The association of non-alcoholic fatty liver disease (NAFLD) with several other diseases has gained increased interest during the recent years. Among them, the association with chronic kidney disease (CKD) has emerged as an important one regarding both its prevalence and significance. The early recognition of this association is important for the prognosis of patients with NAFLD and CKD. Apart from early diagnosis, the accurate assessment of renal function is also crucial in the clinical practice of hepatologists. Several methods have been used in the literature for the evaluation of kidney function in patients with NAFLD up to now. In this respect, calculators (or formulas) for the estimation of Glomerular Filtration Rate (eGFR) and Albumin to Creatinine Ratio (ACR) are simple, practical and easily available methods for this purpose. The aim of this review is to report on the epidemiology and pathophysiology of the relationship between NAFLD and CKD and to describe the different methods of kidney function assessment in patients with NAFLD. The collection of all relevant data regarding this association will provide hepatologists with pertinent knowledge on this topic and allow them to use the most accurate methods for the assessment of kidney function in these patients in their clinical practice.
With the increasing prevalence of obesity, diabetes mellitus and the metabolic syndrome in the general population, non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease [1–3]. The prevalence of NAFLD has been reported to be in the 8–45% range in the general population in various countries and is almost certainly increasing [4]. NAFLD refers to a wide spectrum of liver damage, ranging from simple steatosis to non-alcoholic steatohepatitis, advanced fibrosis and cirrhosis [5–7] as well as hepatocellular carcinoma [2,3,8]. Recent studies have shown that NAFLD is the underlying cause of an increasing number of extrahepatic manifestations. NAFLD is mainly linked to type II diabetes mellitus (T2DM) and cardiovascular disease (CVD), as well as a number of other severe chronic diseases including chronic kidney disease (CKD) [5–7,9]. Several large cross-sectional population and hospital-based studies involving both diabetic patients and patients without diabetes have consistently shown that the prevalence of CKD is increased in people with NAFLD [7]. NAFLD and CKD share some common features, including visceral obesity, T2DM, hypertension and metabolic syndrome and both diseases are also linked to an increased risk of CVD [5].
The possible link between NAFLD and CKD has recently attracted considerable scientific interest. Establishing a link between liver and kidney injury would enhance the earlier identification of kidney disease and allow for the selection of treatments targeting both liver and kidney disease with potentially relevant preventive and therapeutic implications [5]. The ultimate goal of identifying patients with established but also with early kidney damage is to prevent disease progression and minimize complications, to promote quality of life and improve survival [10,11]. Many recent studies, including the meta-analysis from Musso et al. [6], suggest that individuals with NAFLD should be screened for CKD by estimation of GFR and urinalysis even in the absence of classical risk factors for CKD, particularly if NASH and/or advanced fibrosis are suspected [6]. Early recognition of impaired kidney function in patients with NAFLD, may also allow drug dosage adjustment, thus preventing drug accumulation especially in those being treated for obesity-associated co-morbidities [6,7].
The precise evaluation of kidney function in patients with liver disease is of central importance, especially in those with cirrhosis. eGFR (estimated Glomerular Filtration Rate) is one of the monitoring indicators of renal function in these patients and one of the indicative criteria for simultaneous liver-kidney transplantation [12]. The most commonly used methods to estimate GFR in this population are based on creatinine, which is biased by these patients’ low creatinine production, reduced skeletal mass and potentially by elevated serum bilirubin [13,14] and decreased albumin levels [14]. On the other side, cystatin C based formulae seem to be superior in the estimation of GFR in patients with liver disease, particularly in cirrhotics, by demonstrating less bias than creatinine based eGFR [15–19]. But this method has yielded mixed results and further studies are needed to evaluate its utility [14]. However, it is necessary to develop a new modified calculation formula for estimating GFR in patients with liver disease, not only in order to optimize care for these patients, but also to make decisions on organ allocation.
In this article, we discuss the evidence linking nonalcoholic fatty liver disease with CKD, relevant epidemiological data, the possible risk factors and the putative mechanisms by which NAFLD contributes to kidney damage. We also discuss current measuring options for estimating renal function, as well as markers of renal function and its limitations in patients with NAFLD. This knowledge is considered to be a helpful tool for hepatologists, who manage patients with NAFLD.
2Chronic kidney disease: definition and stagingChronic kidney disease (CKD) is defined by the presence of reduced glomerular filtration rate (GFR <60mL/min/1.73m2) and/or evidence of kidney damage (usually indicated by albuminuria or proteinuria) for 3 or more months [20,21]. On the other hand, kidney failure is defined as a GFR of less than 15mL/min per 1.73m2, or the need for treatment with dialysis or transplantation [22]. In clinical practice the most common tests for CKD diagnosis include eGFR estimated from the serum creatinine concentration and albuminuria from the urinary albumin-to-creatinine ratio (ACR). The importance of eGFR and albuminuria as diagnostic tools becomes obvious by their use in classification of CKD patients in stages [10].
On the basis of GFR the disease is classified into five stages: more than 90mL/min per 1.73m2 (stage 1), 60–89mL/min per 1.73m2 (stage 2), 30–59mL/min per 1.73m2 (stage 3), more specific 45–59mL/min per 1.73m2 (stage 3a) and 30–44mL/min per 1.73m2 (stage 3b), 15–29mL/min per 1.73m2 (stage 4) and less than 15mL/min per 1.73m2 (stage 5) [23,24]. Albuminuria as a marker of kidney damage is characterized by increased glomerular permeability and urine ACR>30mg/g. The normal urinary ACR in young adults is <10mg/g. Urine ACR categories 10–29, 30–300 and >300mg are high normal, high, and very high, respectively. Urine ACR >2000mg/g is accompanied by signs and symptoms of nephrotic syndrome (low serum albumin, edema, and high serum cholesterol) [22–24].
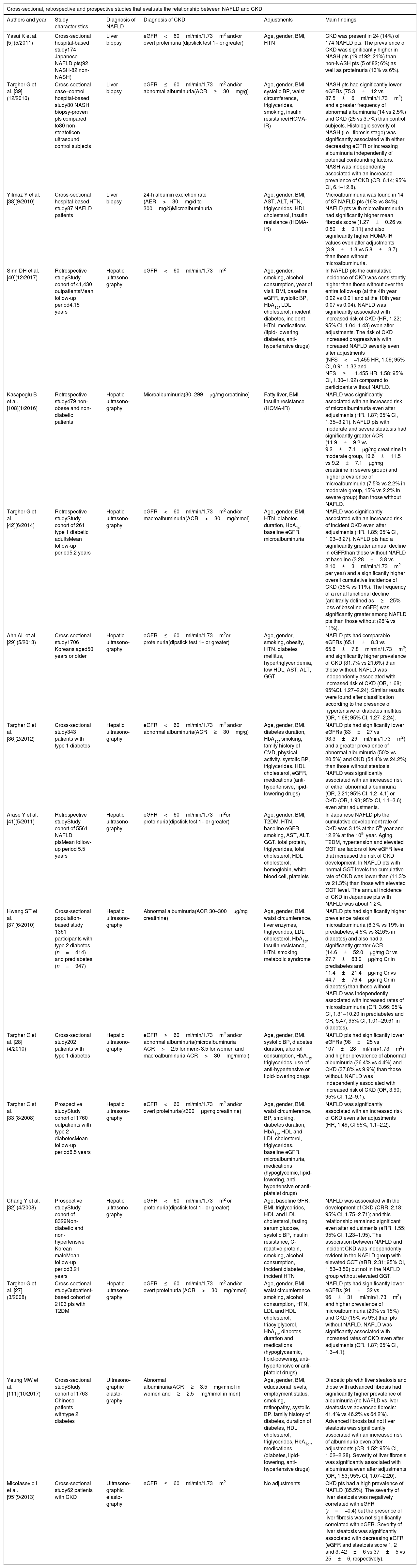
3Epidemiological dataThe scientific community is interested in the recent years in the epidemiological association of NAFLD with CKD and whether NAFLD could be considered as a surrogate marker of CKD. Current population-based prevalence of NAFLD is approximately 30–40% in men and 15–20% in woman and is even higher in people with T2DM, occurring in up to 70% in this group of patients [25]. CKD affects over 25% of the adult population over the age 65 years old in Western countries [26], while it affects up to 16% of the adult population of all ages [10]. According to most studies, the prevalence of CKD is increased in patients with NAFLD independently of age, sex, body mass index (BMI) and other confounders such as diabetes, hypertension, obesity and even in those with normal or near-normal kidney function at baseline [5,27–40]. The prevalence of CKD has been found to be higher in patients with steatohepatitis than those with simple steatosis [5,39,40]. Table 1 summarizes all relevant studies that evaluate the relationship between NAFLD and CKD.
Summary of the studies linking NAFLD and CKD.
| Cross-sectional, retrospective and prospective studies that evaluate the relationship between NAFLD and CKD | |||||
|---|---|---|---|---|---|
| Authors and year | Study characteristics | Diagnosis of NAFLD | Diagnosis of CKD | Adjustments | Main findings |
| Yasui K et al. [5] (5/2011) | Cross-sectional hospital-based study174 Japanese NAFLD pts(92 NASH-82 non-NASH) | Liver biopsy | eGFR<60ml/min/1.73m2 and/or overt proteinuria (dipstick test 1+ or greater) | Age, gender, BMI, HTN | CKD was present in 24 (14%) of 174 NAFLD pts. The prevalence of CKD was significantly higher in NASH pts (19 of 92; 21%) than non-NASH pts (5 of 82; 6%) as well as proteinuria (13% vs 6%). |
| Targher G et al. [39] (12/2010) | Cross-sectional case–control hospital-based study80 NASH biopsy-proven pts compared to80 non-steatoticon ultrasound control subjects | Liver biopsy | eGFR≤60ml/min/1.73m2 and/or abnormal albuminuria(ACR≥30mg/g) | Age, gender, BMI, systolic BP, waist circumference, triglycerides, smoking, insulin resistance(HOMA-IR) | NASH pts had significantly lower eGFRs (75.3±12 vs 87.5±6ml/min/1.73m2) and a greater frequency of abnormal albuminuria (14 vs 2.5%) and CKD (25 vs 3.7%) than control subjects. Histologic severity of NASH (i.e., fibrosis stage) was significantly associated with either decreasing eGFR or increasing albuminuria independently of potential confounding factors. NASH was independently associated with an increased prevalence of CKD (OR, 6.14; 95% CI, 6.1–12.8). |
| Yilmaz Y et al. [38](9/2010) | Cross-sectional hospital-based study87 NAFLD patients | Liver biopsy | 24-h albumin excretion rate (AER>30mg/d to 300mg/d)Microalbuminuria | Age, gender, BMI, AST, ALT, HTN, triglycerides, HDL cholesterol, insulin resistance (HOMA-IR) | Microalbuminuria was found in 14 of 87 NAFLD pts (16% vs 84%). NAFLD pts with microalbuminuria had significantly higher mean fibrosis score (1.27±0.26 vs 0.80±0.11) and also significantly higher HOMA-IR values even after adjustments (3.9±1.3 vs 5.8±3.7) than those without microalbuminuria. |
| Sinn DH et al. [40](12/2017) | Retrospective studyStudy cohort of 41,430 outpatientsMean follow-up period4.15 years | Hepatic ultrasono-graphy | eGFR<60ml/min/1.73m2 | Age, gender, smoking, alcohol consumption, year of visit, BMI, baseline eGFR, systolic BP, HbA1c, LDL cholesterol, incident diabetes, incident HTN, medications (lipid- lowering, diabetes, anti-hypertensive drugs) | In NAFLD pts the cumulative incidence of CKD was consistently higher than those without over the entire follow-up (at the 4th year 0.02 vs 0.01 and at the 10th year 0.07 vs 0.04). NAFLD was significantly associated with increased risk of CKD (HR, 1.22; 95% CI, 1.04–1.43) even after adjustments. The risk of CKD increased progressively with increased NAFLD severity even after adjustments (NFS<−1.455 HR, 1.09; 95% CI, 0.91–1.32 and NFS≥−1.455 HR, 1.58; 95% CI, 1.30–1.92) compared to participants without NAFLD. |
| Kasapoglu B et al. [108](1/2016) | Retrospective study479 non-obese and non-diabetic patients | Hepatic ultrasono-graphy | Microalbuminuria(30–299μg/mg creatinine) | Fatty liver, BMI, insulin resistance (HOMA-IR) | NAFLD was significantly associated with an increased risk of microalbuminuria even after adjustments (HR, 1.87; 95% CI, 1.35–3.21). NAFLD pts with moderate and severe steatosis had significantly greater ACR (11.9±9.2 vs 9.2±7.1μg/mg creatinine in moderate group, 19.6±11.5 vs 9.2±7.1μg/mg creatinine in severe group) and higher prevalence of microalbuminuria (7.5% vs 2.2% in moderate group, 15% vs 2.2% in severe group) than those without NAFLD. |
| Targher G et al. [42](6/2014) | Retrospective studyStudy cohort of 261 type 1 diabetic adultsMean follow-up period5.2 years | Hepatic ultrasono-graphy | eGFR<60ml/min/1.73m2 and/or macroalbuminuria(ACR>30mg/mmol) | Age, gender, BMI, HTN, diabetes duration, HbA1c, baseline eGFR, microalbuminuria | NAFLD was significantly associated with an increased risk of incident CKD even after adjustments (HR, 1.85; 95% CI, 1.03–3.27). NAFLD pts had a significantly greater annual decline in eGFRthan those without NAFLD at baseline (3.28±3.8 vs 2.10±3ml/min/1.73m2 per year) and a significantly higher overall cumulative incidence of CKD (35% vs 11%). The frequency of a renal functional decline (arbitrarily defined as≥25% loss of baseline eGFR) was significantly greater among NAFLD pts than those without (26% vs 11%). |
| Ahn AL et al. [29] (5/2013) | Cross-sectional study1706 Koreans aged50 years or older | Hepatic ultrasono-graphy | eGFR≤60ml/min/1.73m2or proteinuria(dipstick test 1+ or greater) | Age, gender, smoking, obesity, HTN, diabetes mellitus, hypertriglyceridemia, low HDL, AST, ALT, GGT | NAFLD pts had comparable eGFRs (65.1±8.3 vs 65.6±7.8ml/min/1.73m2) and significantly higher prevalence of CKD (31.7% vs 21.6%) than those without. NAFLD was independently associated with increased risk of CKD (OR, 1.68; 95%CI, 1.27–2.24). Similar results were found after classification according to the presence of hypertensive or diabetes mellitus (OR, 1.68; 95% CI, 1.27–2.24). |
| Targher G et al. [36](2/2012) | Cross-sectional study343 patients with type 1 diabetes | Hepatic ultrasono-graphy | eGFR<60ml/min/1.73m2 and/or abnormal albuminuria(ACR≥30mg/g) | Age, gender, BMI, diabetes duration, HbA1c, smoking, family history of CVD, physical activity, systolic BP, triglycerides, HDL cholesterol, eGFR, medications (anti-hypertensive, lipid-lowering drugs) | NAFLD pts had significantly lower eGFRs (83±27 vs 93.3±29ml/min/1.73m2) and a greater prevalence of abnormal albuminuria (50% vs 20.5%) and CKD (54.4% vs 24.2%) than those without steatosis. NAFLD was significantly associated with an increased risk of either abnormal albuminuria (OR, 2.21; 95% CI, 1.2–4.1) or CKD (OR, 1.93; 95% CI, 1.1–3.6) even after adjustments. |
| Arase Y et al. [41](5/2011) | Retrospective studyStudy cohort of 5561 NAFLD ptsMean follow-up period 5.5 years | Hepatic ultrasono-graphy | eGFR<60ml/min/1.73m2or proteinuria(dipstick test 1+ or greater) | Age, gender, BMI, T2DM, HTN, baseline eGFR, smoking, AST, ALT, GGT, total protein, triglycerides, total cholesterol, HDL cholesterol, hemoglobin, white blood cell, platelets | In Japanese NAFLD pts the cumulative development rate of CKD was 3.1% at the 5th year and 12.2% at the 10th year. Aging, T2DM, hypertension and elevated GGT are factors of low eGFR level that increased the risk of CKD development. In NAFLD pts with normal GGT levels the cumulative rate of CKD was lower than (11.3% vs 21.3%) than those with elevated GGT level. The annual incidence of CKD in Japanese pts with NAFLD was about 1.2%. |
| Hwang ST et al. [37](6/2010) | Cross-sectional population-based study 1361 participants with type 2 diabetes (n=414) and prediabetes (n=947) | Hepatic ultrasono-graphy | Abnormal albuminuria(ACR 30–300μg/mg creatinine) | Age, gender, BMI, waist circumference, liver enzymes, triglycerides, LDL cholesterol, HbA1c, insulin resistance, HTN, smoking, metabolic syndrome | NAFLD pts had significantly higher prevalence rates of microalbuminuria (6.3% vs 19% in prediabetes, 4.5% vs 32.6% in diabetes) and also had a significantly greater ACR (14.6±52.0μg/mg Cr vs 27.7±63.9μg/mg Cr in prediabetes and 11.4±21.4μg/mg Cr vs 44.7±76.4μg/mg Cr in diabetes) than those without. NAFLD was independently associated with increased rates of microalbuminuria (OR, 3.66; 95% CI, 1.31–10.20 in prediabetes and OR, 5.47; 95% CI, 1.01–29.61 in diabetes). |
| Targher G et al. [28] (4/2010) | Cross-sectional study202 patients with type 1 diabetes | Hepatic ultrasono-graphy | eGFR≤60ml/min/1.73m2 and/or abnormal albuminuria(microalbuminuria ACR>2.5 for men> 3.5 for women and macroalbuminuria ACR>30mg/mmol) | Age, gender, BMI, systolic BP, diabetes duration, alcohol consumption, HbA1c, triglycerides, use of anti-hypertensive or lipid-lowering drugs | NAFLD pts had significantly lower eGFRs (98±25 vs 107±28ml/min/1.73m2) and higher prevalence of abnormal albuminuria (36.4% vs 4.4%) and CKD (37.8% vs 9.9%) than those without. NAFLD was independently associated with increased risk of CKD (OR, 3.90; 95% CI, 1.2–9.1). |
| Targher G et al. [33](8/2008) | Prospective studyStudy cohort of 1760 outpatients with type 2 diabetesMean follow-up period6.5 years | Hepatic ultrasono-graphy | eGFR<60ml/min/1.73m2 and/or overt proteinuria(≥300μg/mg creatinine) | Age, gender, BMI, waist circumference, BP, smoking, diabetes duration, HbA1c, HDL and LDL cholesterol, triglycerides, baseline eGFR, microalbuminuria, medications (hypoglycemic, lipid-lowering, anti-hypertensive or anti-platelet drugs) | NAFLD was significantly associated with an increased risk of CKD even after adjustments (HR, 1.49; CI 95%, 1.1–2.2). |
| Chang Y et al. [32] (4/2008) | Prospective studyStudy cohort of 8329Non-diabetic and non-hypertensive Korean maleMean follow-up period3.21 years | Hepatic ultrasono-graphy | eGFR<60ml/min/1.73m2 or proteinuria(dipstick test 1+ or greater) | Age, baseline GFR, BMI, triglycerides, HDL and LDL cholesterol, fasting serum glucose, systolic BP, insulin resistance, C-reactive protein, smoking, alcohol consumption, incident diabetes, incident HTN | NAFLD was associated with the development of CKD (CRR, 2.18; 95% CI, 1.75–2.71); and this relationship remained significant even after adjustments (aRR, 1.55; 95% CI, 1.23–1.95). The association between NAFLD and incident CKD was independently evident in the NAFLD group with elevated GGT (aRR, 2.31; 95% CI, 1.53–3.50) but not in the NAFLD group without elevated GGT. |
| Targher G et al. [27] (3/2008) | Cross-sectional studyOutpatient-based cohort of 2103 pts with T2DM | Hepatic ultrasono-graphy | eGFR≤60ml/min/1.73m2 and/or overt proteinuria (ACR>30mg/mmol) | Age, gender, BMI, waist circumference, smoking, alcohol consumption, HTN, LDL and HDL cholesterol, triacylglycerol, HbA1c, diabetes duration and medications (hypoglycaemic, lipid-powering, anti-hypertensive or anti-platelet drugs) | NAFLD pts had significantly lower eGFRs (91±32 vs 96±31ml/min/1.73m2) and higher prevalence of microalbuminuria (20% vs 15%) and CKD (15% vs 9%) than pts without NAFLD. NAFLD was significantly associated with increased rates of CKD even after adjustments (OR, 1.87; 95% CI, 1.3–4.1). |
| Yeung MW et al. [111](10/2017) | Cross-sectional studyStudy cohort of 1763 Chinese patients withtype 2 diabetes | Ultrasono-graphic elasto-graphy | Abnormal albuminuria(ACR≥3.5mg/mmol in women and≥2.5mg/mmol in men) | Age, gender, BMI, educational levels, employment status, smoking, retinopathy, systolic BP, family history of diabetes, duration of diabetes, HDL cholesterol, triglycerides, HbA1c,, medications (diabetes, lipid-lowering, anti-hypertensive drugs) | Diabetic pts with liver steatosis and those with advanced fibrosis had significantly higher prevalence of albuminuria (no NAFLD vs liver steatosis vs advanced fibrosis: 41.4% vs 46.2% vs 64.2%). Advanced fibrosis but not liver steatosis was significantly associated with an increased risk of albuminuria even after adjustments (OR, 1.52; 95% CI, 1.02–2.28). Severity of liver fibrosis was significantly associated with albuminuria even after adjustments (OR, 1.53; 95% CI, 1.07–2.20). |
| Micolasevic I et al. [95](9/2013) | Cross-sectional study62 patients with CKD | Ultrasono-graphic elasto-graphy | eGFR≤60ml/min/1.73m2 | No adjustments | CKD pts had a high prevalence of NAFLD (85.5%). The severity of liver steatosis was negatively correlated with eGFR (r=−0.4) but the presence of liver fibrosis was not significantly correlated with eGFR. Severity of liver steatosis was significantly associated with decreasing eGFR (eGFR and staetosis score 1, 2 and 3: 42±6 vs 37±5 vs 25±6, respectively). |
NAFLD: nonalcoholic fatty liver disease; NASH: nonalcoholic steatohepatitis; CKD: chronic kidney disease; pts: patients; eGFR: estimated glomerular filtration rate; ACR: albumin-creatinine ratio; BMI: body mass index; HTN: hypertension; BP: blood pressure; HOMA-IR: homeostasis model assessment-insulin resistance; OR: odds ratio; CI: confidence interval; AER: albumin excretion rate; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; HbA1c: Hemoglobin A1c; HR: hazard ratio; NFS: NAFLD fibrosis score; GGT: gamma-glutamyltransferase; CVD: cardiovascular disease; T2DM: type 2 diabetes mellitus; CRR: crude relative risk; aRR: adjusted relative risk.
Several large cross-sectional population and hospital-based studies, involving both adults without diabetes and patients with diabetes found that the prevalence of CKD in patients with NAFLD ranged from approximately 20% up to almost 55% compared to 5–35% in patients without NAFLD [7,9,25,32,33,41,42]. Importantly, most of these studies reported that NAFLD was associated independently with the presence and severity of CKD even after adjusting for traditional cardiorenal risk factors [7]. Case-control studies that used liver biopsy (the gold standard) to diagnose NAFLD have shown that patients with histologically defined NAFLD have lower eGFRs and a greater prevalence of CKD or abnormal albuminuria than matched controls [7,39]. Additionally, these studies showed an aggraded positive relationship between the histologic severity of NAFLD and kidney disease independently of several risk factors for CKD, including metabolic syndrome features [7].
In a retrospective study in Japan that included 5.561 NAFLD patients diagnosed by ultrasonography without CKD at baseline the cumulative development rate of CKD was 3.1% at the 5th year and 12.2% at the 10th year. Furthermore, the annual incidence of CKD in Japanese patients with NAFLD was about 1.2%. In this study the co-existence of five factors (low level of eGFR of 60–75mL/min/1.73m, age ≥50 years, T2DM, hypertension and elevated gamma-glutamyltransferase of ≥109IU/L,) increases the risk of developing CKD.
In a meta-analysis performed by Musso et al. [6] NAFLD patients, diagnosed by histology, ultrasound or liver enzymes elevation were shown to have an increased prevalence and incidence of CKD. NASH was associated with a higher prevalence and incidence of CKD than simple steatosis and advanced fibrosis was associated with a higher prevalence and incidence of CKD than non-advanced fibrosis. These associations remained statistically significant in diabetic and non diabetic individuals, as well as in studies adjusting for traditional risk factors for CKD and were independent of whole body/abdominal obesity and insulin resistance. Additionally, it was reported that NAFLD was associated with an approximate two fold increased risk of CKD [6,25]. However, it is important to underline that none of these studies used renal biopsy to examine the pathology of CKD, so whether NAFLD is associated with a specific type of kidney disease is currently unknown [9]. In future, large prospective studies on histologically diagnosed NAFLD patients that include CKD incidence in their long-term outcomes are needed to confirm the causal relationship between NAFLD and CKD [9,43].
4Risk factorsNAFLD is associated with insulin resistance, obesity, glucose intolerance, dyslipidemia and hypertension, which are all risk factors of CVD. Increasing evidence indicate that NAFLD is associated with CVD especially with subclinical atherosclerosis (carotid artery wall thickness) and subclinical CVD outcomes [coronary artery calcium (CAC) and abdominal artery calcium (AAC)] so that it is currently believed that one of the most important causes of death in NAFLD patients are the cardiovascular complications [44–53]. The mechanisms that link the two diseases are not clear. Notably, a 10-year follow-up study performed at Mayo Clinic reported that malignancy (28%) was the most common cause of death in US NAFLD subjects followed by ischemic heart disease (25%) and liver disease (13%) [54]. Additionally, it is believed that NAFLD is associated with a higher prevalence of retinopathy in diabetics [27,28]. Obesity, hyperglycemia, T2DM and hypertriglyceridemia are the best known risk factors for these associations. There is also emerging evidence that NAFLD is linked to other chronic diseases, such as sleep apnea, colorectal cancers, osteoporosis, psoriasis and various endocrinopathies (e.g. polycystic ovary syndrome) [25].
Common metabolic risk factors such as obesity, insulin resistance, dyslipidaimia and hypertension are involved in the development and progression of both NAFLD and CKD. This fact precludes a direct causal association between the two diseases and an assessment of the predictive value of NAFLD for CKD [5,7,55]. Furthermore, the relationship with these risk factors is reciprocal and so it is even more difficult to understand their exact pathophysiological mechanisms [56,57]. In NAFLD arterial hypertension is believed to constitute a relatively modest risk factor as compared to other factors [58]. Obesity is an independent risk factor for both diseases [59,60]and although it is an important risk factor for NAFLD it is currently known that hepatic steatosis is also present in non-overweight patients [61].
T2DM predicts NAFLD independently of age and obesity and supports the role of hepatic insulin resistance in the pathogenesis of this disease [62]. The liver plays a major role in systemic metabolism contributing to the development of insulin resistance and T2DM. The underlying mechanisms are not fully understood but are thought to include hepatic fat accumulation, alterations of energy metabolism, lipotoxins, mitochondrial function, inflammatory cytokines, adipocytokines, intestinal dysbiosis as well as genetic, nutritional and lifestyle factors. It is known that more than 70% of patients with T2DM have NAFLD. On the other hand, NAFLD patients are at increased risk (twofold to fivefold) of developing insulin resistance and T2DM. As a result, not only NAFLD is strongly associated with insulin resistance and T2DM but also T2DM commonly coexists together with NAFLD and especially NASH [63]. Regarding CKD, it is known that T2DM is the most common cause of kidney injury [64,65]. T2DM is one of the common characteristics of NAFLD and CKD, whereas all these disturbances are simultaneously components of the metabolic syndrome.
On the other hand, several studies in diabetic and non diabetic, hypertensive and non-hypertensive individuals demonstrated that NAFLD patients exhibit increased incidence of CKD, independently of important risk factors and potential confounders, such as insulin resistance and metabolic syndrome [32,33]. It is obvious that NAFLD and CKD participate in a complex multisystem condition, in which common risk factors are involved and thus the understanding of a cause-effect relationship is currently extremely challenging.
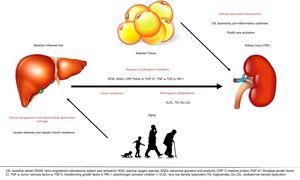
5Potential mechanismsThe underlying mechanisms that are putatively responsible for the observed association between NAFLD and kidney disease are not fully understood [39]. The liver is the key regulator of glucose and lipid metabolism as well as the main source of inflammatory elements and is thought to be involved in the development of cardiovascular and kidney disease [43]. Currently, however, there is mounting evidence to suggest that NAFLD, especially in its necro-inflammatory form (NASH), is not only a marker of kidney damage but it might also be involved in its pathogenesis. Possible mechanisms are the systemic release of pathogenic mediators from the steatotic and inflamed liver, including increased reactive oxygen species, advanced glycation end products, C-reactive protein (CRP) [39], pro-inflammatory, pro-fibrogenic, and anti-fibrinolytic molecules, including fetuin-A, fibroblast growth factor (FGF)-21, tumor necrosis factor (TNF)-a, transforming growth factor (TGF)-b and plasminogen activator inhibitor-1 (PAI-1), that all have the ability to promote kidney injury [6]. The existence of pathways linking liver and kidneys is also supported by the presence of hepatorenal syndrome, which can develop in cirrhotic patients with portal hypertension [9]. Despite the growing evidence linking NAFLD to CKD, their causal association has not been definitely established. Evidence suggest that NAFLD exacerbates insulin resistance, predisposes to atherogenic dyslipidaemia and causes the release of pathogenic mediators that are important in the pathophysiology of CKD [9,66].
NAFLD and CKD share common risk factors and therefore both liver and kidney injury may be driven by obesity-associated mechanisms of disease, including lipotoxicity, oxidative stress, enhanced pro-inflammatory cytokine and renin-angiotensin-aldosterone system (RAAS) axis activation [6]. It is known that obesity is an independent risk factor for CKD and it is associated with the development of proteinuria and pathologic findings of podocyte hypertrophy and focal segmental glomerular sclerosis even in the absence of diabetes and hypertension. In addition, studies have shown that obesity as well as metabolic syndrome is a strong predictor of the development of NAFLD. While the complex “crosstalk” among adipose tissue, the liver, and kidneys make it difficult to delineate the specific processes underlying NAFLD as a cause of CKD, it is not surprising that these diseases are linked [43]. However, NAFLD may promote CKD independently of coexisting risk factors. Furthermore, fatty liver may damage the kidney through VLDL lipoprotein over-secretion and induction of atherogenic dyslipidemia, as triglyceride-rich lipoproteins and oxidized LDLs promote glomerular injury and mesangial cell proliferation [6].
Oxidative stress and particularly mitochondrial dysfunction are thought to play a role in the pathogenesis of NAFLD and the development of CKD by producing reactive oxygen species (ROS) [67,68]. Increased visceral adipose tissue in NAFLD patients is also a significant source of ROS. Generation of ROS impacts insulin signaling pathways leading to insulin resistance and tissue inflammatory infiltrate [67]. Increased levels of ROS, oxidative stress and inflammatory response are thought to be involved in the development and progression of CKD, too [68]. Interestingly, recent data support that biomarkers of oxidative stress and inflammation (malondialdehyde [MDA], advanced oxidant protein products [AOPP]) could identify patients at increased risk of developing fatty liver and CKD [69,70].
Advanced liver disease and liver-related complications in patients with NAFLD are more often in the 6th through 8th decades of life. This finding could be related to increasing rate of fibrotic progression with age or to mitochondrial dysfunction (which causes steatosis and hepatic insulin resistance) developing in the elderly. In addition, genetic factors undoubtedly predispose to NAFLD/NASH, as well. Kind reds with more than 1 family member affected have been reported, whereas a family history of T2DM was found in two thirds of cases [71]All the pathways that are currently believed to explain the association between NAFLD and CKD are shown in Fig. 1. Understanding of all these mechanisms may lead to modifiable risk factors and therapeutic targets for the prevention and treatment of NAFLD and CKD [43].
The belief that NAFLD and CKD share common pathogenetic mechanisms for progression leads to the hypothesis that they can also share potential therapeutic targets [72,73]. During the recent years several therapeutic agents have been tested in phase II and III clinical trials (Obeticholic acid [OCA], elafibranor, ASK1 inhibitor, Cenicriviroc [CVC]) [72,74–79]. Their main goals are to correct relevant metabolic abnormalities and halt hepatic fibrosis development. These agents are thought to protect from renal inflammation and kidney fibrosis, too [72,75–77]. Furthermore, two new classes of anti-diabetic drugs (GLP-1RA and SGLT2 inhibitors) that exert beneficial effects on renal function are currently tested in patients with NASH, too [74,80,81].
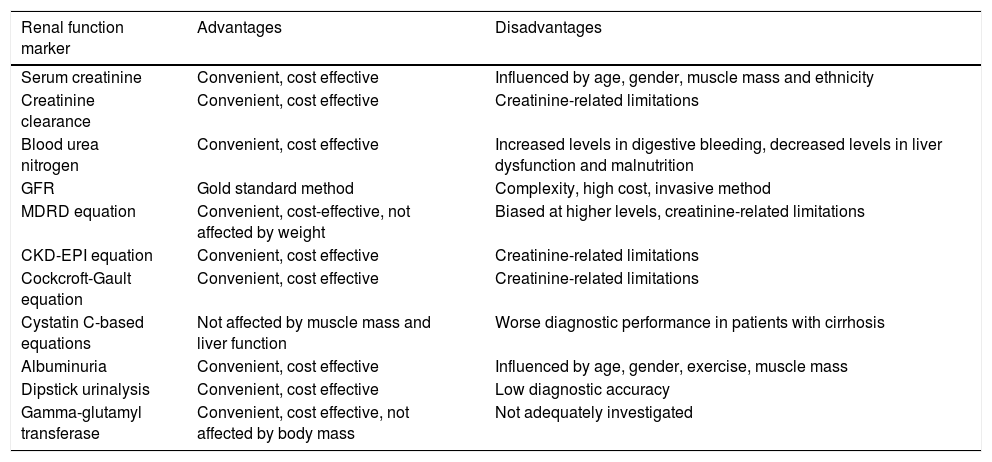
6Renal function markers – applicability in patients with NAFLDThe accurate assessment of renal function in NAFLD patients is of vital importance for their clinical outcome, given the high prevalence of CKD in these patients. However, so far there are not enough published studies evaluating the diagnostic accuracy of renal function markers in these patients. The majority of studies have focused on patients with liver cirrhosis or diabetes. Therefore, most of the following data mainly refer to these patients rather than especially NAFLD patients, although kidney function assessment at least in non-cirrhotic NAFLD patients is expected to have similar results with those found in patients with diabetes. Table 2 summarizes the advantages and disadvantages of each below described renal function marker that we propose to use for kidney function assessment in NAFLD patients in clinical practice.
Comparison of the different markers of renal function in patients with NAFLD.
| Renal function marker | Advantages | Disadvantages |
|---|---|---|
| Serum creatinine | Convenient, cost effective | Influenced by age, gender, muscle mass and ethnicity |
| Creatinine clearance | Convenient, cost effective | Creatinine-related limitations |
| Blood urea nitrogen | Convenient, cost effective | Increased levels in digestive bleeding, decreased levels in liver dysfunction and malnutrition |
| GFR | Gold standard method | Complexity, high cost, invasive method |
| MDRD equation | Convenient, cost-effective, not affected by weight | Biased at higher levels, creatinine-related limitations |
| CKD-EPI equation | Convenient, cost effective | Creatinine-related limitations |
| Cockcroft-Gault equation | Convenient, cost effective | Creatinine-related limitations |
| Cystatin C-based equations | Not affected by muscle mass and liver function | Worse diagnostic performance in patients with cirrhosis |
| Albuminuria | Convenient, cost effective | Influenced by age, gender, exercise, muscle mass |
| Dipstick urinalysis | Convenient, cost effective | Low diagnostic accuracy |
| Gamma-glutamyl transferase | Convenient, cost effective, not affected by body mass | Not adequately investigated |
Creatinine is the breakdown product of creatine, which is synthesized in the liver and primarily stored in muscle tissue. Thus, serum creatinine is highly correlated with body mass and is consequently influenced by a number of variables such as age, gender, muscle mass and ethnicity, leading to major limitations in its interpretation [14]. In an elegant paper Papadakis et al. [82] studied 23 non-azotemic cirrhotic patients with ascites. Patients were divided into three groups on the basis of initial GFR: group I consisted of patients with supra normal filtration rates (mean 183ml/min), patients with normal filtration rates (mean 92ml/min) constituted group II whereas group III comprised of patients with severely impaired filtration rates (mean 32ml/min). Serum creatinine level was below 1.5mg/dl in all three groups and frequently failed to rise above normal even when the glomerular filtration rate was very low (less than 25ml/min) [82].
The reference method for creatinine clearance determination is based on the measurement of creatinine concentrations in urine and serum (mCrCl). Several formulas are available for the assessment of creatinine clearance such as Cockcroft-Gault (CG), Simplified Modification of Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology (CKD-EPI) [83]. According to Beben and Rifkin, creatinine clearance estimation based on a 24-h urine sample is not a reliable method to estimate GFR. Even though it would help to adjust for the low creatinine production in cirrhotic patients, this method has been shown to overestimate measured GFR (mGFR) in many studies, as creatinine is not only filtered at the glomerulus but also secreted by the tubules [15,16,84].
Blood Urea Nitrogen (BUN) is considered a less accurate biomarker of renal function, especially in cases of impaired liver function, where the production rate of urea is unstable. BUN level can rise after a digestive bleeding or decrease as a consequence of liver dysfunction due to impaired urea cycle or malnutrition [85].
GFR, MDRD equation, CKD-EPI equationGFR is considered the best overall index of kidney function [86]. The gold standard method for the measurement of GFR is the indirect determination of exogenous filtration markers, such as inulin, iothalamate, or radioactive tracers that are excreted by the kidney only by glomerular filtration [14,87]. However, this method is not routinely available because of the complexity of measurement protocols [88]. Instead, clinicians usually rely on endogenous creatinine clearance. The GFR can be estimated from serum levels of endogenous filtration markers, such as creatinine and cystatin C, using certain equations, without requiring calculation of clearance [87]. The most commonly used equations are MDRD and CKD-EPI [87]. Variables included in the CKD-EPI equation for estimating log GFR are log serum creatinine, sex, race and age on the natural scale (GFR=141×min (Scr/κ, 1)α×max (Scr/κ, 1)−1.209×0.993Age×1.018 [if female]×1.159 [if black], where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 [86]. MDRD Study equation is calculated using log serum creatinine without a spline, sex, race and age on the log scale (estimated GFR=175×standardized Scr−1.154×age−0.203×0.742 [if female]×1.212 [if black], where GFR is expressed as mL/min/1.73m2 of body surface area and Scr is expressed in mg/dL [89]. Given that the MDRD equation was developed in people with CKD, it is characterized by systematic underestimation of measured GFR (bias) at higher levels, in contrast to CKD-EPI equation [86]. Both equations are based on serum creatinine levels. Thus, both methods suffer from the limitations of this marker [14].
Cystatin C, a low molecular mass plasma protein, has, recently, been proposed as a potential replacement for serum creatinine as a filtration marker [90,91]. Several studies have demonstrated that cystatin C is a sensitive marker of renal function. In a study performed on 52 Caucasian T2DM patients, Mussap et al. [91] suggested that cystatin C is a more accurate serum marker than serum creatinine or the Cockcroft and Gault estimated GFR in discriminating T2DM patients with reduced GFR from those with normal GFR. In a 4-year follow up study of 30 participants with T2DM, Perkins et al. [92] concluded that cystatin C is an accurate alternative to gold standard GFR, providing sufficient precision for detecting longitudinal trends in GFR in the normal and hyperfiltration ranges, unlike creatinine-based estimates. Considering that most patients with NAFLD are obese, the question arises as to whether conventional markers of renal function are accurate in these patients. According to Gary et al. [93], application of the MDRD equation to individuals with extremes of body size or muscle mass could lead to inaccurate GFR estimation, as it has not been tested in such groups. However, in 2010, Michels et al. [94] demonstrated that both MDRD and CKD-EPI equations were not influenced by BMI.
As previously stated, T2DM is present in many patients with NAFLD [95]. According to the Kidney Disease Outcomes Quality Initiative (KDOQI) guideline, the MDRD equations have no limitations in patients with diabetes [23]. In a study of 166 patients with CKD, 91 of whom with T2DM, the MDRD formula was found more accurate than the equations based on cystatin C in patients with diabetes [96]. In 2004, Ibrahim et al. [97] concluded that the Cockcroft-Gault estimate had minimal bias and was more accurate at a GFR of 60–120ml/min per 1.73m2, whereas the MDRD equation estimate was more accurate at GFR>120ml/min per 1.73m2.
The diagnostic accuracy of creatinine-based equations has not been fully studied in NAFLD as most studies have focused on patients with liver cirrhosis. All methods of estimating GFR that employ creatinine have well recognized limitations in cirrhotic patients. As mentioned above, serum creatinine is highly correlated with body mass. Cirrhotic patients tend to produce less creatine, and consequently creatinine due to impaired liver function, low muscle mass, and protein malnutrition [14]. Additionally, these patients are characterized by increased volume of creatinine distribution in the presence of ascites and altered body composition due to muscle wasting that affect standard normalization for body surface area [14]. Furthermore, cirrhotic patients with the lowest GFRs tend to secrete more creatinine in their tubules. As a result, all creatinine based equations tend to overestimate GFR in patients with liver disease and the degree of overestimation is highest at lower GFR values and in more severe liver disease [14]. Several studies have demonstrated that the MDRD4 equation has less bias and better accuracy as compared to the Cockcroft Gault [98–100], whereas the CKD-EPI equation has conflicting results [14].
In contrast to conventional creatinine based equations, cystatin C is unrelated to muscle volume and liver function [19]. Many studies have revealed that cystatin C based eGFR equations have superior diagnostic accuracy for estimating GFR as compared to creatinine based equations. Especially, CKD-EPI equation that combines serum creatinine and cystatin C measurements has the best performance, particularly at advanced stages of cirrhosis [16–19,100] although this was not shown in all relevant studies [17]. Of note, some studies have shown that cystatin C is not a superior marker of renal function compared to creatinine based eGFR in cirrhosis, suggesting that new specific formulas are needed [101,102]. In a study of 129 patients with decompensated cirrhosis, Cholongitas et al. [103] demonstrated that all conventional serum creatinine-based and serum cystatin C-based formulae had major limitations in patients with decompensated cirrhosis and proposed a new eGFR formula that was based on age, serum creatinine, and cystatin C (New GFR=163−[creatinine×(19.6)]−[Cystatin C×(11.8)]−[age×(0.86)]). This new formula had a strong correlation with 51Chromium-ethylenediaminetetraacetic acid GFR and significantly lower bias, compared to the other commonly used eGFRs, providing a more accurate evaluation of renal function in cirrhotic patients and certainly needs further assessment to confirm its accuracy.
7AlbuminuriaAlbuminuria, defined as urine albumin/creatinine ratio (ACR) ≥30mg/g, is a diagnostic component of CKD [21]. Albumin is not filtered at the glomerulus and its presence in the urine at concentration above 30mg/day is suggestive of glomerular damage [104]. In fact, several studies suggest that albumin may be involved in the pathophysiology of renal failure [105]. The preferred method for assessment of albuminuria is urine ACR measurement in the first-void spot urine specimen [106]. In cases where this method is impossible, a random spot urine specimen is acceptable, although some studies showed that ACR measured on random urine samples appears to overestimate the prevalence of albuminuria compared to the first morning urine collections [106,107]. Urinary ACR is affected by several factors such as age, gender, exercise, muscle mass and others [23].
Microalbuminuria, which is defined as the urinary albumin excretion of 30–300mg/24, has been strongly associated with T2DM and it has been included by the World Health Organization as a possible component of the metabolic syndrome [108,109]. However, it yet remains undefined whether microalbuminuria is associated with NAFLD among patients with prediabetes or diabetes. Several studies have revealed a positive correlation between microalbuminuria and NAFLD. In 2009, Casoinic et al. [110] found that microalbuminuria was significantly more frequent in NAFLD patients with T2DM than in controls. In a study of 1361 patients with prediabetes and diabetes, Hwang et al. [37] demonstrated that NAFLD patients had higher levels of microalbuminuria and greater ACR than those without NAFLD. Yeung et al. [111] also reported that advanced liver fibrosis, but not steatosis, is independently associated with albuminuria in T2DM patients. However, in another study of 87 non diabetic biopsy-proven NAFLD patients, Yilmaz et al. [38] failed to prove any association between the presence of microalbuminuria and the degree of hepatic steatosis. Nonetheless, the researchers found that mean fibrosis scores were higher in patients with microalbuminuria than those without[38].
8Dipstick urinalysisDipstick urinalysis is a convenient and cost-effective alternative method for proteinuria assessment. Nevertheless, its diagnostic accuracy has not yet been sufficiently evaluated. According to the National Kidney Foundation, this diagnostic modality is only suggested for adults at low risk for developing CKD [112]. Patients at high risk for developing CKD, especially diabetic patients, who have a negative result for protein on a standard dipstick test should be tested with either an albumin-specific dipstick or an untimed urine measurement for ACR [112]. In a study of 11.247 individuals, White et al. [113] highlighted the high negative predictive value of a dipstick test result <1+ or less than trace in the general community setting, as well as its high false-positive rates, suggesting laboratory confirmation of positive results. Several studies have evaluated the diagnostic accuracy of urine dipsticks in diabetic patients, with controversial results. Nagrebetsky et al. [114] studied 98 patients with T2DM, concluding that dipstick testing did not reliably identify diabetes patients with microalbuminuria. However, Nah et al. [115] suggested that ACR strip test could be used to screen for albuminuria in cases of prediabetes and diabetes, showing high sensitivity, specificity, and negative predictive value in a study of 501 patients with prediabetes and diabetes.
9Gamma-glutamyl transferase (GGT)Recently, GGT has been proposed as an early predictor for the development of CKD. In 2007, Ryu et al. [35] studied 10.337 nonhypertensive and nondiabetic males, demonstrating an increased risk for CKD with an increasing quartile of serum GGT. Given that patients with diabetes and hypertension were excluded from this study, GGT appears to be independently associated with CKD. In 2008, Chang et al. [32] studied 8.329 NAFLD patients and healthy controls and revealed an association between NAFLD and CKD in patients with elevated GGT, that was not noted in the NAFLD group without elevated GGT, implying an independent association between GGT serum level and CKD. Further investigation is required in order to establish whether GGT could be used as a predictor of CKD in NAFLD patients.
10ConclusionThe link between NAFLD and CKD is an emerging association with potentially significant impact on the prognosis of patients that are diagnosed with both conditions. Common metabolic risk factors for both diseases can explain the occurrence of this association. The early recognition of this association through accurate assessment of renal function is mandatory for the effective management of these patients. Formulas for the estimation of GFR and assessment of albuminuria through calculation of ACR seem to be currently the most relevant methods for this purpose. As pointed out throughout this review, these conclusions are mainly based on studies including non-cirrhotic patients with or without diabetes mellitus as the comparative diagnostic accuracy of the discussed methods specifically in patients with NAFLD has not been extensively studied. Thus, there is an undoubted need for large, prospective studies, in order to draw safer conclusions , with direct applicability in daily clinical practice.AbbreviationsNAFLD non-alcoholic fatty liver disease type II diabetes mellitus cardiovascular disease CVD chronic kidney disease glomerular Filtration Rate estimated glomerular filtration rate non-alcoholic steatohepatitis albumin-to-creatinine ratio body mass index coronary artery calcium abdominal artery calcium C-reactive protein fibroblast growth factor-21 tumor necrosis factor-a transforming growth factor-b plasminogen activator inhibitor-1 reactive oxygen species malondialdehyde advanced oxidant protein products renin-angiotensin-aldosterone system very-low density lipoproteins low-density lipoproteins Obeticholic acid Cenicriviroc measurement of creatinine concentrations in urine and serum Cockcroft-Gault simplified modification of diet in renal disease chronic kidney disease epidemiology measured GFR blood urea nitrogen kidney disease outcomes quality initiative gamma-glutamyl transferase
No financial support.
Conflict of interestThe authors have no conflicts of interest to declare.