Among all immune cells, natural killer (NK) cells play an important role as the first line of defense against tumor. The purpose of our study is to observe whether the NK cell counts can predict the overall survival of patients with hepatocellular carcinoma (HCC).
MethodsTo develop a novel model, from January 2010 to June 2015, HCC patients enrolled in Beijing Ditan hospital were divided into training and validation cohort. Cox multiple regression analysis was used to analyze the independent risk factors for 1-year, 3-year and 5-year overall survival (OS) of patients with HCC, and the nomogram was used to establish the prediction model. In addition, the decision tree was established to verify the contribution of NK cell counts to the survival of patients with HCC.
ResultsThe model used in predicting overall survival of HCC included six variables (namely, NK cell counts, albumin (ALB) level, alpha-fetoprotein (AFP) level, portal vein tumor thrombus (PVTT), tumor number and treatment). The C-index of nomogram model in HCC patients predicting 1-year, 3-year and 5-year overall survival was 0.858, 0.788 and 0.782 respectively, which was higher than tumor–lymph node–metastasis (TNM) staging system, Okuda, model for end-stage liver disease (MELD), MELD-Na, the Chinese University Prognostic Index (CUPI) and Japan Integrated Staging (JIS) scores (p < 0.001). The decision tree showed the specific 5-year OS probability of HCC patients under different risk factors, and found that NK cell counts were the third in the column contribution.
ConclusionsOur study emphasizes the utility of NK cell counts for exploring interactions between long-term survival of HCC patients and predictor variables.
According to the 2020 global cancer statistics [1], the mortality rate of hepatocellular carcinoma (HCC) ranks third in the world. Every year, there are about 841,000 new cases of HCC, and the number of deaths is approximately 782,000, while the mortality rate of HCC in China accounts for half of the global population.
Immunotherapy is a major breakthrough in the field of cancer therapy in recent years, and the potential of natural killer (NK) cells for cancer immunotherapy has gradually become well known. NK cells play an important role in the innate immune response. When infected with a virus and the tumor microenvironment, NK cells play a particularly important role as the first line of defense [2, 3]. In the tumor microenvironment, the immune surveillance function of NK cells is impaired. Furthermore, the failure of NK cells is mainly manifested as a decrease in NK cell counts and function [4, 5]. A number of previous studies have shown that the number of NK cells in patients with HCC is reduced [6, 7], and a decrease in the proportion of NK cell subsets is associated with poor prognosis [8, 9]. However, the effect of reduced NK cell count on long-term prognosis in patients with HCC is unclear. Therefore, we aimed to analyze the correlation between NK cell counts detected by clinical objective laboratory indicators and the survival outcome of patients with HCC.
Currently, several well-known tumor prediction models are commonly used clinical prediction tools, including the Barcelona Clinic Liver Cancer (BCLC) [10], tumor–lymph node–metastasis (TNM) staging system [11], Okuda staging system [12], model for end-stage liver disease (MELD) [13], Cancer of the Liver Italian Program (CLIP) [14], the Chinese University Prognostic Index (CUPI) [15], and Japan Integrated Staging (JIS) [16]. These classic and recognized models only consider clinically relevant indicators (including liver function and tumor-related indicators), but have not yet involved immune indicators and the tumor microenvironment [17–19].
Nomograms are currently considered a simple visual and reliable tool for predicting risk by incorporating important related factors [20, 21]. The aim of the current study was to combine clinical and immunological indicators that are associated with the overall survival of patients with HCC into a prediction model. It also aimed to determine the value of immunological indicators (i.e., NK cell counts) in predicting the survival of patients with HCC.
2Patients and methods2.1Study design and populationThe retrospective cohort (n = 190) was divided into training and validation cohorts. The training cohort comprised patients with HCC from Beijing Ditan Hospital between January 1, 2010, and December 31, 2013, and the validation cohort included patients with HCC from Beijing Ditan Hospital between January 1, 2014, and June 30, 2015. Overall survival (OS) was defined as the interval between the date of diagnosis and the occurrence of death or the end of follow-up. The survival group was defined as having no deaths during the follow-up period. The death group was defined as death from any cause between the date of diagnosis and the end of the follow-up period. Our follow-up method was to follow up every three months by telephone and consult the medical record system. The inclusion criteria were as follows: (1) patients with HCC who were diagnosed according to the 2019 China guidelines (based on biopsy, radiology, and alpha-fetoprotein [AFP level ≥ 400 ng/mL] serology results) [22]; (2) those with complete clinical data, including immunoassays (NK cell counts); and (3) those with more than 5 years of follow-up in the retrospective cohort study. The exclusion criteria were as follows: (1) pregnant women, (2) those younger than 18 years, (3) those with incomplete data, and (4) those with <5 years of follow-up. The study was approved by the Ethics Committee of Beijing Ditan Hospital.
2.2Calculation of NK cell countsBefore treatment, 5ml fasting venous blood were collected from the patients and were placed in EDTA anticoagulant blood collection tubes. Absolute cell counts were determined using TruCOUNT Tubes (BD) and a lyse-no-wash method according to the manufacturer's instructions by FACS Calibur flow cytometer (BD Biosciences). NK cell counts were detected using a MulitiTEST IMK kit (CD3/CD16/+CD56/CD45/CD19 Reagent, BD FACS Iysing solution) and TruCOUNT tubes, and analyzed automatically using FACSMULTISET software. NK cell-surface markers were detected using the following reagents for flow cytometry: anti-human CD3-BV786, anti-CD56-BV510, and anti-CD16-BV711 antibodies. NK cells were CD3−CD16+ and/or CD56+ lymphocytes.
2.3Demographic and clinical dataWe included important variables related to OS of patients with HCC, including age, sex, etiology of HCC, HBV DNA, antiviral therapy, portal vein tumor thrombus at baseline, cirrhosis, metastasis, Child-Pugh stage, BCLC stage, tumor number, tumor size, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), γ-glutamyl transferase (γ-GGT), total bilirubin (TBIL), albumin (ALB), prothrombin time activity (PTA), alanine aminotransferase (ALT), alpha-fetoproteinlevel (AFP), NK/lymphocytes, and NK absolute count. Consistent with our previous research, the treatment methods were classified as follows [23]: palliative treatment, minimally invasive treatment, and resection treatment. Palliative treatment was defined as supportive treatment. Minimally invasive treatment was considered to be local treatment of cancer cells or areas surrounding the cancer cells, which mainly included radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and microwave ablation (MWA). Resection treatment was defined ascurative resection, including partial resection and liver transplantation. Baseline data were obtained at the first HCC diagnosis in the Beijing Ditan Hospital.
2.4Statistical analysisAll statistical analyses were performed using SPSS 22.0. Categorical variables were analyzed using the χ2 test or Fisher's exact probability test. Cox proportional regression analysis was performed to identify the independent prognostic factors of HCC. The 5-year OS rates were compared using the Kaplan–Meier method. Based on the multivariate analysis results, the nomogram model was established using rms in the R project version 3.4.2 package (http://www.rproject.org/). A calibration curve was used to evaluate the calibration degree between the predicted probability and actual probability of the nomogram model. The concordance index (C-index) was used to test the degree of discrimination of the model.
Subsequently, the decision tree was drawn with JMP pro version 15.2, as a method of machine learning, which can directly show the mapping relationship between predictors and outcomes. Decision trees visually demonstrate the 5-year OS rate of patients affected by different risk factors for HCC.
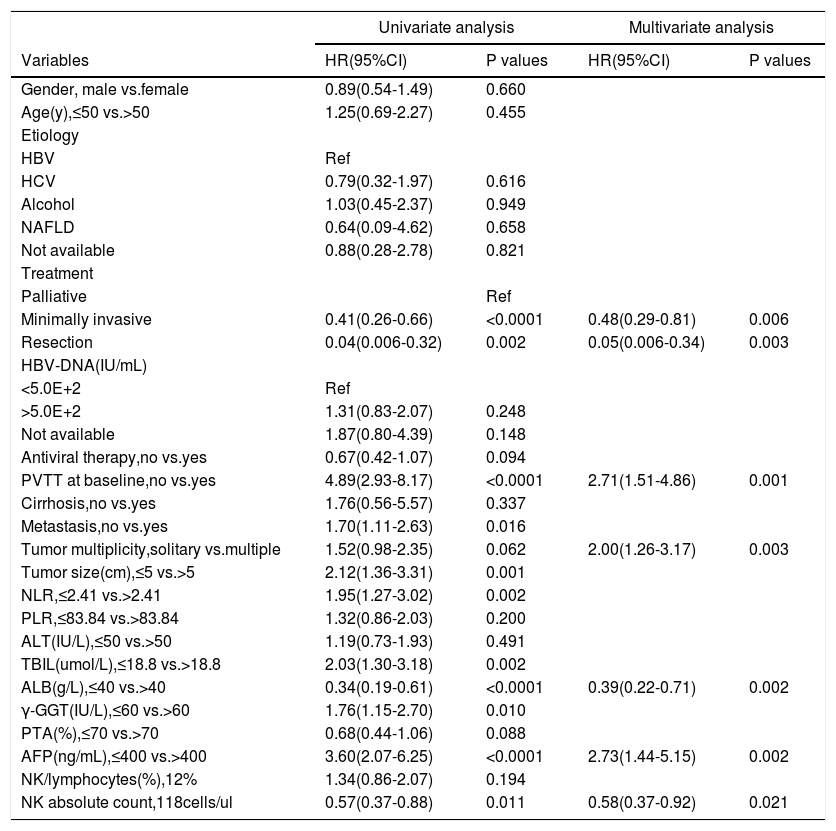
3Results3.1Characteristics of the retrospective cohort of patients with HCCThe retrospective cohort (n = 190) was divided into training (n = 139) and validation cohorts (n = 51). The characteristics of patients with HCC are presented in detail in Table 1. All demographic and clinical values of the two groups were balanced and comparable (p > 0.05). Among all patients with HCC, hepatitis B-related HCC accounted for 82.1%, and 143 (75.3%) received antiviral therapy. Among the different treatment methods, 68.9% of patients with HCC received local minimally invasive treatment, the remaining 10.6% received resection treatment, and 20.5% received palliative treatment. In the training cohort, of the 139 patients whose age ranged from 20 to 89 years, 81.3% were over 50 years and 106 (76.3%) were male, which showed male and elderly dominance. Overall, 114 (82.0%) patients with HCC were caused by HBV infection, and 104 (74.8%) patients were treated with antiviral therapy. The treatment method was minimally invasive (71.2%). According to the clinical outcome, they were divided into the survival group (n = 55) and the death group (n = 84). In the death group, 28.6% of HCC patients received conservative treatment, 21 (25%) had PVTT at baseline, 38 (45.2%) had metastasis, 12 (14.3%) had Child-Pugh C, 31 (36.9%) had BCLC stage C-D, and 31 (36.9%) had tumor size>5 cm. Meanwhile, compared with patients in the survival group, patients in the death group had higher levels of NLR, TBIL, γ-GGT, and AFP, while the levels of ALB and NK cell counts were lower than those in the survival group (Table S1, p < 0.05).
Characteristics of patients with hepatocellular carcinoma
| Demographic and clinical values | Total | Training Cohort | Validation Cohort | P Value |
|---|---|---|---|---|
| n=190(%) | n=139(%) | n=51(%) | ||
| Gender | 0.232 | |||
| Male | 149(78.4) | 106(76.3) | 43(84.3) | |
| Female | 41(21.6) | 33(23.7) | 8(15.7) | |
| Age(y) | 0.461 | |||
| ≤50 | 38(20.0) | 26(18.7) | 12(23.5) | |
| >50 | 152(80.0) | 113(81.3) | 39(76.5) | |
| Etiology | 0.872 | |||
| HBV | 156(82.1) | 114(82.0) | 42(82.3) | |
| HCV | 12(6.3) | 9(6.5) | 3(5.9) | |
| Alcohol | 12(6.3) | 9(6.5) | 3(5.9) | |
| NAFLD | 2(1.1) | 2(1.4) | 0(0.0) | |
| Not available | 8(4.2) | 5(3.6) | 3(5.9) | |
| HBV-DNA(IU/mL) | 0.729 | |||
| <5.0E+2 | 110(57.9) | 82(59.0) | 28(54.9) | |
| >5.0E+2 | 69(36.3) | 50(36.0) | 19(37.3) | |
| Not available | 11(5.8) | 7(5.0) | 4(7.8) | |
| Treatment | 0.151 | |||
| Palliative | 39(20.5) | 29(20.9) | 10(19.6) | |
| Minimally invasive | 131(68.9) | 99(71.2) | 32(62.7) | |
| Resection | 20(10.6) | 11(7.9) | 9(17.7) | |
| Antiviral therapy(yes) | 143(75.3) | 104(74.8) | 39(76.5) | 0.815 |
| PVTT at baseline(yes) | 27(14.2) | 22(15.8) | 5(9.8) | 0.292 |
| Cirrhosis(yes) | 181(95.3) | 132(95.0) | 49(96.1) | 0.749 |
| Metastasis(yes) | 69(36.3) | 50(36.0) | 19(37.3) | 0.870 |
| Child-Pugh Stage | 0.284 | |||
| A | 109(57.4) | 75(54.0) | 34(66.7) | |
| B | 65(34.2) | 51(36.7) | 14(27.5) | |
| C | 16(8.4) | 13(9.3) | 3(5.8) | |
| BCLC stage | 0.546 | |||
| 0-B | 147(77.4) | 106(76.3) | 41(80.4) | |
| C-D | 43(22.6) | 33(23.7) | 10(19.6) | |
| Tumor multiplicity | 0.398 | |||
| Solitary | 125(65.8) | 89(64.0) | 36(70.6) | |
| Multiple | 65(34.2) | 50(36.0) | 15(29.4) | |
| Tumor size(cm) | 0.611 | |||
| ≤5 | 132(69.5) | 98(70.5) | 34(66.7) | |
| >5 | 58(30.5) | 41(29.5) | 17(33.3) | |
| NLR | 0.752 | |||
| ≤2.41 | 97(51.1) | 70(50.4) | 27(52.9) | |
| >2.41 | 93(48.9) | 69(49.6) | 24(47.1) | |
| PLR | 0.377 | |||
| ≤83.84 | 92(48.4) | 70(50.4) | 22(43.1) | |
| >83.84 | 98(51.6) | 69(49.6) | 29(56.9) | |
| ALT (U/L) | 0.607 | |||
| Normal ≤50 | 144(75.8) | 104(74.8) | 40(78.4) | |
| High>50 | 46(24.2) | 35(25.2) | 11(21.6) | |
| TBIL(umol/L) | 0.141 | |||
| Normal ≤18.8 | 95(50.0) | 65(46.8) | 30(58.8) | |
| High>18.8 | 95(50.0) | 74(53.2) | 21(41.2) | |
| ALB(g/L) | 0.204 | |||
| Normal ≤40 | 129(67.9) | 98(70.5) | 31(60.8) | |
| High>40 | 61(32.1) | 41(29.5) | 20(39.2) | |
| γ-GGT(IU/L) | 0.651 | |||
| Normal≤60 | 102(53.7) | 76(54.7) | 26(51.0) | |
| High>60 | 88(46.3) | 63(45.3) | 25(49.0) | |
| PTA(%) | 0.274 | |||
| Low≤70 | 60(31.6) | 47(33.8) | 13(25.5) | |
| High>70 | 130(68.4) | 92(66.2) | 38(74.5) | |
| AFP(ng/mL) | 0.054 | |||
| Low≤400 | 158(83.2) | 120(86.3) | 38(74.5) | |
| High>400 | 32(16.8) | 19(13.7) | 13(25.5) | |
| NK/lymphocytes(%) | 0.506 | |||
| Low≤12 | 82(43.2) | 62(44.6) | 20(39.2) | |
| High>12 | 108(56.8) | 77(55.4) | 31(60.8) | |
| NK absolute count(cells/uL) | 0.520 | |||
| Low≤118 | 93(48.9) | 70(50.4) | 23(45.1) | |
| High>118 | 97(51.1) | 69(49.6) | 28(54.9) | |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; BCLC, Barcelona Clinic Liver Cancer classification system; PVTT, portal vein tumor thrombus; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ALT, alanine aminotransferase; TBIL, total bilirubin; ALB, albumin; γ-GGT, γ-glutamyl transferase; PTA, prothrombin time activity; AFP, alpha-fetoprotein.
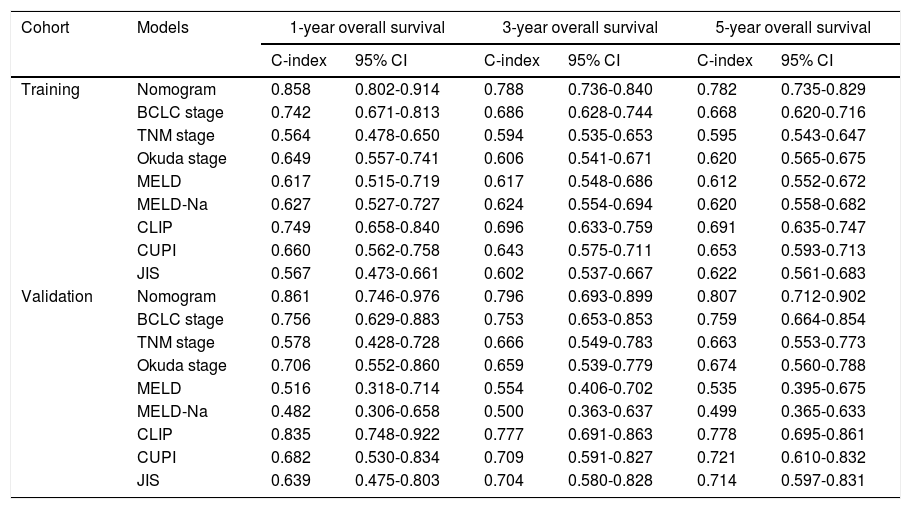
Cox proportional regression analysis was performed to evaluate the effect of clinical variables on the outcomes of patients with HCC. Univariate analysis showed that treatment, PVTT at baseline, antiviral therapy, metastasis, tumor number, tumor size, NLR, ALB, TBIL, γ-GGT, PTA, AFP, and NK cell absolute counts were significantly associated with survival in the training cohort (p< 0.1, Table 2). The above variables were entered into Cox multivariate analysis. This analysis revealed that treatment (minimally invasive, hazard ratio [HR] = 0.48, 95% confidence interval [CI]: 0.29-0.81, p = 0.006; resection treatment, HR = 0.05; 95% CI: 0.006-0.34, p = 0.003), PVTT at baseline (HR = 2.71, 95% CI: 1.51-4.86, p = 0.001), tumor multiplicity (HR = 2.00, 95% CI: 1.26-3.17, p = 0.003), ALB level (>40g/L; HR = 0.39, 95% CI: 0.22-0.71, p = 0.002), AFP level (> 400 ng/mL, HR = 2.73, 95% CI: 1.44-5.15, p = 0.002), and NK cell absolute count (>118 cells/μL, HR = 0.58, 95% CI: 0.37-0.92, p = 0.021) were the independent prognostic predictors of HCC (Table 2).
Univariate and multivariate Cox regression analyses for 5-year OS in patients with HCC
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR(95%CI) | P values | HR(95%CI) | P values |
| Gender, male vs.female | 0.89(0.54-1.49) | 0.660 | ||
| Age(y),≤50 vs.>50 | 1.25(0.69-2.27) | 0.455 | ||
| Etiology | ||||
| HBV | Ref | |||
| HCV | 0.79(0.32-1.97) | 0.616 | ||
| Alcohol | 1.03(0.45-2.37) | 0.949 | ||
| NAFLD | 0.64(0.09-4.62) | 0.658 | ||
| Not available | 0.88(0.28-2.78) | 0.821 | ||
| Treatment | ||||
| Palliative | Ref | |||
| Minimally invasive | 0.41(0.26-0.66) | <0.0001 | 0.48(0.29-0.81) | 0.006 |
| Resection | 0.04(0.006-0.32) | 0.002 | 0.05(0.006-0.34) | 0.003 |
| HBV-DNA(IU/mL) | ||||
| <5.0E+2 | Ref | |||
| >5.0E+2 | 1.31(0.83-2.07) | 0.248 | ||
| Not available | 1.87(0.80-4.39) | 0.148 | ||
| Antiviral therapy,no vs.yes | 0.67(0.42-1.07) | 0.094 | ||
| PVTT at baseline,no vs.yes | 4.89(2.93-8.17) | <0.0001 | 2.71(1.51-4.86) | 0.001 |
| Cirrhosis,no vs.yes | 1.76(0.56-5.57) | 0.337 | ||
| Metastasis,no vs.yes | 1.70(1.11-2.63) | 0.016 | ||
| Tumor multiplicity,solitary vs.multiple | 1.52(0.98-2.35) | 0.062 | 2.00(1.26-3.17) | 0.003 |
| Tumor size(cm),≤5 vs.>5 | 2.12(1.36-3.31) | 0.001 | ||
| NLR,≤2.41 vs.>2.41 | 1.95(1.27-3.02) | 0.002 | ||
| PLR,≤83.84 vs.>83.84 | 1.32(0.86-2.03) | 0.200 | ||
| ALT(IU/L),≤50 vs.>50 | 1.19(0.73-1.93) | 0.491 | ||
| TBIL(umol/L),≤18.8 vs.>18.8 | 2.03(1.30-3.18) | 0.002 | ||
| ALB(g/L),≤40 vs.>40 | 0.34(0.19-0.61) | <0.0001 | 0.39(0.22-0.71) | 0.002 |
| γ-GGT(IU/L),≤60 vs.>60 | 1.76(1.15-2.70) | 0.010 | ||
| PTA(%),≤70 vs.>70 | 0.68(0.44-1.06) | 0.088 | ||
| AFP(ng/mL),≤400 vs.>400 | 3.60(2.07-6.25) | <0.0001 | 2.73(1.44-5.15) | 0.002 |
| NK/lymphocytes(%),12% | 1.34(0.86-2.07) | 0.194 | ||
| NK absolute count,118cells/ul | 0.57(0.37-0.88) | 0.011 | 0.58(0.37-0.92) | 0.021 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; PVTT, portal vein tumor thrombus; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ALT, alanine aminotransferase; TBIL, total bilirubin; ALB, albumin; γ-GGT, γ-glutamyl transferase; PTA, prothrombin time activity; AFP, alpha-fetoprotein.
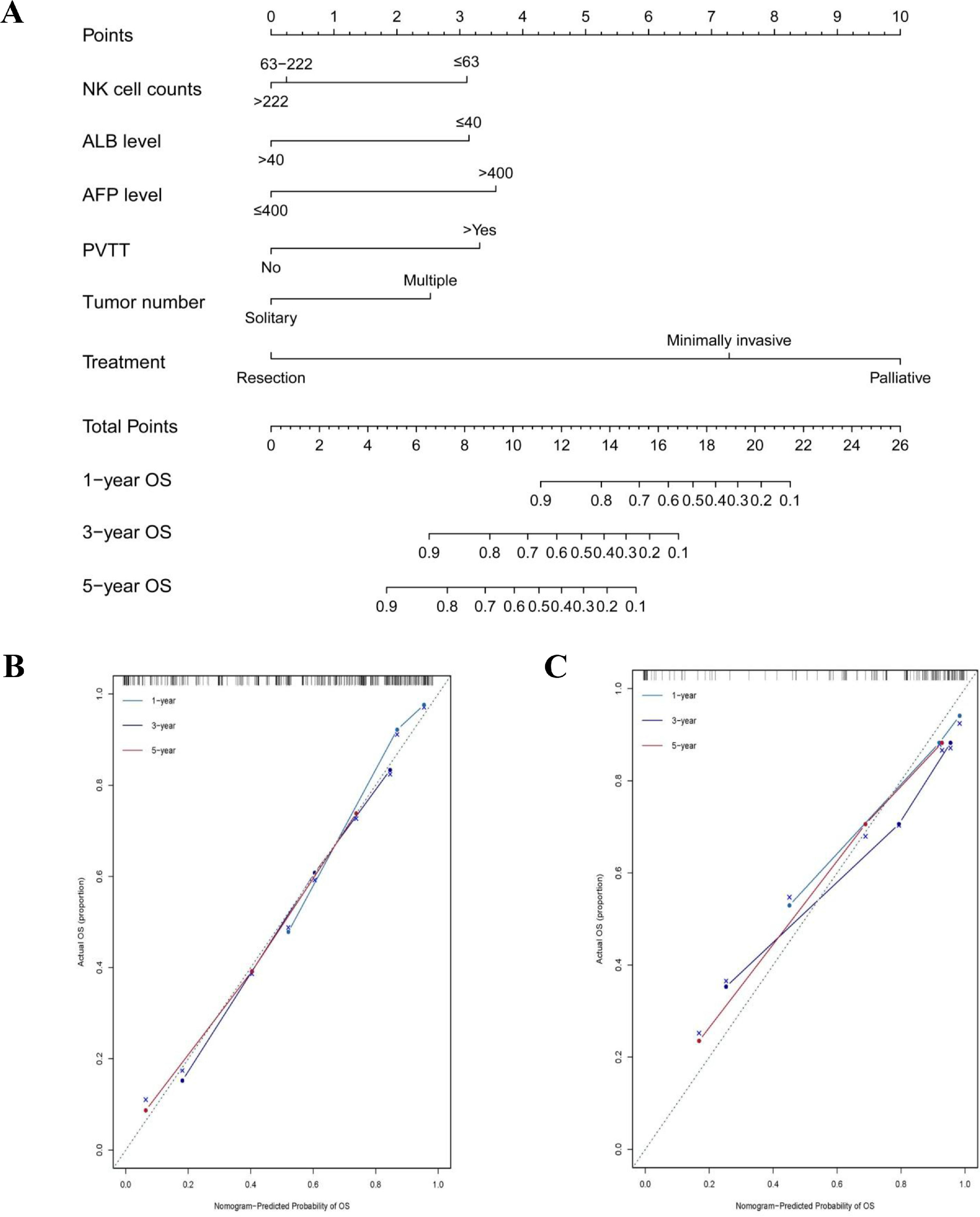
The model was further established using a nomogram for 1-year, 3-year, and 5-year OS by combining NK cell counts, ALB level, AFP level, PVTT, tumor number, and treatment identified via the Cox multivariate analyses (Figure 1A). The nomogram included five parameters related to liver function and the tumor (ALB level, AFP level, PVTT, tumor number, and treatment) and one immunological parameter (NK cell counts).
Nomogram prediction model and calibration curve. (A) Nomogram predicted overall survival (OS) for HCC patients. To use the nomogram, the value of an individual patient is located on each variable axis, and a line is drawn upward to determine the number of points received for the value of each variable. The sum of these numbers is located on the total point axis, and a line is drawn downward to OS axes to determine the likelihood of 1-year OS, 3-year OS and 5-year OS. The calibration curves for 1-year OS, 3-year OS and 5-year OS were identified in the training cohort (B) and in the validation cohort (C).
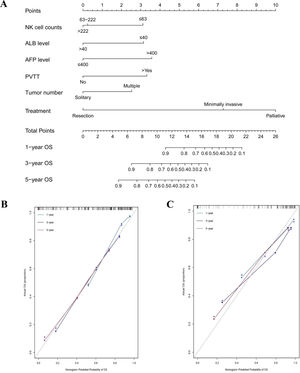
In the training cohort, the C-indexes of the nomogram model predicting 1-year, 3-year, and 5-year OS were 0.858 (95% CI: 0.802-0.914), 0.788 (95% CI: 0.736-0.840) and 0.782 (95% CI: 0.735-0.829), respectively, which were higher than those of TNM, Okuda, MELD, MELD-Na, CUPI, and JIS scores (p < 0.001). The C-indexes predicting the 1-year, 3-year, and 5-year OS in the validation cohort were 0.861, 0.796, and 0.807, respectively, and were found to be better than the other models (Table 3).
Comparison of the discriminative ability among the nomogram and other models
| Cohort | Models | 1-year overall survival | 3-year overall survival | 5-year overall survival | |||
|---|---|---|---|---|---|---|---|
| C-index | 95% CI | C-index | 95% CI | C-index | 95% CI | ||
| Training | Nomogram | 0.858 | 0.802-0.914 | 0.788 | 0.736-0.840 | 0.782 | 0.735-0.829 |
| BCLC stage | 0.742 | 0.671-0.813 | 0.686 | 0.628-0.744 | 0.668 | 0.620-0.716 | |
| TNM stage | 0.564 | 0.478-0.650 | 0.594 | 0.535-0.653 | 0.595 | 0.543-0.647 | |
| Okuda stage | 0.649 | 0.557-0.741 | 0.606 | 0.541-0.671 | 0.620 | 0.565-0.675 | |
| MELD | 0.617 | 0.515-0.719 | 0.617 | 0.548-0.686 | 0.612 | 0.552-0.672 | |
| MELD-Na | 0.627 | 0.527-0.727 | 0.624 | 0.554-0.694 | 0.620 | 0.558-0.682 | |
| CLIP | 0.749 | 0.658-0.840 | 0.696 | 0.633-0.759 | 0.691 | 0.635-0.747 | |
| CUPI | 0.660 | 0.562-0.758 | 0.643 | 0.575-0.711 | 0.653 | 0.593-0.713 | |
| JIS | 0.567 | 0.473-0.661 | 0.602 | 0.537-0.667 | 0.622 | 0.561-0.683 | |
| Validation | Nomogram | 0.861 | 0.746-0.976 | 0.796 | 0.693-0.899 | 0.807 | 0.712-0.902 |
| BCLC stage | 0.756 | 0.629-0.883 | 0.753 | 0.653-0.853 | 0.759 | 0.664-0.854 | |
| TNM stage | 0.578 | 0.428-0.728 | 0.666 | 0.549-0.783 | 0.663 | 0.553-0.773 | |
| Okuda stage | 0.706 | 0.552-0.860 | 0.659 | 0.539-0.779 | 0.674 | 0.560-0.788 | |
| MELD | 0.516 | 0.318-0.714 | 0.554 | 0.406-0.702 | 0.535 | 0.395-0.675 | |
| MELD-Na | 0.482 | 0.306-0.658 | 0.500 | 0.363-0.637 | 0.499 | 0.365-0.633 | |
| CLIP | 0.835 | 0.748-0.922 | 0.777 | 0.691-0.863 | 0.778 | 0.695-0.861 | |
| CUPI | 0.682 | 0.530-0.834 | 0.709 | 0.591-0.827 | 0.721 | 0.610-0.832 | |
| JIS | 0.639 | 0.475-0.803 | 0.704 | 0.580-0.828 | 0.714 | 0.597-0.831 | |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer classification system; TNM, tumor–lymph node–metastasis staging system; MELD, model for end-stage liver disease; CLIP, Cancer of the Liver Italian Program; CUPI, Chinese University Prognostic Index; JIS, Japan Integrated Staging.
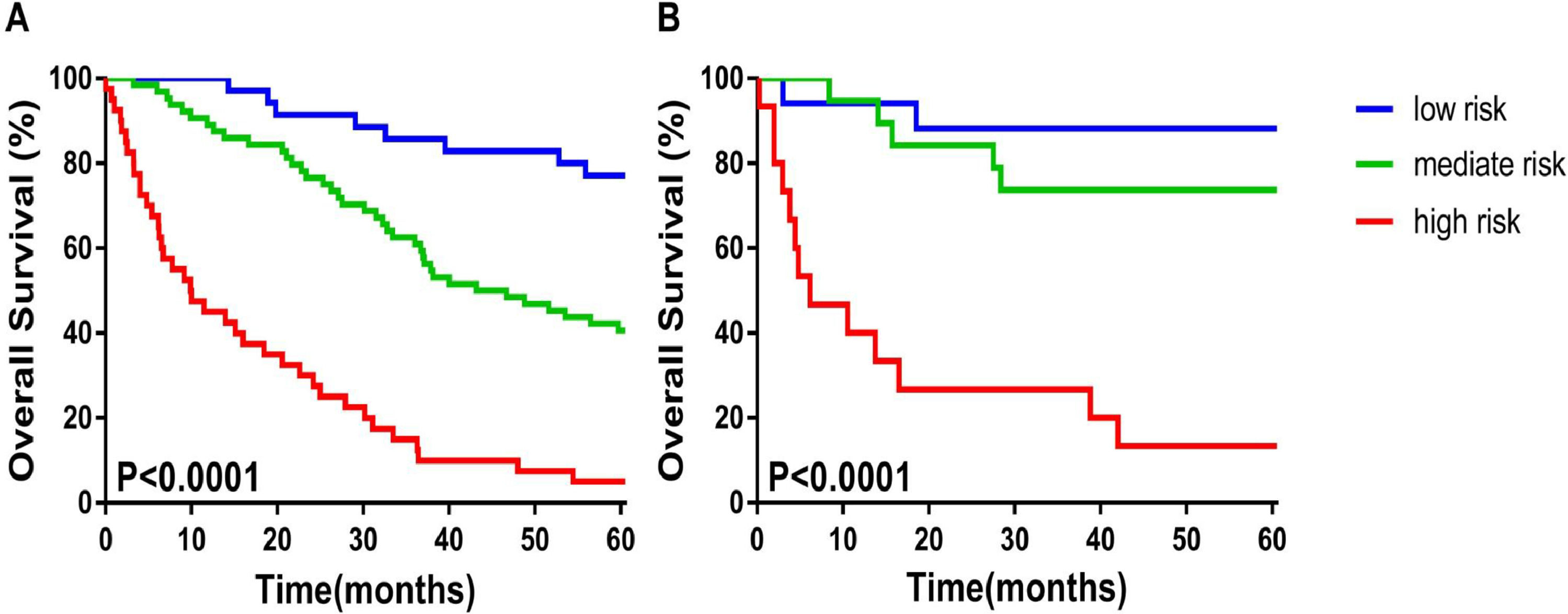
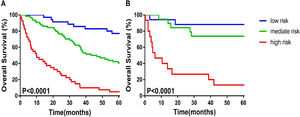
The calibration plot for the probabilities of 1-year, 3-year, 5-year OS fitted well between the actual observation and the prediction of the model using a nomogram in the training and validation cohorts (Figure 1B and C). According to the nomogram scores patients were divided into, and low-risk (≤ 10.2 scores), medium-risk (10.2-14.4 scores), and high-risk (≥ 14.4 scores) groups. The Kaplan–Meier survival curve showed that the nomogram could distinguish the low-, medium-, and high-risk groups well in both the training and validation cohorts (p< 0.0001) (Figure 2A and B).
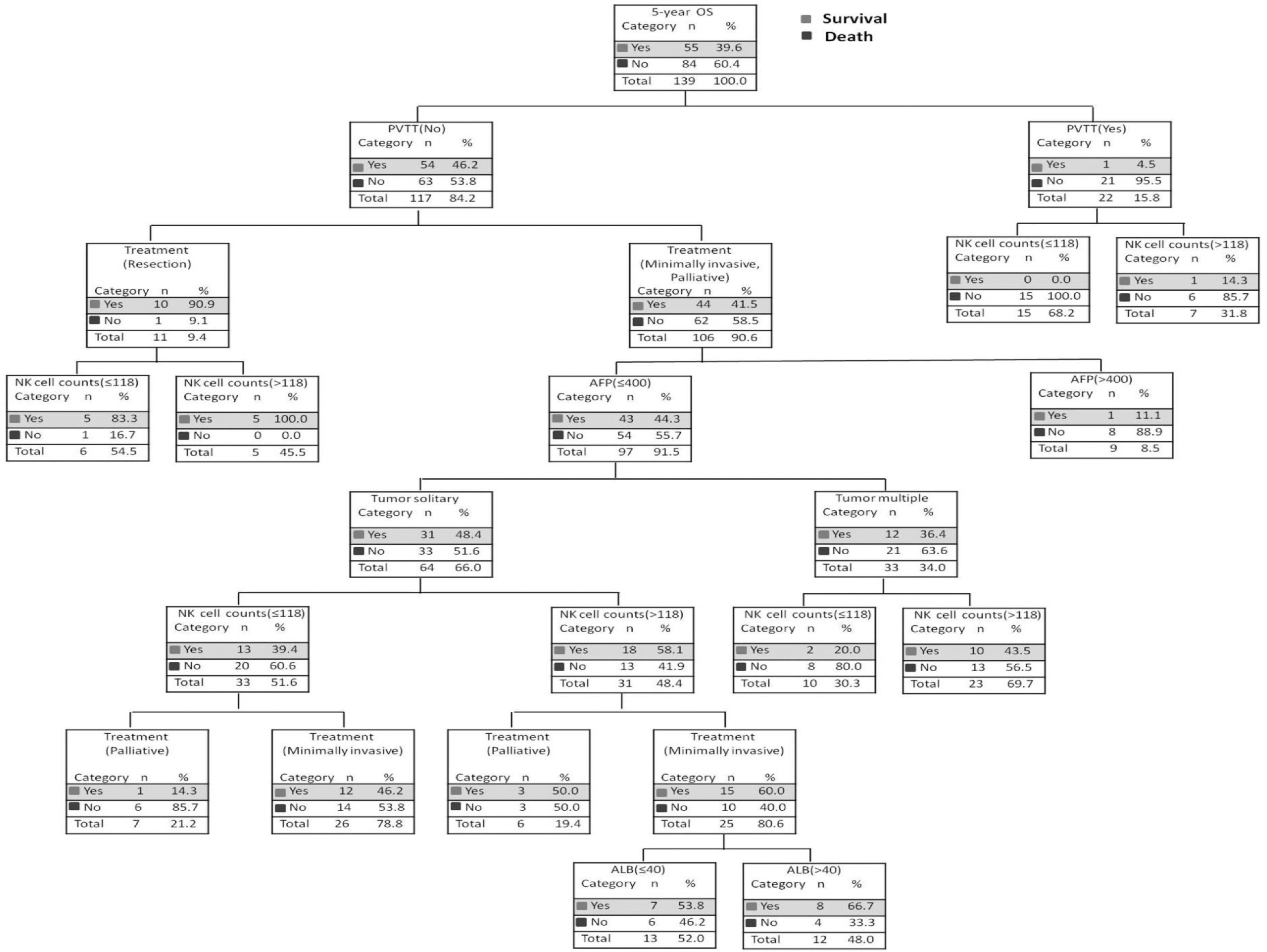
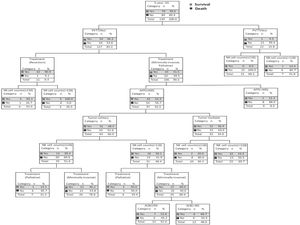
3.4Survival probability of decision tree under different risk factorsSix independent risk factors, including NK cell counts (≤ 118 cells/μL vs. >118 cells/μL), ALB level (≤ 40g/L vs. > 40g/L), AFP level (≤ 400ng/mL vs. > 400ng/mL), PVTT at baseline (No vs. Yes), tumor number (solitary vs. multiple) and treatment (palliative vs. minimally invasive, resection) were put into the decision tree, which was divided into seven layers according to the column contribution of variables to the outcome (Figure 3). The decision tree showed that the 5-year OS rate of patients with HCC in the PVTT (No) and the PVTT (Yes) groups were 46.2% and 4.5%, respectively. In addition, the 5-year OS rate of patients with NK cell counts≤118 cells/μL and those with NK cell counts > 118 cells/μL were 0.0% and 14.3% in the PVTT (Yes) group. As can be seen intuitively, the PVTT was in the first layer, and its column contribution was the largest. The treatment ranks second regarding column contribution. As an important immune index, NK cell count ranks third in column contribution, higher than the AFP level, tumor number, and ALB level (Table S2).
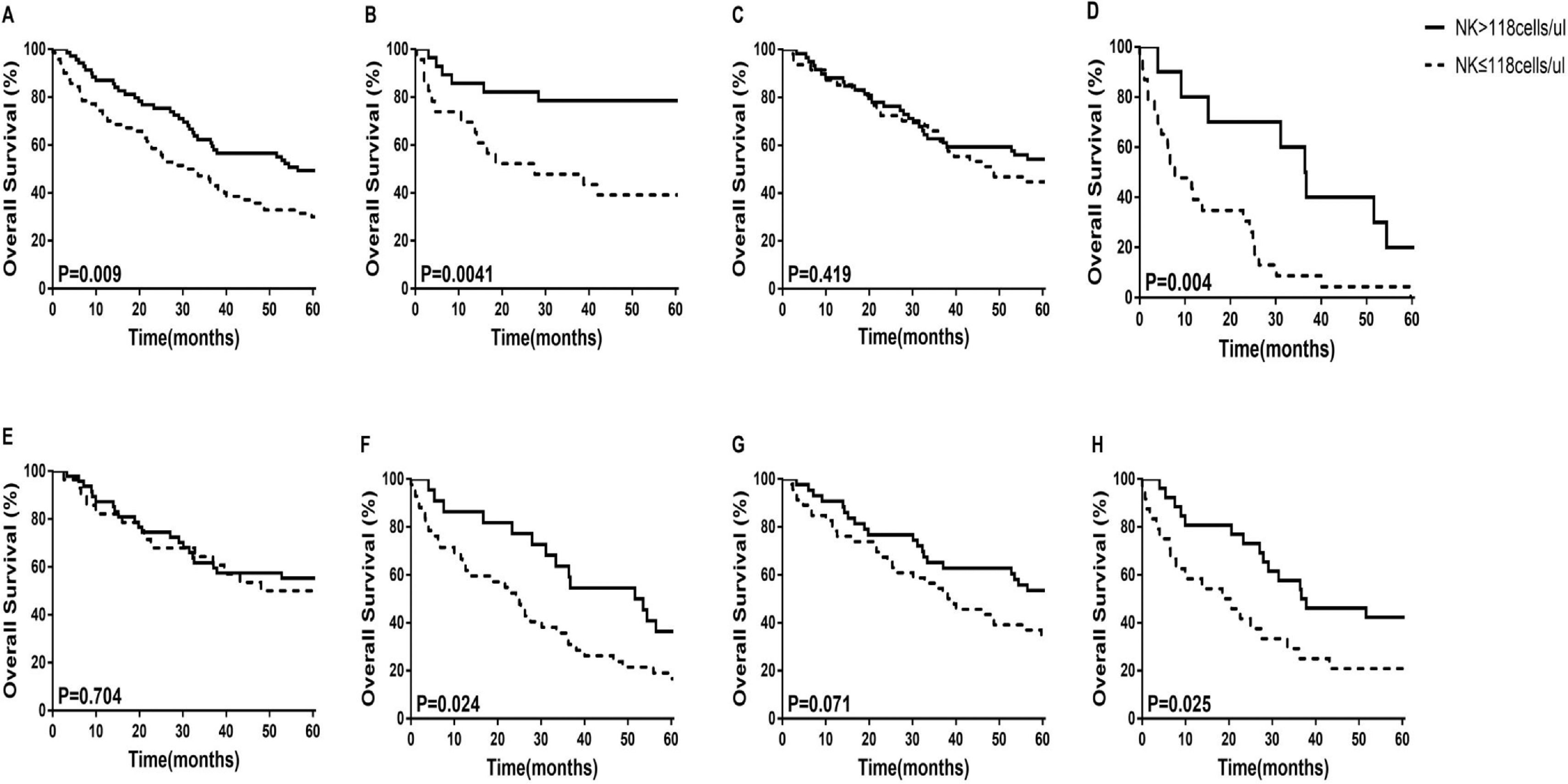
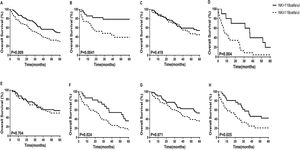
3.5Prognostic value of NK cell counts in patients with HCCWe used the median NK cell counts of 118 as the cut-off value, and divided all patients into two groups. The 5-year OS of patients with HCC were compared using the Kaplan–Meier curves. We found that the 5-year OS rate of the high NK cell group (NK > 118 cells/μL) was significantly higher than that of the low NK cell group (NK ≤118 cells/μL) in both the training and validation cohorts (Figure 4 A and B). We further examined whether the low or the high NK cell group is closely related to the OS of patients with HCC. We found that in BCLC stage C-D, Child-Pugh B+C, and tumor multiple subgroups, HCC patients with high NK cell counts had higher survival rates (p = 0.004, p = 0.024, p = 0.025, respectively) (Figure 4D, F, and H). In BCLC stage 0-B, Child-Pugh A, and tumor solitary subgroups, there was no statistically significant difference in survival between patients with high NK cell counts and those with low NK cell counts (Figure 4C, E, and G).
The 5-year overall survival analysis between HCC patients with NK cell counts > 118 cells/μL and NK cell counts ≤118 cells/μL in the retrospective cohort. (A) Nomogram in the Training Cohort; (B) Nomogram in the Validation Cohort; (C)BCLC stage 0-B; (D)BCLC stage C-D; (E) Child-Pugh A; (F)Child-Pugh B+C; (G) Tumor solitary; (H) Tumor multiple.
Research has been focused on the prognosis of patients with HCC, especially for long-term survival. In previous studies, models related to the prognosis of HCC have been reported, and the factors included in the models were related more to the liver function and the tumor [17-19, 24]. The influence of liver function and tumor-related factors on the occurrence and prognosis of patients with HCC is obvious. However, and the effect of other non-hepatic and non-tumor factors affecting the prognosis of patients with HCC should also be considered and confirmed.
In this study, the model included five liver function- and tumor-related factors, namely ALB level, AFP level, PVTT, tumor number, and treatment, as well as an immune-related factor, that is, NK cell counts. The effect of NK cell count on the OS rate of patients with HCC is worthy of attention. In addition, all the above factors are objective indicators obtained directly through clinical laboratory examinations.
NK cells are enriched in liver lymphocytes and have specific phenotypes and functional characteristics. As an important part of the liver innate immune system, NK cells play a vital role in tumor development through natural cytotoxicity and cytokine production [25]. Previous studies have shown that NK cells play an important role in the control of viral hepatitis, liver fibrosis, and liver cancer [26–28]. In patients with HCC, NK cells can inhibit the occurrence and development of tumors through different pathways and mechanisms, which are mainly mediated by perforin, granzyme, and IFN-γ. However, the function of NK cells is often inhibited in conditions of inflammation, fibrosis, and cirrhosis, even in the tumor microenvironment. Previous studies have found that compared with healthy subjects, NK cell counts in peripheral blood of patients with HCC are decreased, especially the number of CD56dimNK cell killing subsets is significantly reduced [6], which is consistent with the findings of this study. Zhang et al. found that CD11b−CD27− (DN) NK cells accumulate in the tumor tissues of patients with HCC. Immunosuppressive factors (IL-10 and TGF-β) change the phenotype of NK cells and inhibit the immune surveillance function, resulting in immune escape of tumors [29]. Sun et al. found that the expression of CD96+NK cells increased in the tumor tissues of patients with HCC, and their functions were exhausted. Blocking TGF-β can restore NK cell function, which indicates that CD96 and other immunosuppressive receptors can play a more powerful role in anti-tumor immune response [30]. We found that the decrease in NK cell counts detected by clinical laboratory tests is an independent risk factor affecting the long-term prognosis of patients with HCC that cannot be ignored. Therefore, it is of great significance to improve the immune monitoring function of patients with HCC by increasing NK cell counts and activity.
A nomogram is a prediction model widely used in clinics; it is simple and visual. It can directly predict the survival rate of a single clinical variable and the entire model and promote clinical decision-making [31]. In recent years, the nomogram model has become a reliable clinical tool for quantifying the risk and evaluating the prognosis of lung cancer, breast cancer, prostate cancer, rectal cancer, and gastric cancer [32–36]. In this study, a nomogram was used to synthesize the best predictors of prognosis of patients with HCC, so that the 1-year, 3-year, and 5-year OS rates can be accurately predicted. At the same time, a calibration curve was used to evaluate the correlation between the predicted and actual risks.
As a method of machine learning, decision trees can directly show the mapping relationship between predictors and outcomes [37]. The algorithm of the decision tree is complex, and the software can provide it directly. We only need to pay attention to the levels and nodes displayed by the decision tree. The level is the contribution of different predictors to the outcome, and the node is the best way to divide the variables. Our previous study found that high numbers of CD4 T lymphocytes associate with longer progression-free survival (PFS) and OS in hepatocellular carcinoma [23]. CD4 T cells not only enhance the function of CD8 T cells, but also recruit NK cells to the tumor microenvironment. Therefore, immune cells play an important role in the survival of HCC [38, 39]. In this study, the decision tree intuitively shows the influence of different risk factors on the 5-year OS rate of patients with HCC. It can be seen that the NK cell count is in the third place of the column contribution of the decision tree. We consider that the impact of the reduction in NK cell counts on the outcome is still significant.
This study has the following limitations: First, the sample size is small. However, we used two modeling methods to prove the predictive ability of NK cell counts for the survival of patients with HCC. Second, the lack of external verification may affect the applicability of the model. Third, the decision tree lacks stability.
5ConclusionsA visual nomogram was used to establish a prediction model based on NK cell counts to predict the OS of patients with HCC, providing a simple and quick decision-making tool for clinical practice. In the future, NK cell count is worthy of the attention as clinical index, and it needs further verification regarding its clinical value.
DeclarationsEthics approval and consent to participateThis study is in accordance with the Declaration of Helsinki and has been approved by the ethical committee of Beijing Ditan Hospital, Capital Medical University. This study is a retrospective research. The ethics committee approved it to be used in this study while ensuring patient privacy, not interfering with the patient's treatment methods, and not bringing risks to the patient's physiology. Retrospective researches are unable to obtain written informed consent. The main reasons are: (1) we only collect the clinical laboratory data from patients, and the risks are manageable; (2) some patients have lost contact or died and are unable to track their informed consent signatures. This study was based on the approval of the ethics committee and the oral informed consent of the patients or their families who survived and could be contacted during the regular follow-up period.
Authors' contributionsLihua Yu: data curation, methodology, formal analysis, writing-original draft, writing-review and editing. Xiaoli Liu: study design, data curation. Xinhui Wang, Dongdong Zhou, Huiwen Yan, Yuqing Xie, and Qing Pu: data acquisition. Ke Zhang: study design, corrected the final version of the manuscript. Zhiyun Yang: final approval of the version to be submitted. The definitive version has been read and approved by all authors.
FundingThis study was supported by the Special Fund of Capital Health Research and Development (No. 2020-2-2173), the National Natural Science Foundation of China (No. 81874435), Dengfeng Talent Support Program of Beijing Municipal Administration of Hospitals (No. DFL20191803), Fund for Beijing Science & Technology Development of TCM (No. JJ-2020-52), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (ZYLX202127).