One of the evolutionary complications of hepatic echinococcosis (HE) is cholangiohydatidosis, a rare cause of obstructive jaundice and cholangitis. The aim of this study was to describe the results of surgical treatment on a group of patients with cholangiohydatidosis and secondary cholangitis in terms of post-operative morbidity (POM).
Material and methodCase series of patients operated on for cholangiohydatidosis and cholangitis in the Department at Surgery of the Universidad de La Frontera and the Clínica Mayor in Temuco, Chile between 2004 and 2014. The minimum follow-up time was six months. The principal outcome variable was the development of POM. Other variables of interest were age, sex, cyst diameter, hematocrit, leukocytes, total bi-lirubin, alkaline phosphatase and transaminases, type of surgery, existence of concomitant evolutionary complications in the cyst, length of hospital stay, need for surgical re-intervention and mortality. Descriptive statistics were calculated.
ResultsA total of 20 patients were studied characterized by a median age of 53 years, 50.0% female and 20.0% having two or more cysts with a mean diameter of 13.3 ± 6.3 cm. A median hospital stay of six days and follow-up of 34 months was recorded. POM was 30.0%, re-intervention rate was 10.0% and mortality rate was 5.0%.
ConclusionCholangiohydatidosis is a rare cause of obstructive jaundice and cholangitis associated with significant rates of POM and mortality.
Intrabiliary rupture of hepatic echinococcosis (HE) is one of the most frequent evolutionary complications of this pathological entity. Once this rupture occurs and there is communication between a cyst and the biliary tree, the conditions are created that allow the further migration of the parasitic structures (pieces of germinal layer or daughter vesicles) into the biliary tract.1,2 This situation (cholangiohydatidosis) may produce a secondary cholan-gitis3 and even infection of the cyst with liver abscess.4 Obviously, any of these situations might increase the risk of post-operative morbidity (POM).3-5
The reported prevalence of intrabiliary rupture of HE is between 9.0% and 42.0%,2,6-9 but the frequency of cholangiohydatidosis and secondary cholangitis is unclear. There are few related reports; most are about intrabiliary rupture, but not about cholangiohydatidosis and secondary cholangitis, about which we found only 15 articles.3,10-23 The information available is scarce and with a low level of evidence, so collecting specific data regarding the prevalence or incidence of cholangitis due to cholangiohydati-dosis is virtually impossible.
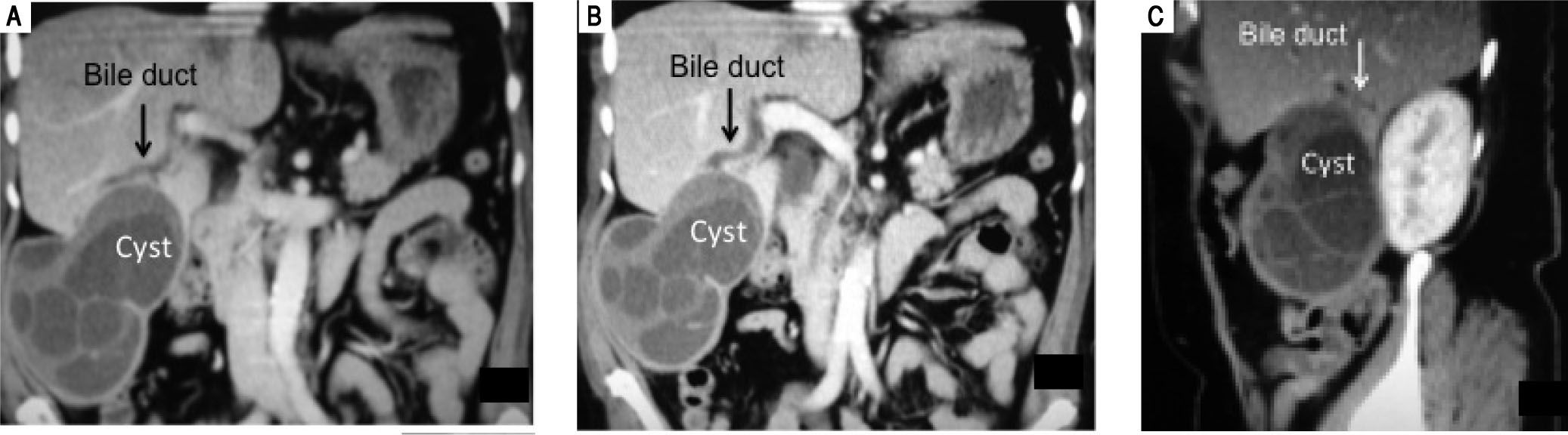
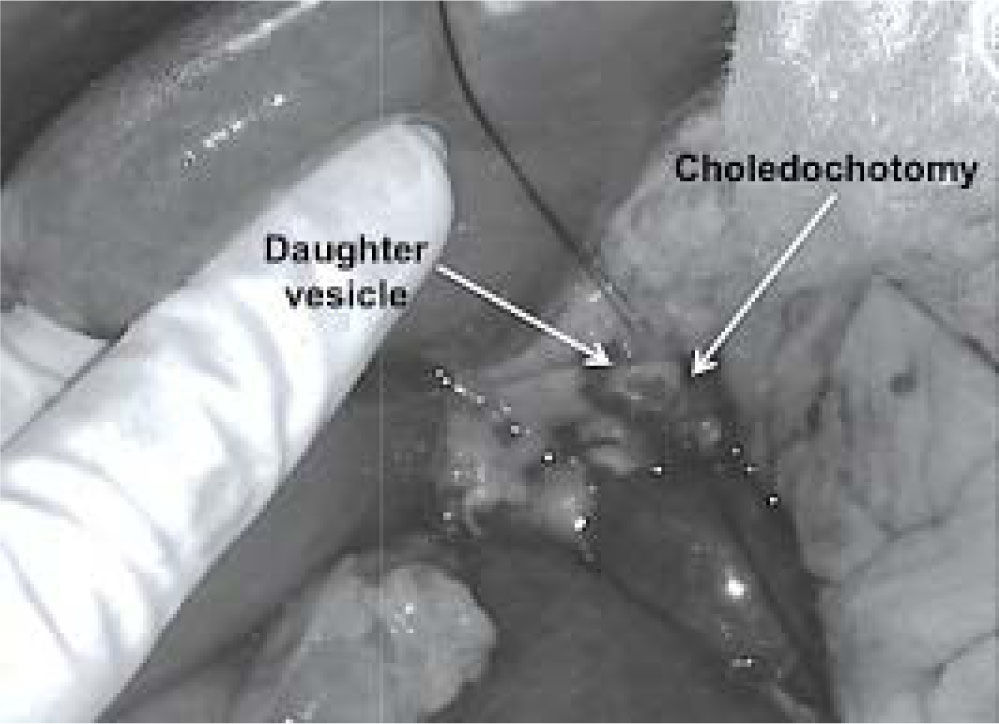
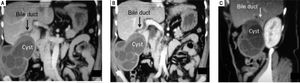
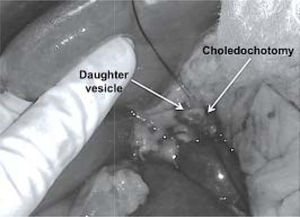
However, a preoperative diagnosis of cholangiohy-datidosis and secondary cholangitis is difficult unless it can be observed in images such as those in Figure 1. Diagnosis is usually intraoperative once a dilatation of the bile duct has been confirmed, where tension bile or bilious pus and parasite remnants may be flowing in the interior (Figure 2).
Female 66-year-old patient presented with four days’ evolution of right upper abdominal pain, fever and jaundice. Abdominal CT scan verified a heterogeneous cystic mass that represented germinative membrane residues and some daughter vesicles. The intimate relation between the cyst and the right biliary tree can also be seen, specifically with its anterior branches. The biliary communication at this level (segment VI) gave rise to cholangiohydatidosis and secondary acute cholangitis.
The aim of this study was to describe the results of surgical treatment on a group of patients with cholangiohy-datidosis and secondary cholangitis in terms of POM.
This report was written on the basis of the MINCIR Statement (Methodology and Investigation in Surgery) for reporting observational descriptive studies.24
Material and MethodsStudy designA case series of patients operated on for cholangiohy-datidosis and secondary cholangitis, who are part of a concurrent cohort of subjects operated on for HE.
SettingThe study was conducted at the Department of Surgery of the Universidad de La Frontera and the Clínica Mayor in Temuco, Chile. The recruitment period was between January 2004 and December 2014. The minimum follow-up time was six months.
ParticipantsPatients operated on for HE (only E. granulosus variety) by the first author (CM) during the study period were included. Patients with a history of prior surgery for HE or other concomitant hepatic pathologies were excluded. A non-probabilistic sampling of consecutive cases was used.
Diagnostic criteriaDiagnosis of cholangiohydatidosis and secondary cholangitis was suspected by images (abdominal CT-Scan or IMR) in all patients and confirmed intraoperatively by the observation of a solution of continuity in the bile duct and the existence of pus and parasitic structures in the biliary ducts. Typification of echinococcosis was made post-operatively at a parasitology laboratory, using direct microscope observation of protoscolices, and histological study of surgical specimens confirmed the preoperative diagnosis in all the patients.
Surgical procedureAll patients underwent emergency surgery with prior antibiotic prophylaxis. The criterion for open surgery in acute cholangitis in our center is: patient with Charcot triad associated with dilatation of the biliary tract verified by abdomi-ationn.al ultrasonography when endoscopic surgery is not available. Surgery consisted of the exploration of the bile duct, removal of hydatid structures, choledochal drainage (Kehr drain) and concomitant treatment of the cyst with pericystectomy or liver resection. Then, all patients continued with antibiotics postoperatively for seven days.
Study protocolAfter hospital discharge, all patients were followed with strict check-ups at least at months 1 and 6 and then once per year (where possible). At these check-ups, clinical evaluation was performed as well as general laboratory and liver function tests, immunodiagnostics and abdominal ultrasound.
VariablesThe principal outcome variable was “development of POM”, measured at least six months after surgery. This was considered dichotomously (present or absent), and from the point of view of the severity of POM, applying the Clavien-Dindo surgical complications classification.25 Other variables of interest were age, sex, cyst diameter, hematocrit, leukocytes, total bilirubin, alkaline phos-phatase and transaminases, type of surgery (categorized in pericystectomy and liver resection), existence of concomitant evolutionary complications in the cyst (dichotomized as yes or no), length of hospital stay, need for surgical re-intervention and operative mortality.
BiasesIn the recruitment and follow-up stage, biases were reduced with a complete follow-up and blinded data collection. Then, data were encoded and not revealed until the analysis period was complete.
Study sizeSince it is an observational and descriptive study, no sample size was estimated.
Statistical methodsThe data were analyzed with the Stata 11.0/SE® program. Descriptive statistics were used to calculate percentages, averages and standard deviations, medians and extreme values.
EthicsWritten informed consent was obtained from each patient. All the ethical guidelines for research on human beings as defined by the Helsinki Declaration26 were observed.
FundingThere was no special funding for this study.
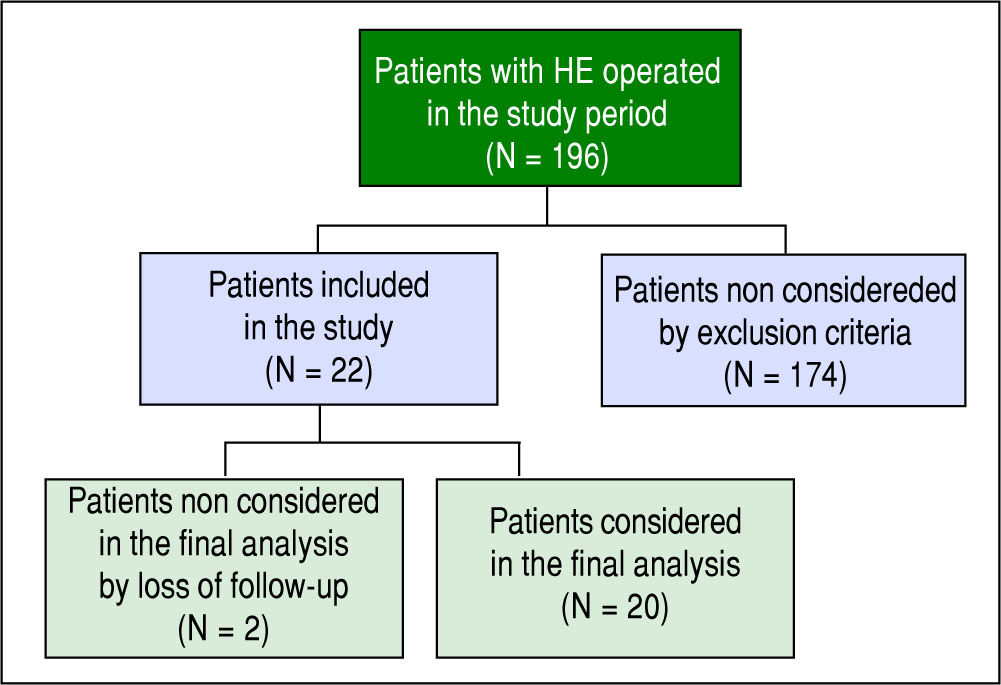
ResultsA total of 22 patients operated on for cholangiohydatido-sis were recruited in this period. Two were not included in the final analysis due to a lack of follow-up (Figure 3).
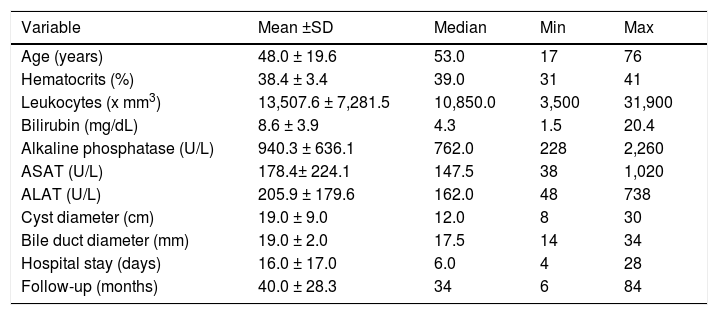
The patients comprising the series are characterized by a median age of 53 years, 50.0% female and 20.0% of them having two or more cysts with a mean diameter of 13.3 ± 6.3 cm (Table 1).
Distribution of clinical data in the study patients (N = 20).
| Variable | Mean ±SD | Median | Min | Max |
|---|---|---|---|---|
| Age (years) | 48.0 ± 19.6 | 53.0 | 17 | 76 |
| Hematocrits (%) | 38.4 ± 3.4 | 39.0 | 31 | 41 |
| Leukocytes (x mm3) | 13,507.6 ± 7,281.5 | 10,850.0 | 3,500 | 31,900 |
| Bilirubin (mg/dL) | 8.6 ± 3.9 | 4.3 | 1.5 | 20.4 |
| Alkaline phosphatase (U/L) | 940.3 ± 636.1 | 762.0 | 228 | 2,260 |
| ASAT (U/L) | 178.4± 224.1 | 147.5 | 38 | 1,020 |
| ALAT (U/L) | 205.9 ± 179.6 | 162.0 | 48 | 738 |
| Cyst diameter (cm) | 19.0 ± 9.0 | 12.0 | 8 | 30 |
| Bile duct diameter (mm) | 19.0 ± 2.0 | 17.5 | 14 | 34 |
| Hospital stay (days) | 16.0 ± 17.0 | 6.0 | 4 | 28 |
| Follow-up (months) | 40.0 ± 28.3 | 34 | 6 | 84 |
ASAT: aspartate aminotransferase. ALAT: alanine aminotransferase.
Two patients (10.0%) had a history of previous surgery for hepatic hydatid disease and another due to pulmonary hydatid disease. Five patients (25.0%) had comorbidities: heart disease (2 cases), diabetes mellitus (2 cases) and cholelithiasis (1 case).
Nine subjects (45.0%) had concomitant parasitic diseases: hepatic abscess of hydatid origin (7 cases, 35.0%), and hepatothoracic transit (2 cases, 10.0%).
Laboratory tests revealed anemia (5 cases, 25.0%), leu-kocytosis (11 cases, 55.0%), signs of cholestasis (17 cases, 85.0%) and hepatocyte cytolysis (17 cases, 85.0%) (Table 1).
The existence of concomitant hydatid disease in the peritoneum (2 cases) and spleen (one case) was verified. The most common location of cysts communicated to the bile duct was the right hepatic lobe (12 cases, 60.0%) (Table 2).
Distribution of clinical data and surgical characteristics in the study patients (N = 20).
| Variable | % |
|---|---|
| Sex (%) | |
| Female | 50.0 |
| Male | 50.0 |
| Other evolutionary complication in the cyst (%) | |
| No | 65.0 |
| Yes | 35.0 |
| Number of cysts (%) | |
| One | 80.0 |
| Two or more | 20.0 |
| Surgery performed (%) | |
| Lobectomy | 30.0 |
| Morbidity (%) | |
| No | 70.0 |
| Yes | 30.0 |
| Need for re-operation (%) | |
| No | 90.0 |
| Yes | 10.0 |
| Mortality (%) | |
| No | 95.0 |
| Yes | 5.0 |
The mean diameter of the common bile duct was 18.5 ± 2.1 mm and biliary communications were found in all patients (Tables 1 and 3).
A median hospital stay of six days was recorded with a median follow-up of 34 months (Table 1), 30.0% morbidity and a re-intervention rate of 10.0% (Table 2). The related morbidity was secondary to pulmonary, cardiac and metabolic complications (1 case of each), infection at the surgical site, incisional hernia, biliary fistula (1 case of each) (Table 3).
Distribution of surgical characteristics in the study patients (N = 20).
| Variable | % |
|---|---|
| Location of the cyst (%) | |
| Right lobe | 60.0 |
| Left lobe | 25.0 |
| Bilateral | 15.0 |
| Biliary communications (%) | |
| One | 65.0 |
| Two | 25.0 |
| Three or more | 10.0 |
| Treatment of the residual cavity (%) | |
| None | 60.0 |
| Capitonnage | 25.0 |
| Omentoplasty | 15.0 |
| Other surgeries performed concomitantly (%) | |
| Cholecystectomy | 15.0 |
| Cholecystectomy and choledocostomy | 20.0 |
| Cholecystectomy + choledocostomy + | 60.0 |
| splenectomy and peritoneal hydatidosis surgery | 5.0 |
| Morbidity according to Dindo & Clavien | |
| 0 | 70.0 |
| 1 | 10.0 |
| 2 | 10.0 |
| 4 | 5.0 |
| 5 | 5.0 |
Mortality rate was 5.0%. This was a 76-year-old patient with a coronary cardiopathy who presented episodes of projectile vomiting, and then developed aspiration pneumonia, secondary respiratory insufficiency and finally multiple organ failure.
All the diagnosed cases of acute cholangitis due to cholangiohydatidosis in the reported period were treated according to the protocol described in material and methods section. There were no cases of medical or endoscop-ic treatment.
DiscussionThere are few reports regarding the study of cholan-giohydatidosis as an associated factor for POM in HE surgery, and most are retrospective case series, contributing evidence level 4. Reports in the literature are scarce because it is a rare entity, with a low incidence among patients who have undergone surgery for HE. In general, there have only been isolated case reports and small case series (single case report,13,15,16,18-20,22,23 between 4 and 6 cases of endoscopic treatment10-12,21), 13 cases reported by our group in 20013, 25 cases treated endoscopically14 and the most numerous series (80 cases).17 However, secondary cholangitis is not clearly reported in any of them.
In addition to being a rare entity, the biggest problems are the complications which cholangiohydatidosis can cause: biliary obstructive syndrome,3,14 acute suppurated cholangitis,3,13,14 and even acute secondary pancreatitis.13,15
The fact that 35.0% of cases concomitantly presented a liver abscess of hydatid origin,4 and 10.0% also had a hepatotho-racic transit,27 underscores these patients’ severe condition.
The mean diameter of the cysts was 19.0 ± 9.0 cm, with a mostly heterogeneous sonographic appearance, and these were generally complicated cysts. This fact at least partly undermines the thesis of exclusive endoscopic treatment for this problema,12,14 because if so, we would be rendering treatment for the original lesion null, which, as discussed in this manuscript, in more than 30% of cases can be found abscessed as well. For this reason, we believe that surgery remains the treatment of choice for cholan-giohydatidosis and secondary cholangitis, and endoscopic drainage may be useful only in select cases,16,28,29 and more as a bridge procedure to drain the bile duct while awaiting definitive surgery.
Clinical presentation and laboratory findings are not specific, and may simulate an obstructive jaundice and acute cholangitis (predominantly lithiasic in Chile), which is similar to that reported by other groups.16,29
The existence of biliary communications in all patients explains the genesis of cholangiohydatidosis, because it is through these communications that parasitic structures migrate into the bile duct.3
Treatment of cholangiohydatidosis and secondary cholangitis is to drain and clean the bile duct, which can be performed by open (conventional) surgery or closed surgery (endoscopic papillotomy); however, as discussed above, while it is true that endoscopic papillotomy offers all the advantages of a minimally invasive surgery (particularly useful in high-risk patients), it does not allow a rational and definitive treatment of the whole problem. Therefore, in general, surgery remains the option of choice through biliary drainage (choledochostomy or choledocoduodenoanastomosis) and simultaneous resection of the hydatid cyst.16,28,29
It would be of great interest to ascertain the effectiveness of endoscopic and laparoscopic treatments in patients with cholangitis secondary to cholangiohydatidosis. However, the actual number of available cases is insufficient to conduct a comparative study with a methodologically adequate power and level of confidence.
We believe that the reported morbidity (30.0%) is relevant; however, the clinical severity in these patients and their concomitant comorbidities must also be consid-ered3,17, as should the mortality of the series (5.0%), which guides a clinical entity of poor prognosis for which no effort should be spared to make an early diagnosis and take prompt and appropriate action.3,28
However, we are aware that the study presents some potential sources of bias that merit comment. One of these has to do with the heterogeneity of the population. Another is related to the sample size, because there are no more cases to report. The loss of follow-up at less than 10% and an extensive follow-up time, however, are tools that increase the quality of the data.
With respect to the external validity of this study, it is worth noting that the study results correspond to the reality of one center of reference for hepatobiliary surgery located in an endemic region of echinococcosis; therefore, the reader must assess the external validity and reproduci-bility of the results with caution.
The protocol treatment did not include surgery prophylaxis with chemotherapy for Echinococcus Granulosus. This is a controversial point; however, we chose not to use it because of a previous study where we described al-bendazole as having no effect on the viability of proto-scoleces in patients treated with this drug preoperatively.30 With respect to medical treatment with albendazole, there is strong evidence (systematic reviews and clinical trials) that indicate that anthelmintic treatment alone is not ideal for liver hydatid cysts.31-33 On the other hand, the World Health Organization carried out multicenter trials in Europe to compare albendazole and mebendazole and verified that both drugs had similar efficacy, but mebendazole required higher doses and a different treatment with longer periods of adverse reactions.34,35
Finally, it is important to note that cholangiohydatidosis is a rare cause of obstructive jaundice and cholangitis associated with important rates of POM and mortality which occurs in the context of complex patients, so it must be treated in centers with experience in such diseases.
Abbreviations- •
HE: hepatic echinococcosis.
- •
POM: post-operative morbidity.
Financial Support
None.