The purpose of this study was to investigate the expression levels and prognostic roles of α-fetoprotein (AFP), carcinoembryonic antigen (CEA), and Ki67 in tumor tissues of intrahepatic cholangiocarcinoma (ICC) patients.
Patients or materials and methodsThe study involved ninety-two ICC patients with complete clinicopathological data and follow-up information, who had previously undergone radical surgery. AFP, CEA, CD10, CD34, and Ki67 were detected in tumor tissues using immunohistochemistry. Statistical tests were used to identify independent risk factors and their associations with overall survival (OS) and disease-free survival (DFS).
ResultsAFP, CEA and Ki67 were strongly correlated with prognosis. Univariate analysis indicated that higher AFP (P = 0.002), CEA (P < 0.0001), Ki67 (P < 0.0001), CA19-9 (P = 0.039), and CA12−5 (P = 0.002), and larger tumor size (P = 0.001), as well as more advanced tumor node metastasis (TNM) staging (P < 0.0001) were all associated with worse OS. Meanwhile, higher AFP (P = 0.002), CEA (P = 0.001), and Ki67 (P < 0.0001), as well as more advanced TNM staging (P = 0.005) were associated with worse DFS. Multivariate analysis showed that higher AFP (HR = 2.004, 95%CI: 1.146-3.504 P = 0.015), CEA (HR = 2.226, 95%CI: 1.283−3.861 P = 0.004), and Ki67 (HR = 3.785, 95%CI: 2.073−6.909 P < 0.0001), as well as more advanced TNM staging (HR = 2.900, 95%CI: 1.498−5.757 P = 0.002) had associations with worse OS. Furthermore, higher AFP (HR = 2.172, 95%Cl: 1.291−3.654 P = 0.003), CEA (HR = 1.934, 95%Cl: 1.180−3.169 P = 0.009), and Ki67 (HR = 2.203, 95%Cl: 1.291−3.761 P = 0.004) had associations with worse DFS.
ConclusionHigh AFP, CEA, and Ki67 are significant prognostic indicators in ICC patients, and can be used to evaluate ICC biological behavior and prognosis.
Intrahepatic cholangiocarcinoma (ICC), which is considered as the second most general primary liver malignancy, has been associated with a staggering increase in mortality over the past twenty years [1,2]. Compared with hepatocellular carcinoma (HCC), ICC is more malignant and has worse prognosis. Data from the recent five years have shown that the survival rate of ICC patients is less than 5% [3]. In addition, because of its high recurrence rate, the five-year survival rate after surgery is only 22–24% [4,5]. Hence, in the current study, we focused on the parameters which may predict the prognosis of ICC patients, such as α-fetoprotein (AFP), carcinoembryonic antigen (CEA), endothelial cell marker (CD10), endothelial cell marker (CD34), and Ki67. We retrospectively assessed 92 ICC cases, which had undergone surgical treatment in the Henan Province People’s Hospital in Zhengzhou, China. Based on the information regarding patient clinical characteristics, pathology, treatment, and prognosis, we determined whether these parameters can be used for a better understanding of the prognosis of patients. The objective of this study was to find novel prognostic factors for ICC in order to improve the prognosis of patients through the regulation of these factors.
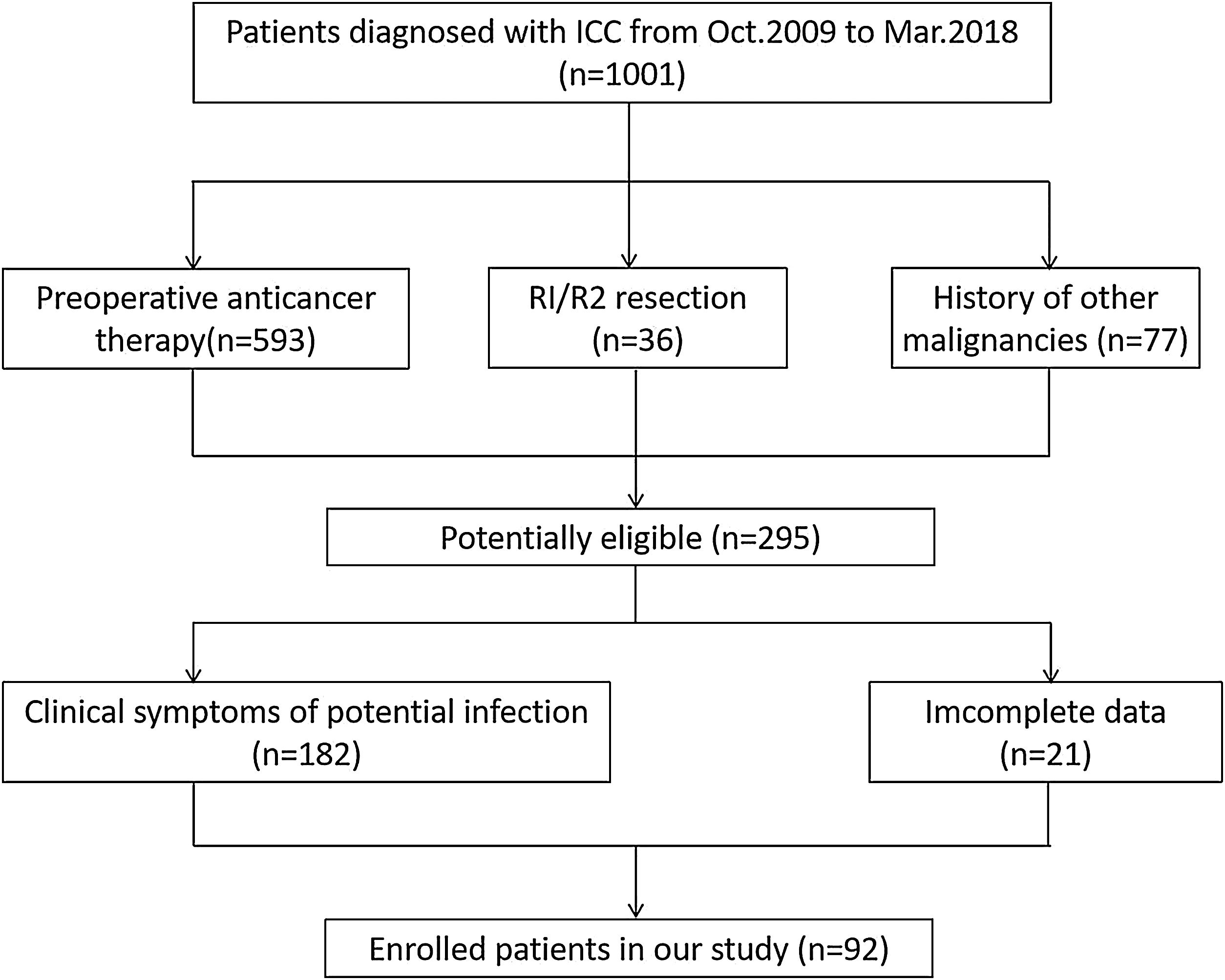
2Materials and methods2.1PatientsThe institutional review committee of the Henan University People’s Hospital board approved this retrospective research and ensured the consent of all patients. The research protocol was in accordance with the moral standard of the 1975 Declaration of Helsinki. Approximately 1001 patients with ICC who had underwent surgical treatment in the Henan Province People’s Hospital from October 2009 to March 2018 participated were followed up. The inclusion criteria were met as follows: (1) patients being diagnosed with ICC by CT, MRI and clinicopathological examinations, (2) without preoperative anticancer therapy, (3) without clinical symptoms of potential infection, (4) no history of other malignancies, (5) with complete resection, (6) with complete data. Exclusion criteria were those who were diagnosed as extrahepatic CCA or hepatocellular carcinoma. Finally, 92 patients were enrolled in this research (Fig. 1). Clinical datas such as age, gender, operation date, surgical resection range, tumor size, tumor number, vascular invasion, lymph node metastases, chemotherapy, some routine tumor marker expressions (including AFP, CD10, CD34, CEA, and Ki67), and survival status were recorded.
2.2Immunohistochemistry (IHC)Expression levels of AFP, CEA, and Ki67, as well as infiltrations of CD10 and CD34 cells, were evaluated by immunohistochemistry in 92 resected ICC samples. After continuous sectioning, tumor tissues were fixed with formaldehyde and embedded in paraffin. Then, the tissue chip was de-waxed and antigenic repair was carried out. The following antibodies were used in this study: anti-human AFP mouse monoclonal antibody (ADI, San Antonio, USA), anti-human CEA mouse monoclonal antibody, anti-CD10 rabbit monoclonal antibody, anti-CD34 rabbit monoclonal antibody, and rabbit polyclonal anti-Ki67 (Seebio, Shanghai, China). The experiment adopted the streptavidin–peroxidase method (SP-900, SP link detection kit; ZSGB-Bio, Beijing, China). Subsequently, 3,3’-diaminobiphenylamine (DAB) was used for color development and hematoxylin was used for 3 min. Then, the samples were dehydrated and dried using an ethanol gradient, treated with xylene, and sealed with neutral gum. Finally, the images were observed and analyzed under a light microscope (Olympus, CX31-LV320, Japan).
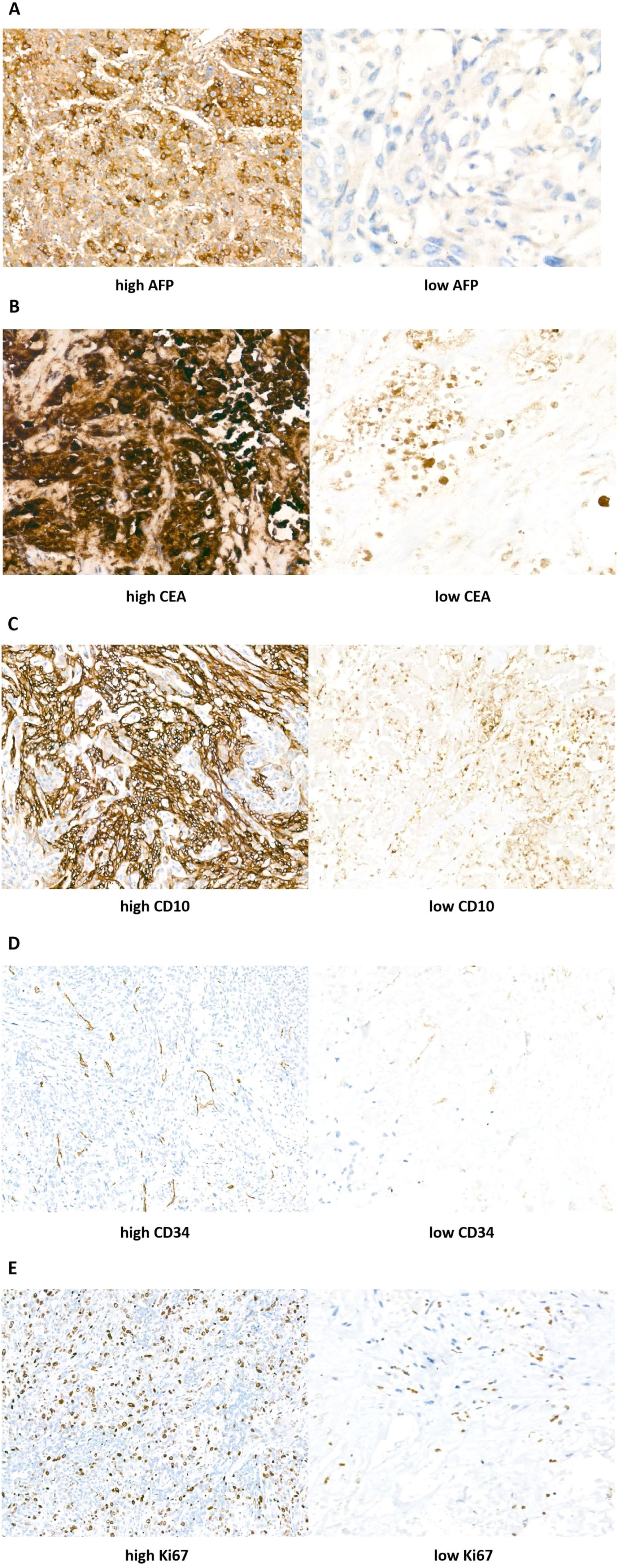
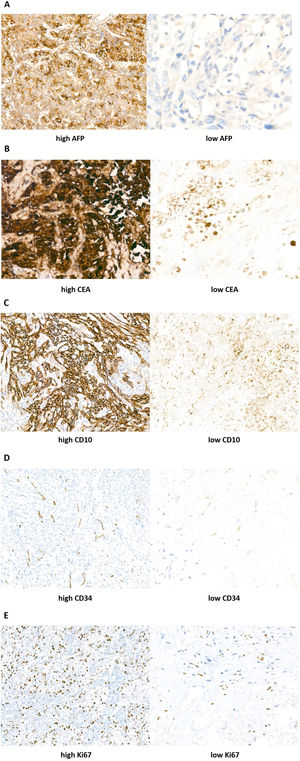
2.3IHC scoreSemi-quantitative IHC scores were determined by combining immunostaining intensity and the percentage of positive cells. The rate of positive staining refers to the percentage of positive cells within a population of stained cells. All tissue samples were examined and graded by three pathologists. The results were assessed as follows: the staining intensity was rated from 0 to 3 points corresponding to absent, weak, medium, and strong staining, respectively. The rate of positive staining was rated from 0% to 100%: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The immunohistochemistry score was obtained by multiplying the staining intensity by the rate of positive staining. The result would be considered as positively immuno-reactive if the immunohistochemistry score was greater than 2. Samples were then split into high expression group and low expression group according to the immunohistochemistry score (Fig. 2).
Immunohistochemistry (IHC) staining results of AFP, CEA, CD10, CD34, and Ki67 in ICC tumor tissues (Streptavidin–peroxidase conjugated method, 400× magnification). According to the intensity scores: (A) AFP expression was divided into high left. and low right. (B) CEA expression was divided into high left. and low right. (C) CD10 expression was divided into high left. and low right. (D) CD34 expression was divided into high left. and low right. (E) Ki67 expression was divided into high left. and low right.
Using univariate analysis to evaluate categorical variables, survival curves of OS and DFS were constructed using Kaplan–Meier curves. Survival differences were analyzed by log-rank tests. Multivariate Cox proportional hazard models were used to identify independent factors in the development of ICC. Hazard ratios (HR) were calculated using the Cox analysis and expressed as relative risks with corresponding 95% confidence intervals (CI). Statistical analyses were performed using SPSS 22.0 software (IBM Corp, Armonk, NY, USA). A P < 0.05 was considered statistically significant.
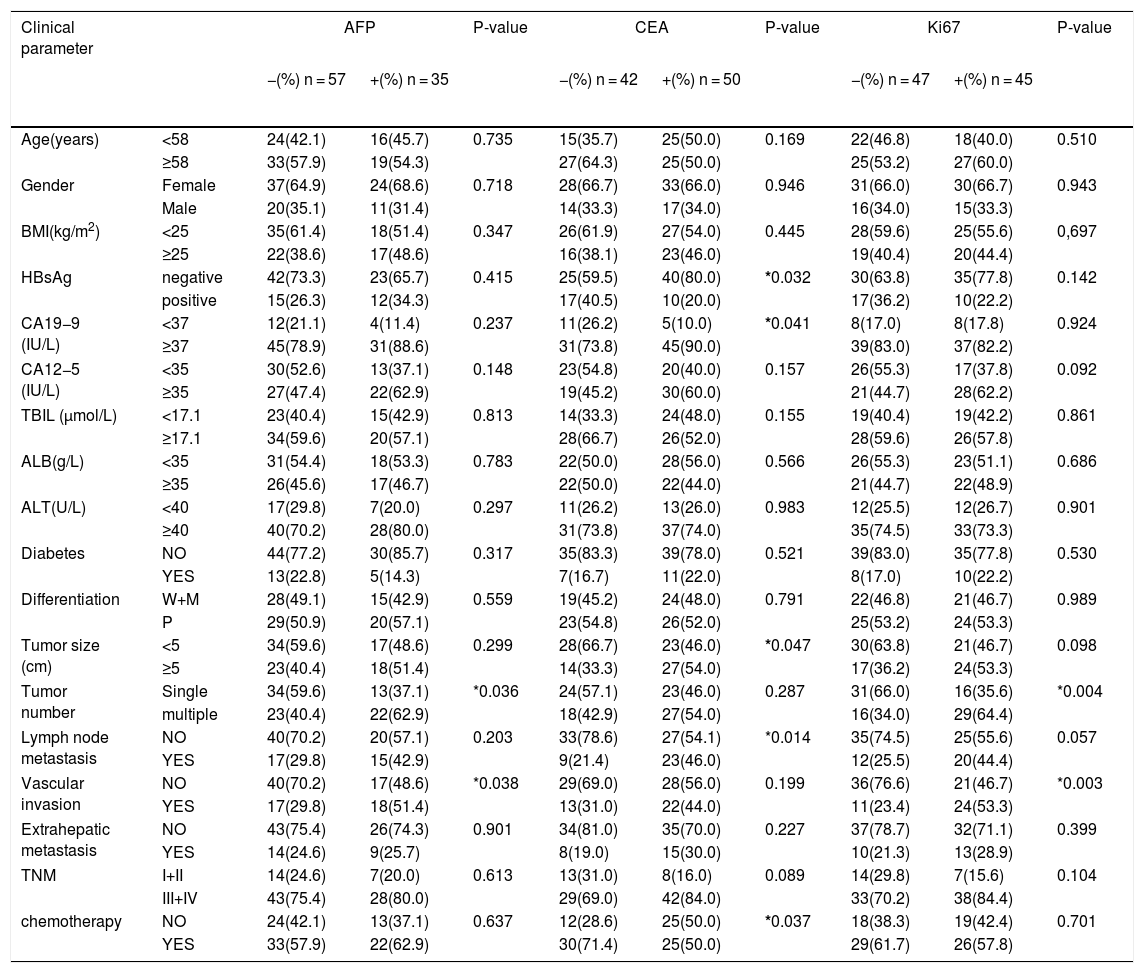
3Results3.1Patient clinical characteristicsWe explored the relation between AFP, CEA, KI67 and clinicopathological features in patients with Intrahepatic cholangiocarcinoma. Chi-square test showed that high expression of AFP was closely related to vascular invasion (P = 0.0038) and tumor number (P < 0.0036), but there was no significant correlation with age, gender, tumor size or lymph node metastasis. High expression of CEA was significantly associated with tumor size, CA19−9, HBV infection, chemotherapy and lymph node metastasis, but not with other parameters. In addition, in tumor-related factors, there were more patients with multiple primary tumors (P = 0.004) and vascular invasion (P = 0.003) in the high Ki67 group than in the low group. No significant differences were found between the two groups of Ki67 with respect to age, gender, tumor size, TNM, CA19−9, CA12−5, HBV infection or lymph node metastasis (Table 1).
Comparison of clinicopathological characteristics and intrahepatic cholangiocarcinoma tumor-related factors according to AFP, CEA, Ki67 expression.
| Clinical parameter | AFP | P-value | CEA | P-value | Ki67 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| −(%) n = 57 | +(%) n = 35 | −(%) n = 42 | +(%) n = 50 | −(%) n = 47 | +(%) n = 45 | |||||
| Age(years) | <58 | 24(42.1) | 16(45.7) | 0.735 | 15(35.7) | 25(50.0) | 0.169 | 22(46.8) | 18(40.0) | 0.510 |
| ≥58 | 33(57.9) | 19(54.3) | 27(64.3) | 25(50.0) | 25(53.2) | 27(60.0) | ||||
| Gender | Female | 37(64.9) | 24(68.6) | 0.718 | 28(66.7) | 33(66.0) | 0.946 | 31(66.0) | 30(66.7) | 0.943 |
| Male | 20(35.1) | 11(31.4) | 14(33.3) | 17(34.0) | 16(34.0) | 15(33.3) | ||||
| BMI(kg/m2) | <25 | 35(61.4) | 18(51.4) | 0.347 | 26(61.9) | 27(54.0) | 0.445 | 28(59.6) | 25(55.6) | 0,697 |
| ≥25 | 22(38.6) | 17(48.6) | 16(38.1) | 23(46.0) | 19(40.4) | 20(44.4) | ||||
| HBsAg | negative | 42(73.3) | 23(65.7) | 0.415 | 25(59.5) | 40(80.0) | *0.032 | 30(63.8) | 35(77.8) | 0.142 |
| positive | 15(26.3) | 12(34.3) | 17(40.5) | 10(20.0) | 17(36.2) | 10(22.2) | ||||
| CA19−9 (IU/L) | <37 | 12(21.1) | 4(11.4) | 0.237 | 11(26.2) | 5(10.0) | *0.041 | 8(17.0) | 8(17.8) | 0.924 |
| ≥37 | 45(78.9) | 31(88.6) | 31(73.8) | 45(90.0) | 39(83.0) | 37(82.2) | ||||
| CA12−5 (IU/L) | <35 | 30(52.6) | 13(37.1) | 0.148 | 23(54.8) | 20(40.0) | 0.157 | 26(55.3) | 17(37.8) | 0.092 |
| ≥35 | 27(47.4) | 22(62.9) | 19(45.2) | 30(60.0) | 21(44.7) | 28(62.2) | ||||
| TBIL (μmol/L) | <17.1 | 23(40.4) | 15(42.9) | 0.813 | 14(33.3) | 24(48.0) | 0.155 | 19(40.4) | 19(42.2) | 0.861 |
| ≥17.1 | 34(59.6) | 20(57.1) | 28(66.7) | 26(52.0) | 28(59.6) | 26(57.8) | ||||
| ALB(g/L) | <35 | 31(54.4) | 18(53.3) | 0.783 | 22(50.0) | 28(56.0) | 0.566 | 26(55.3) | 23(51.1) | 0.686 |
| ≥35 | 26(45.6) | 17(46.7) | 22(50.0) | 22(44.0) | 21(44.7) | 22(48.9) | ||||
| ALT(U/L) | <40 | 17(29.8) | 7(20.0) | 0.297 | 11(26.2) | 13(26.0) | 0.983 | 12(25.5) | 12(26.7) | 0.901 |
| ≥40 | 40(70.2) | 28(80.0) | 31(73.8) | 37(74.0) | 35(74.5) | 33(73.3) | ||||
| Diabetes | NO | 44(77.2) | 30(85.7) | 0.317 | 35(83.3) | 39(78.0) | 0.521 | 39(83.0) | 35(77.8) | 0.530 |
| YES | 13(22.8) | 5(14.3) | 7(16.7) | 11(22.0) | 8(17.0) | 10(22.2) | ||||
| Differentiation | W+M | 28(49.1) | 15(42.9) | 0.559 | 19(45.2) | 24(48.0) | 0.791 | 22(46.8) | 21(46.7) | 0.989 |
| P | 29(50.9) | 20(57.1) | 23(54.8) | 26(52.0) | 25(53.2) | 24(53.3) | ||||
| Tumor size (cm) | <5 | 34(59.6) | 17(48.6) | 0.299 | 28(66.7) | 23(46.0) | *0.047 | 30(63.8) | 21(46.7) | 0.098 |
| ≥5 | 23(40.4) | 18(51.4) | 14(33.3) | 27(54.0) | 17(36.2) | 24(53.3) | ||||
| Tumor number | Single | 34(59.6) | 13(37.1) | *0.036 | 24(57.1) | 23(46.0) | 0.287 | 31(66.0) | 16(35.6) | *0.004 |
| multiple | 23(40.4) | 22(62.9) | 18(42.9) | 27(54.0) | 16(34.0) | 29(64.4) | ||||
| Lymph node metastasis | NO | 40(70.2) | 20(57.1) | 0.203 | 33(78.6) | 27(54.1) | *0.014 | 35(74.5) | 25(55.6) | 0.057 |
| YES | 17(29.8) | 15(42.9) | 9(21.4) | 23(46.0) | 12(25.5) | 20(44.4) | ||||
| Vascular invasion | NO | 40(70.2) | 17(48.6) | *0.038 | 29(69.0) | 28(56.0) | 0.199 | 36(76.6) | 21(46.7) | *0.003 |
| YES | 17(29.8) | 18(51.4) | 13(31.0) | 22(44.0) | 11(23.4) | 24(53.3) | ||||
| Extrahepatic metastasis | NO | 43(75.4) | 26(74.3) | 0.901 | 34(81.0) | 35(70.0) | 0.227 | 37(78.7) | 32(71.1) | 0.399 |
| YES | 14(24.6) | 9(25.7) | 8(19.0) | 15(30.0) | 10(21.3) | 13(28.9) | ||||
| TNM | I+II | 14(24.6) | 7(20.0) | 0.613 | 13(31.0) | 8(16.0) | 0.089 | 14(29.8) | 7(15.6) | 0.104 |
| III+IV | 43(75.4) | 28(80.0) | 29(69.0) | 42(84.0) | 33(70.2) | 38(84.4) | ||||
| chemotherapy | NO | 24(42.1) | 13(37.1) | 0.637 | 12(28.6) | 25(50.0) | *0.037 | 18(38.3) | 19(42.4) | 0.701 |
| YES | 33(57.9) | 22(62.9) | 30(71.4) | 25(50.0) | 29(61.7) | 26(57.8) | ||||
Statistically significant correlation. The association between AFP, CEA, Ki67 and clinical variables were performed with Chi-square test. A P value <0.05 was regarded to be statistically significant. BMI: body mass index; HBsAg: Hepatitis B surface antigen; DM: Diabetes Mellitus; TNM: Tumor node metastasis; W: Well differentiated; M: Moderately differentiated; P: Poorly differentiated; ALB: albumin; TBIL: total bilirubin; ALT: alanine aminotransferase.
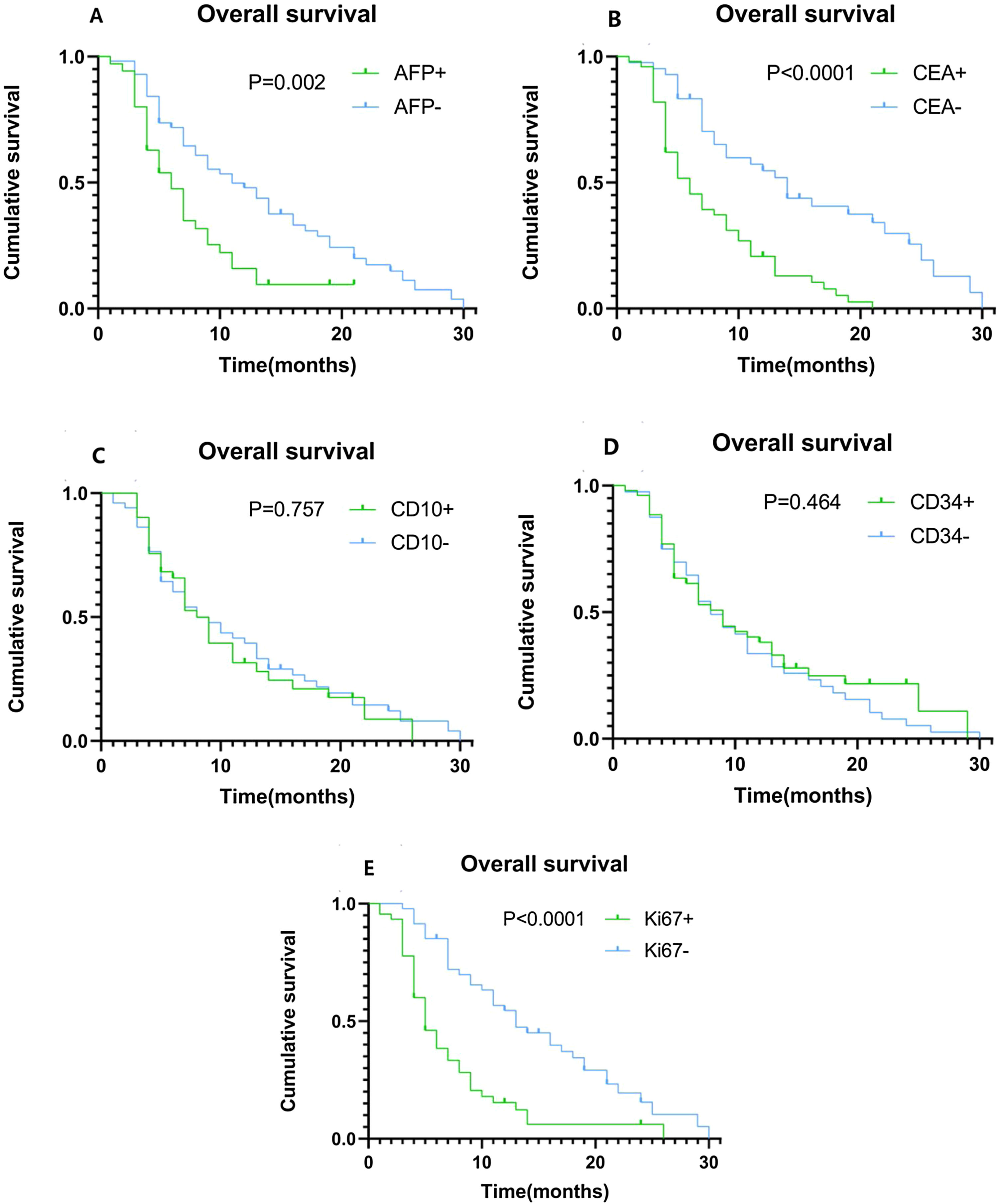
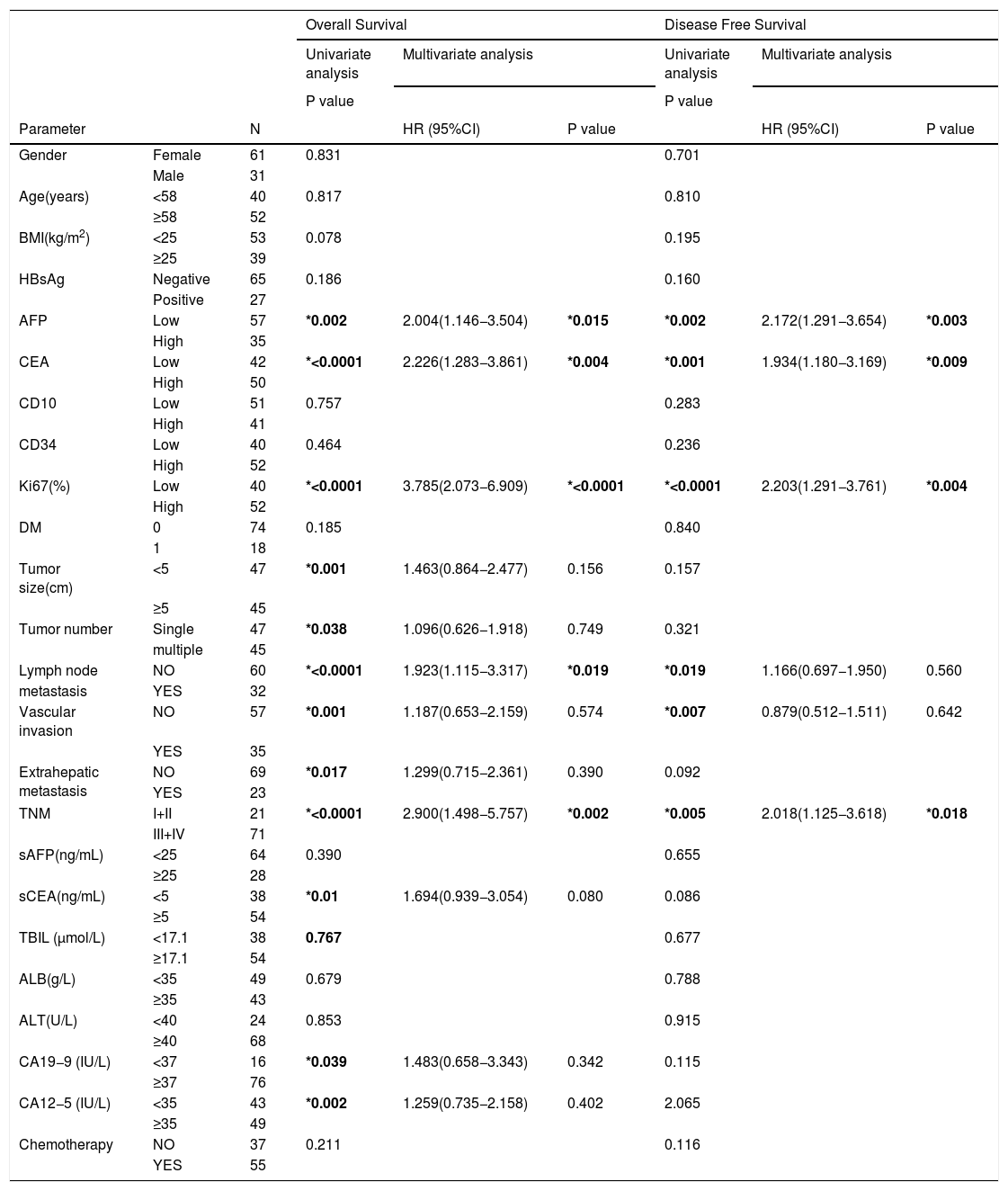
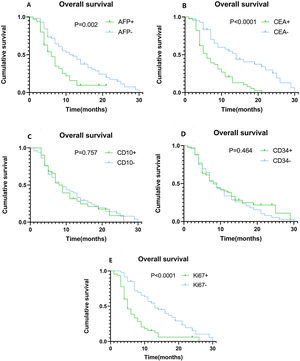
Using univariate analysis, we found that tumor size, tumor number, lymph node metastasis, vascular invasion, extrahepatic metastasis, TNM staging, as well as the expression levels of AFP, CEA, and Ki67 may be risk factors for ICC development. Age, gender, BMI, HBV infection, diabetes mellitus (DM), chemotherapy, tumor differentiation, CD10, and CD34 expression levels were not correlated with the risk of ICC development. The Cox regression proportional hazards model for multivariate analysis showed that AFP expression levels (HR = 2.004, 95%CI: 1.146−3.504 P = 0.015), CEA expression levels (HR = 2.226, 95%CI: 1.283−3.861 P = 0.004), Ki67 expression levels (HR = 3.785, 95%CI: 2.073−6.909 P < 0.0001), and TNM staging (HR = 2.900, 95%CI: 1.498−5.757 P = 0.002) are independent prognostic factors (Table 2). Kaplan–Meier plots of OS compared the high and low expression of AFP (Fig. 3A), CEA (Fig. 3B), CD10 (Fig. 3C), CD34 (Fig. 3D), and Ki67 (Fig. 3E). The OS time of ICC patients with high expression of AFP, CEA and Ki67 was significantly shorter than that of ICC patients with low expression of AFP, CEA and Ki67. When the same analysis was performed using CD10 and CD34, their expression levels did not clearly segregate the survival time.
Univariate and multivariate analysis of risk factors in relation to DFS and OS in ICC.
| Overall Survival | Disease Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| P value | P value | |||||||
| Parameter | N | HR (95%CI) | P value | HR (95%CI) | P value | |||
| Gender | Female | 61 | 0.831 | 0.701 | ||||
| Male | 31 | |||||||
| Age(years) | <58 | 40 | 0.817 | 0.810 | ||||
| ≥58 | 52 | |||||||
| BMI(kg/m2) | <25 | 53 | 0.078 | 0.195 | ||||
| ≥25 | 39 | |||||||
| HBsAg | Negative | 65 | 0.186 | 0.160 | ||||
| Positive | 27 | |||||||
| AFP | Low | 57 | *0.002 | 2.004(1.146−3.504) | *0.015 | *0.002 | 2.172(1.291−3.654) | *0.003 |
| High | 35 | |||||||
| CEA | Low | 42 | *<0.0001 | 2.226(1.283−3.861) | *0.004 | *0.001 | 1.934(1.180−3.169) | *0.009 |
| High | 50 | |||||||
| CD10 | Low | 51 | 0.757 | 0.283 | ||||
| High | 41 | |||||||
| CD34 | Low | 40 | 0.464 | 0.236 | ||||
| High | 52 | |||||||
| Ki67(%) | Low | 40 | *<0.0001 | 3.785(2.073−6.909) | *<0.0001 | *<0.0001 | 2.203(1.291−3.761) | *0.004 |
| High | 52 | |||||||
| DM | 0 | 74 | 0.185 | 0.840 | ||||
| 1 | 18 | |||||||
| Tumor size(cm) | <5 | 47 | *0.001 | 1.463(0.864−2.477) | 0.156 | 0.157 | ||
| ≥5 | 45 | |||||||
| Tumor number | Single | 47 | *0.038 | 1.096(0.626−1.918) | 0.749 | 0.321 | ||
| multiple | 45 | |||||||
| Lymph node | NO | 60 | *<0.0001 | 1.923(1.115−3.317) | *0.019 | *0.019 | 1.166(0.697−1.950) | 0.560 |
| metastasis | YES | 32 | ||||||
| Vascular invasion | NO | 57 | *0.001 | 1.187(0.653−2.159) | 0.574 | *0.007 | 0.879(0.512−1.511) | 0.642 |
| YES | 35 | |||||||
| Extrahepatic metastasis | NO | 69 | *0.017 | 1.299(0.715−2.361) | 0.390 | 0.092 | ||
| YES | 23 | |||||||
| TNM | I+II | 21 | *<0.0001 | 2.900(1.498−5.757) | *0.002 | *0.005 | 2.018(1.125−3.618) | *0.018 |
| III+IV | 71 | |||||||
| sAFP(ng/mL) | <25 | 64 | 0.390 | 0.655 | ||||
| ≥25 | 28 | |||||||
| sCEA(ng/mL) | <5 | 38 | *0.01 | 1.694(0.939−3.054) | 0.080 | 0.086 | ||
| ≥5 | 54 | |||||||
| TBIL (μmol/L) | <17.1 | 38 | 0.767 | 0.677 | ||||
| ≥17.1 | 54 | |||||||
| ALB(g/L) | <35 | 49 | 0.679 | 0.788 | ||||
| ≥35 | 43 | |||||||
| ALT(U/L) | <40 | 24 | 0.853 | 0.915 | ||||
| ≥40 | 68 | |||||||
| CA19−9 (IU/L) | <37 | 16 | *0.039 | 1.483(0.658−3.343) | 0.342 | 0.115 | ||
| ≥37 | 76 | |||||||
| CA12−5 (IU/L) | <35 | 43 | *0.002 | 1.259(0.735−2.158) | 0.402 | 2.065 | ||
| ≥35 | 49 | |||||||
| Chemotherapy | NO | 37 | 0.211 | 0.116 | ||||
| YES | 55 | |||||||
Statistically significant correlation. BMI: body mass index; sAFP: preoperative serum AFP; sCEA: preoperative serum CEA; AFP: α-fetoprotein; CEA: Carcinoembryonic antigen;HBsAg: Hepatitis B surface antigen; DM: Diabetes Mellitus; TNM: Tumor node metastasis; ALB: albumin; TBIL: total bilirubin; ALT: alanine aminotransferase.
Kaplan–Meier plots of overall survival (OS) contrasting positive versus negative expression of AFP, CEA, CD10, CD34, and Ki67. (A) Association between OS and AFP expression. Patients with high AFP expression had significantly shorter OS (P = 0.002). (B) Association between OS and CEA expression. Patients with high CEA expression had significantly shorter OS (P < 0.0001). (C) Association between OS and CD10 expression, showing no statistically significant difference. (D) Association between OS and CD34 expression, showing no statistically significant difference. (E) Association between OS and Ki67 expression. Patients with high Ki67 expression had significantly shorter OS (P < 0.0001).
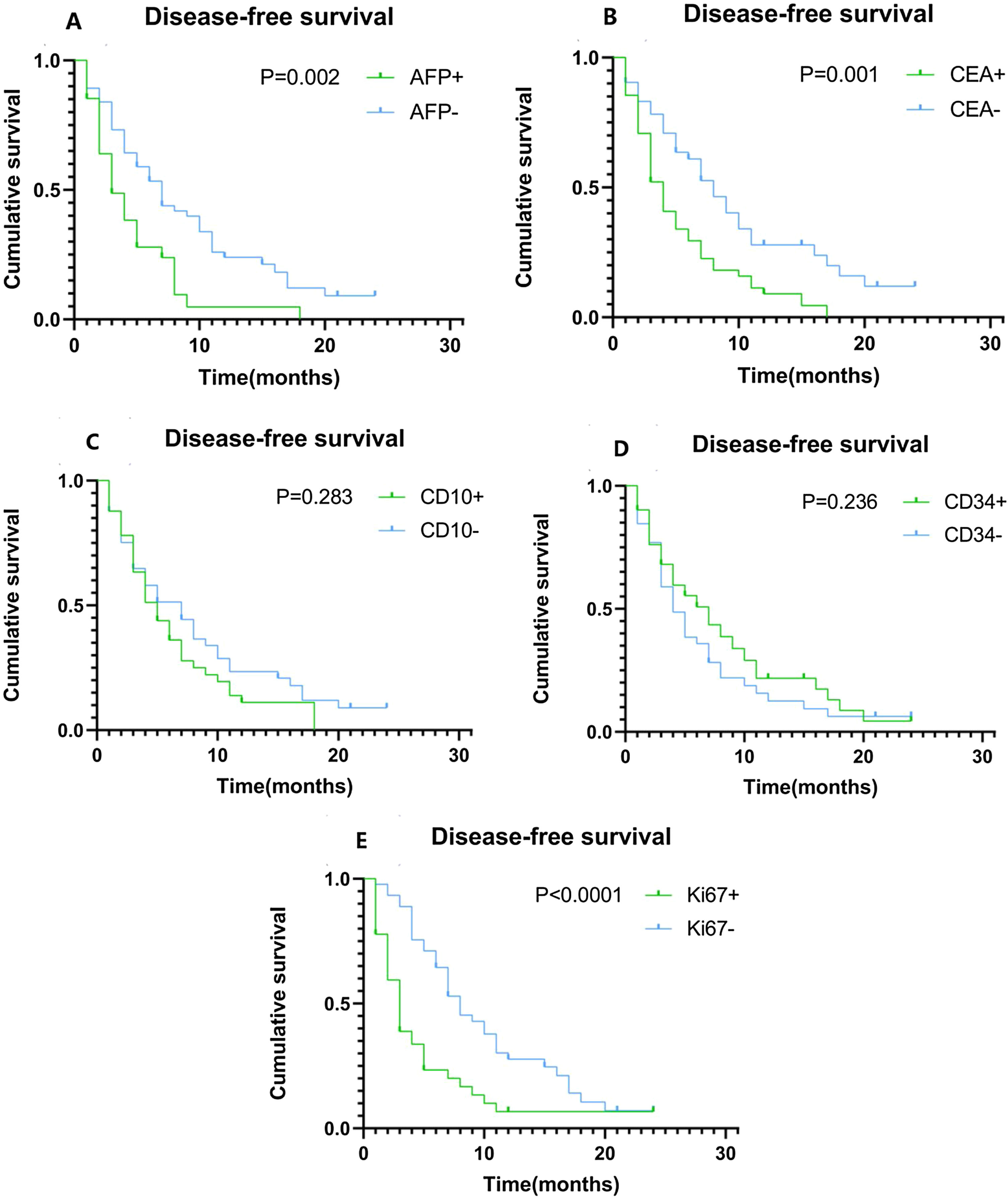
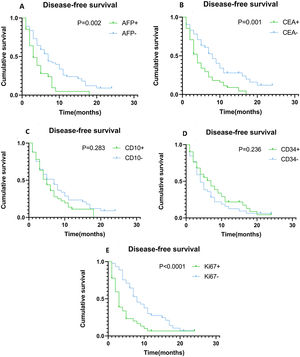
Univariate analysis showed that lymph node metastasis, vascular invasion and TNM, as well as the expression levels of AFP, CEA, and Ki67 are potential risk elements contributing to the development of ICC. The Cox regression proportional hazards model for multivariate analysis showed that AFP (HR = 2.172, 95%Cl: 1.291-3.654 P = 0.003), CEA (HR = 1.934, 95%Cl: 1.180−3.169 P = 0.009) and Ki67 (HR = 2.203, 95%Cl: 1.291−3.761 P = 0.004) are independent risk factors (Table 2). Hence, we speculate that they are the key factors to distinguish the recurrence and metastasis of the tumor. Kaplan–Meier plots of DFS compared high and low expression of AFP (Fig. 4A), CEA (Fig. 4B), CD10 (Fig. 4C), CD34 (Fig. 4D), and Ki67 (Fig. 4E). The DFS time of ICC patients with high expression of AFP, CEA and Ki67 was significantly shorter than that of ICC patients with low expression of AFP, CEA and Ki67. When the same analysis was performed for CD10 and CD34, there was no significant correlation between their expression levels and DFS time.
Kaplan–Meier plots of disease-free survival (DFS) contrasting high versus low expression of AFP, CEA, CD10, CD34, and Ki67. (A) Association between DFS and AFP expression. Patients with high AFP expression had significantly shorter DFS (P = 0.002). (B) Association between DFS and CEA expression. Patients with high CEA expression had significantly shorter DFS (P = 0.001). (C) Association between DFS and CD10 expression, showing no statistically significant difference. (D) Association between DFS and CD34 expression, showing no statistically significant difference. (E) Association between DFS and Ki67 expression. Patients with high Ki67 expression had significantly shorter DFS (P < 0.0001).
ICC is a hepatobiliary malignancy, which shows high invasive and poor clinical outcomes. The incidence and mortality rates of ICC have recently been increasing around the world. Therefore, it is vital to study the expression of proto-oncogenes, tumor suppressor genes, and related proteins during the development of ICC, and to identify markers that might be conducive to diagnosis, treatment, improvement of prognosis, and reduction of mortality. The relationship between different markers and clinical outcome of ICC has already been reported in many studies, however, the exact correlation remains controversial.
Ki67 is a nuclear antigen that was discovered by Gerdes et al. [6] in 1983; it is expressed in proliferating cells and due to its short half-life, it has become the most reliable indicator of cell proliferation. Many studies have shown that the overexpression of Ki67 is closely related to malignant biological behavior and the prognosis of skin cancer, gastric cancer, breast cancer, and other malignant tumors [7–9]. Nakagawa et al. [10] reported that the expression of Ki67 was significantly positively correlated with the differentiation of cholangiocarcinoma cells and negatively correlated with prognosis. Guo et al. [11] reported that Ki67 indirectly promoted proliferation and invasion of bile duct cancer cells by promoting the expression of CXCL7. Our results are aligned with these studies and show that high Ki67 is a strong independent factor associated with worse clinical outcome and a higher risk of relapse. We speculated that high expression levels of Ki67 may indicate an active proliferative stage in tumor cells resulting in the tumor being strongly aggressive with a high degree of metastasis. Besides, Ki67 is highly expressed in cancer cells but not in normal cells, so it might be considered as a novel therapeutic target for treating cancer. In previous studies, CD10 and CD34 have been used as first-line markers for the differential diagnoses of hepatocellular carcinoma and ICC, but have not shown any prognostic value [12]. In this study, we also evaluated the relationship between molecular biomarkers, such as CD10 and CD34, and the postoperative prognosis of ICC patients. However, we failed to conclude that these molecular biomarkers might have value in studying the prognosis of ICC patients. This might be due to the small sample size used in the current study.
No studies have particularly evaluated the prognostic value of AFP and CEA in ICC. AFP and CEA are common tumor markers that are used in clinical practice; they are mainly used for the detection of tumors in the breast as well as in the digestive and respiratory tracts [13–15]. When liver cells become cancerous, some cells recover the function of producing AFP, resulting in increased serum AFP levels. Therefore, AFP is widely used in the diagnosis of liver cancer [16]. Huang et al. [17] found that serum levels of AFP can independently used to study OS in HBV‐related ICC patients. Additionally, serum AFP level was positively correlated with its expression level in cancer tissues and the degree of differentiation of tumor tissues [18]. CEA is a tumor-related antigen extracted from colorectal adenocarcinoma and fetal intestinal tissue, and is a spectral tumor marker used for studying metastatic gastric carcinoma, hepatic carcinoma, lung carcinoma, and colorectal carcinoma [19]. At present, AFP and CEA are generally used in the clinical diagnosis of hepatobiliary malignancies, but their expression levels in tumor tissues have only been used to predict patient prognosis in few studies [13,20]. We were the first to in-depth discuss the distinctive prognostic role of AFP in ICC. In this study, we found that patients with high expression of AFP and CEA in tumor tissues had poorer OS. Notably, we also found that patients with high CEA expression in primary lesions had a higher incidence of lymph node metastasis, so we speculated that high CEA expression might be associated with distant metastasis of tumors. Therefore, more studies are needed on the role of AFP and CEA in tumor invasion, metastasis and proliferation.
The TNM staging system is the most commonly used tumor staging system in the world. It was first proposed by Pierre Denoix [21]. We found that TNM staging was also an independent risk factor for the prognosis of ICC patients. Shen et al. [22] suggested that resection range, lymph node metastasis, and gross cancer embolus were all factors that can influence ICC prognosis. Jong et al. [23] considered that the prognosis was related to tumor number, venous invasion, and lymph node metastasis. In addition, Wang et al. [24] also reported that serum CEA, CA 19−9, tumor diameter and number, venous invasion, and lymph node metastasis were independent prognostic factors. In the present study, we only found lymph node metastasis was an independent prognostic factor. Hence, further investigation is required to probe this question.
This study has the following limitations: (a) The exact mechanisms of AFP, CEA, and Ki67 in tumor progression in ICC remain to be further elucidated. (b) This paper was based on a single-center-based cohort study, which inevitably led to case selection bias. Hence, it will be more convincing if the results were confirmed by another multicenter cohort study. (c) This was a retrospective study on people who had been diagnosed with ICC. Some patients entered the second stage during the study period, causing the results to be biased.
5ConclusionsIn summary, the results of our study provide a new basis for the diagnosis, treatment, and prognosis of ICC patients. According to our univariate and multivariate analyses, AFP, CEA, and Ki67 were found to be strong and independent predictors of poor prognosis in patients diagnosed with ICC. And it may enable an exact prediction of tumor recurrence after operation by assessing AFP, CEA, Ki67. The biological roles of AFP, CEA Ki67 in physiological and pathological state still remains unclear. Therefore, the roles of CEA, AFP and Ki67 in tumor progression deserve further investigation.
Authors’ contributionsQZ is the first author. QZ and YH designed the study. QZ, ZW, and HY collected the data. QZ and TL were involved in data cleaning, mortality follow-up, and verification. ZW and DK analyzed the data. QZ drafted the manuscript. QZ and YH contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors have read and approved the final manuscript. QZ and YH are the study guarantors. All authors read and approved the final manuscript.
FundingThis study was supported by grants from the Henan Medical Science and Technology Research Project, the effect of ADAR1 on the proliferation, invasion and metastasis of liver cancer by regulating microRNA, (201502018).
Ethics approval and consent to participateThis retrospective study was approved by the Ethics Committee of Henan University People’s Hospital All methods were conducted in accordance with the approved guidelines.
Conflict of interestThe authors have no conflicts of interest to declare.