39 (1.5) | 51 (3.5) | 11 (5.9) | <0.001 |
| Physical ActivityNumber (percentage) | ||||
| Walk or bicycle | 679 (26.9) | 303 (20.8) | 39 (20.7) | 0.04 |
| Vigorous recreational activities | 791 (31.3) | 279 (19.1) | 30 (16.0) | <0.001 |
| Moderate recreational activities | 1133 (44.8) | 546 (37.4) | 57 (30.3) | 0.002 |
| Minutes sedentary activityMean (std deviation) | 334.6 (186.7) | 355.1 (203.7) | 417.6 (252.2) | 0.008 |
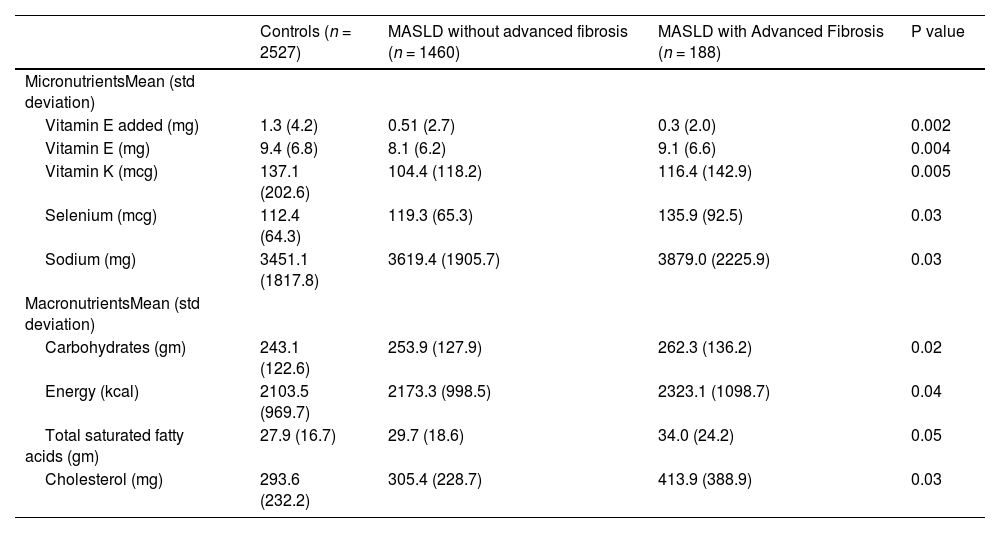
When comparing nutritional variables, we found that total vitamin E (P = 0.004), added vitamin E (P = 0.002), vitamin K (p = 0.005) were reduced in the MASLD cohort compared to the control group and selenium intake was higher in the MASLD group, particularly the MASLD advanced fibrosis cohort (P = 0.03). Carbohydrates (P = 0.02), energy (kcal intake) (P = 0.04), total saturated fatty acids (P = 0.05), cholesterol (P = 0.03) were all increased in the MASLD cohort compared to control. Other micronutrients like folate, iron, vitamin D and carotenes did not have an association with MASLD in our study, nor did caffeine intake. Micro and macronutrient data is summarized in Table 3.
Macro and Micro nutrient intake MASLD.
| Controls (n = 2527) | MASLD without advanced fibrosis (n = 1460) | MASLD with Advanced Fibrosis (n = 188) | P value | |
|---|---|---|---|---|
| MicronutrientsMean (std deviation) | ||||
| Vitamin E added (mg) | 1.3 (4.2) | 0.51 (2.7) | 0.3 (2.0) | 0.002 |
| Vitamin E (mg) | 9.4 (6.8) | 8.1 (6.2) | 9.1 (6.6) | 0.004 |
| Vitamin K (mcg) | 137.1 (202.6) | 104.4 (118.2) | 116.4 (142.9) | 0.005 |
| Selenium (mcg) | 112.4 (64.3) | 119.3 (65.3) | 135.9 (92.5) | 0.03 |
| Sodium (mg) | 3451.1 (1817.8) | 3619.4 (1905.7) | 3879.0 (2225.9) | 0.03 |
| MacronutrientsMean (std deviation) | ||||
| Carbohydrates (gm) | 243.1 (122.6) | 253.9 (127.9) | 262.3 (136.2) | 0.02 |
| Energy (kcal) | 2103.5 (969.7) | 2173.3 (998.5) | 2323.1 (1098.7) | 0.04 |
| Total saturated fatty acids (gm) | 27.9 (16.7) | 29.7 (18.6) | 34.0 (24.2) | 0.05 |
| Cholesterol (mg) | 293.6 (232.2) | 305.4 (228.7) | 413.9 (388.9) | 0.03 |
Kcal= Kilocalories, mg+ milligrams, mcg= micorgrams.
References range:.
Vitamin E added: 0–55 mg, vitamin E: 0–123 mg, vitamin K 0–2237 mcg.
Selenium 0–2550 mcg, Sodium 0–13,608 mg, Carbohydrates 0–719 gm.
Energy 0–3523 kcal, Total Saturated fatty acids 0–115 gm, Cholesterol 0–2319 mg.
We analyzed nutritional and demographic correlates of MASLD in a cohort of adults in the US general population. Individuals with MASLD were more likely to be older, male, married or living with a partner, of Mexican or Hispanic ethnicity, and have an educational level less than college. The pattern of activity in individuals with MASLD is that they are more sedentary and less likely to participate in moderate to vigorous recreational activities. The increased prevalence of MASLD among individuals of Mexican or Hispanic ethnicity may be attributed to dietary and higher prevalence of genetic factors, including well-characterized polymorphisms (PNPLA3) [23–25]. The MASLD cohort was less likely to have a college education. However, income level was not significantly different between MASLD and control groups. This risk may be tied to lower health literacy among MASLD group, which is a strong predictor of health outcomes rather than the higher cost of healthier diets [26,27]. Cross-sectional studies have shown that non-married people have lower BMI than their married counterparts, which could explain our finding of increased prevalence of MASLD among married individuals [28–31]. Finally, MASLD patients are more often on special diets, which may reflect prior provider counseling on diet changes to improve health [32,33]. Besides the increased prevalence of metabolic diseases like diabetes, hypertension, dyslipidemia and obesity, individuals with MASLD had evidence of a pro-inflammatory state with elevated hs-CRP and ferritin levels. In addition, ferritin is also an acute phase reactant that is associated with advanced fibrosis in MASLD [34–36].
Our primary objective was to compare micro and macronutrient intake in MASLD cohort within a general population to identify areas of further research and inform targeted dietary counseling for treatment and prevention of MASLD. Vitamin E intake was reduced in MASLD cohort, consistent with previous clinical studies [37–40]. MASLD is a state of oxidative stress and inflammation with increased denovo lipogenesis and triglyceride hepatic deposition [41,42]. Vitamin E is an antioxidant that can inhibit reactive oxygen species and may play a protective role in MASLD pathogenesis [14,43]. Other potential roles for vitamin E include anti-apoptotic and anti-inflammatory roles that may be salutary in MASLD. In the US, the PIVENs randomized controlled trial demonstrated that vitamin E supplementation was associated with improvement in histologic steatohepatitis in non-diabetic individuals [44]. In a large population-based study of over 200 K individuals in a UK biobank, vitamin E supplementation was linked to reduced MASLD diagnoses in diabetic overweight individuals with reduced mortality [45].
Vitamin K intake was reduced in the MASLD cohort. While not previously reported in a Western population, a Korean hospital-based study showed vitamin K supplementation significantly lowered the risk of MASLD [46]. Vitamin K intake may reflect an overall higher vegetable intake, which has been shown to be protective of MASLD, putatively through antioxidant pathways [47]. Vitamin K supplementation has been associated with reduced insulin resistance in older individuals and higher vitamin K levels were associated with reduced inflammation in the Framingham cohort [48,49]. Vitamin K has been associated with increased beta cell insulin secretion and adiponectin expression in mice with reduced lipopolysaccharide (LPS) induced inflammation, which may improve insulin sensitivity [50,51].
Selenium intake was higher in MASLD group and highest in MASLD with advanced fibrosis (P = 0.001). Selenium is a trace mineral that protects against oxidative stress and is found in animal proteins, breads and cereals. Selenium deficiency is rare. Most individuals in the US consume more than the recommended daily allowance of 55 micrograms/day. More than 150 micrograms/day can be harmful. Prior studies have shown a dose-related relationship between selenium and MASLD, with harmful associations found at serum selenium levels >130 μg/L and <130 μg/L serum selenium had no association with MASLD [52]. This association between high selenium levels and liver fibrosis has also been seen in epidemiologic studies [53]. The exact mechanism remains unclear, but studies suggest that high levels of selenium are associated with insulin resistance, increased lipogenesis, and oxidative stress [53]. In a randomized controlled trial of selenium supplementation for prostate cancer prevention, selenium supplementation was associated with an increased incidence of type 2 diabetes [54]. The higher selenium intake in individuals with MASLD could also be higher due to their greater consumption of food in general (higher caloric intake), including bread, meat and cereals. Although we did not find an association in our studies, reduced folate intake and lower vitamin D have been inconsistently reported in association with MASLD [55,56].
In an analysis of macronutrients, the MASLD group had an increased intake of carbohydrates, calories, cholesterol and total saturated fatty acids. Epidemiologic and experimental studies have shown saturated fats and cholesterol intake are associated with MASLD [57,58]. Mice fed a high fat and cholesterol diet developed sequential progression of hepatic steatosis, steatohepatitis, fibrosis and eventually liver cancer [59,60]. Prior studies have suggested that free fatty acids and their derivates trigger several intracellular responses, including cytokine release and macrophage activation, leading to lipotoxic stress and pro-inflammatory states [61,62]. Saturated fat has been shown to provoke insulin resistance and endotoxinemia associated with MASLD and increase harmful plasma ceramides compared to high carbohydrate or unsaturated fat diets [63]. Excess caloric intake and carbohydrates have also been linked to MASLD, particularly fructose (either found naturally, as added sugar, or as high fructose corn syrup). Elevated triglyceride levels and denovo lipogenesis have been seen in carbohydrate and fructose enriched diets [64,65]. However, the role of total caloric intake versus carbohydrate intake remains a challenging one, with some studies suggesting energy or caloric deficit is the mainstay of decreased liver fat accumulation with no extra benefit of carbohydrate restriction [66].
The role of specific diets in MASLD remains an area of active investigation. Weight loss seems to be beneficial in preventing or even reversing MASH and fibrosis [67]. Caloric restriction can be effective, whether achieved through hypocaloric diets or intermittent fasting. The role of very low carbohydrate diets (ketogenic diets), although resulting in weight loss, remains controversial for MASH reversal, especially in the long term. An increase in vegetable proteins rather than animal proteins may be beneficial, and the Mediterranean diet has been shown to be effective in reducing liver fat, even if not resulting in weight loss [65].
The limitations of our study include the NHANES cross-sectional design, due to which causality cannot be conclusively proven and there could be confounding factors. Given the nature of dietary intake collection, there could be recall and reporting bias. While there is no established CAP cutoff score to diagnose MASLD, 280 dB/m is a widely accepted threshold that has been used in prior studies. The major strengths of our study include a large, nationwide study population that is representative of the general United States population. Dietary intake was taken via a validated method on two separate occasions and Fibroscan® data was obtained via trained technicians with appropriately sized wands. At least 10 valid measurements were obtained with an interquartile range/median (IQR/M) of less than 30 % to reduce sampling error. Future animal and prospective studies would help better characterize the association between our findings.
5ConclusionsWe found that MASLD patients in the US general population are more often on special diets, suggesting increased awareness and interest in utilizing diet changes to improve health. We also found that certain nutritional variables, including vitamin E and vitamin K, may play a protective role against MASLD. Excessive intake of selenium in the US diet may be associated with an increased risk of MASLD, and increased intake of macronutrients, such as carbohydrates, cholesterol, total saturated fatty acids, and total caloric intake, may promote the development of MASLD. This research should prompt further prospective studies to confirm these findings. Such an association, if confirmed, would have the potential to offer specific dietary recommendations for individuals with MASLD as well as potential nutraceutical options.
FundingNone.
Author contributionsAll authors contributed to the conception and design of the study, interpretation of data, and drafting of the article, and provided final approval of the version to be submitted. Fay Osman and Adnan Said participated in data analysis, while Adnan Said also contributed to critically revising the article for important intellectual content.