IApproximately 30% of patients with chronic HCV infection have persistently normal alanine aminotransferase levels (PNALT). Most of these patients have minimal or mild inflammation and absent or minimal fibrosis, although occasionally cirrhosis and hepatocarcinoma may be seen. Overall, liver histology is significantly less severe than in patients with elevated ALT levels, and most follow-up studies have reported stability of the disease, with minimal fibrosis progression over years, and thus a disease with a favorable prognosis. Nevertheless, a few studies have shown more recently that many patients with PNALT, may have elevations in ALT over time, and almost 20-30% have a significant progression of fibrosis, being eligible for antiviral therapy. During the last decade it has been demonstrated that in chronic HCV infection with PNALT, combination antiviral therapy with peg interferon-alpha plus ribavirin is efficacious, safe, and associated with significant improvements in health-related quality of life, and the decision whether to treat or not this patients should be based on multiple factors including: age, HCV genotype, histology, patients motivation and adherence, symptoms and comorbidity, rather than on ALT levels alone. (182 words).
Approximately 3% of people worldwide (about 200 million people) are infected with hepatitis C virus (HCV).1,2 More than 80% of infected patients will develop chronic infection, and 10-20% evolves with spontaneous viral clearance through natural immunity.3 Most patients with chronic HCV have asymptomatic mild elevations of serum aminotransferase levels, without any clinical sign of liver disease.4
Around 25-30% of chronically HCV infected patients have repeatedly normal levels of serum alani-ne aminotransferase (ALT) and aspartate aminotransferase (AST), which may vary over time when patients are followed-up for many years.5-11 The natural history and management of this sub-group of patients with normal ALT, is a matter of controversy.
Definition of Normal Aminotransaminase LevelsElevations of serum ALT and AST levels usually serve as important markers for liver injury, although there is little correlation between serum ALT elevation and the degree of hepatic injury assessed though biopsy. Aminotransferase levels are measured by fluorometric assays and are influenced by many known factors, making possible certain variability between laboratories.12
The definition of normal aminotransferase levels has changed over time. Reference ranges for serum ALT and AST levels vary widely between different laboratories, usually considering “upper normal limits”, when levels are up to 40 U/L, making slight differences for women. Recent data has shown that when considering a “healthy” population, the upper limit of normal ALT should be around 30 U/L for males and 19 U/L for females,13 which would improve significantly the sensitivity of the test for the detection of patients with chronic asymptomatic liver diseases, but decreases the specificity of the test.
For clinical purposes, most studies evaluating management and treatment of the subgroup of patients with chronic HCV with normal ALT, have considered the historical definition by local laboratories. The concept of “persistently normal ALT” (PNALT) in patients with chronic HCV infection, varies in the literature, but most authors have defined PNALT when ALT measurements persisted less than 40 U/L on 2 or 3 different occasions separated by at least lmonth, over a period of 6 months.3 Some groups have extended further this period of observation of normal ALT, to up 18 months.14 In the following paragraphs we will discuss the natural history of this subgroup of patients with PNALT and their clinical management.
Natural History of Chronic Hcv Patients With PnaltThe natural history of chronic HCV infection is poorly understood.15,16 Most studies are retrospective, frequently being difficult to determine the onset of the viral infection, the liver damage progression is not linear over time, and many co-factors (obesity, coinfection, alcohol intake, etc) may contribute and affect the speed of development of fibrosis, cirrhosis and hepatocarcinoma. More recently an effective combined antiviral therapy (Peg-Interferon and Ribavirin) has been able to change its overall prognosis.
The histological progression of chronic hepatitis C over time has always been considered a matter of debate. Long-term follow-up studies have shown that around 20-30% of patients with chronic HCV infection, (prospectively followed-up after acute transfusion-associated non-A, non-B hepatitis), with elevated aminotransferase levels, developed cirrhosis after 30 years of follow-up, and once cirrhosis has developed, the risk of hepatocelular carcinoma is 14% per year.15-17 Most patients will die from other causes not related to liver disease, but rather from co-morbid conditions often accompanying this infec-tion.
Between the subgroup of patients with chronic HCV infection with PNALT, most studies have shown that liver disease is, on average, significantly less severe than in HCV patients with abnormal ALT.7,8,12,18-20 A review of 16 published biopsy studies that included 447 patients with normal ALT levels showed normal histology or nonspecific changes in 24%, chronic hepatitis with mild inflammation in 54%, chronic hepatitis with moderate inflammation with minimal or no fibrosis in 21%, and cirrhosis in less than 1% of cases21(Table 1). Most HCV carriers with PNALT levels are females.18,20 up to 50% have an infection due to non-1 genotype HCV,20 and the serum HCV RNA titer was significantly lower than in patients with elevated ALT levels.20
Initial biopsy findings from some studies including patients with chronic HCV infection with PNALT.
| Study (Reference) | Year | Patients With biopsy histology | (%) Patients with normal histology | (%) with moderate to severe chronic hepatitis histology | (%) with cirrhosis |
|---|---|---|---|---|---|
| Puoti C (8) | 1997 | 46 | 0% | 23% | 1% |
| Persico M (18) | 2000 | 37 | 8% | Not specified | Not specified |
| Martinot-Peignoux M (19) | 2001 | 108 | 11% | 40% | 0% |
| Renou C (47) | 2002 | 83 | 6% | Not specified | 0% |
| Hui CK (48) | 2007 | 82 | 3% | Not specified | 0% |
| Jamal MM (49) | 1999 | 75 | Not specified | Not specified | 6% |
| Puoti C (24) | 2002 | 691 | 17% | 4% | 1% |
| Okanoue T (32) | 2008 | 129 | 13% | 7% | 0% |
| Hervé S (50) | 2001 | 80 | 9% | Not specified | 1% |
| Puoti C (41) | 2009 | 72 | 19% | 18% | 1% |
Around 15-20% of HCV carriers with PNALT may have normal or near normal liver histology (Table 1). Liver damage in most HCV carriers with PNALT, is usually stable over time, with minimal or mild liver necroinflammation and minimal or absent fibrosis (cirrhosis is rare), making this subgroup of patients, a group with a favorable overall prognosis.18-22 The progression rate of liver fibrosis has been described as slower than in chronic HCV patients with elevated ALT levels.18-22 Nevertheless, 20-30% of these patients may eventually progress to significant inflammation and fibrosis over time, despite normal ALT.23 This is the reason why in the last few years, this subgroup of patients have been considered more often as candidates for antiviral treatment.
Persico, et al.,18 studied a cohort of 37 patients with chronic HCV with PNALT levels over a period of 7 years. Most patients persisted with normal ALT (73%) during the follow-up. The majority of patients had an initial liver biopsy showing mild chronic hepatitis, which did not change significantly during the follow-up, suggesting a benign course of these patients and a very slow or absent progression to cirrhosis. An extended updated follow-up of 24 of these PNALT patients for up to 12 years, confirmed that histological stage did not significantly change over time, and progression to cirrhosis was slow or absent as compared to HCV patients with increased ALT, where steatosis and staging were significantly higher.22 Similar results have been described by Martinot-Peignoux, et al.,19 after following 135 anti-HCV positive patients with PNALT during a mean of 3.6 years.
Puoti C, et al.,24 described that in a cohort of 691 Italian patients with chronic HCV with PNALT, 17% of patients had a normal liver histology, 34% minimal chronic hepatitis, 44% mild hepatitis, 4% moderate to severe hepatitis, and only 1% had cirrhosis.
In contrast with the previous authors, Okanoue, et al.,23 although agrees with the initial benign liver biopsy of most HCV patients with PNALT (90% had normal to mild liver histology not requiring initial therapy), demonstrated that during a 5 year follow-up, almost 30% of 129 patients, developed symptomatic chronic HCV, becoming at that time, real candidates for antiviral therapy. Lawson, et al.,25 also found lower Ishak fibrosis and necroinflamma-tory scores in the first liver biopsies of PNALT patients when compared to abnormal ALT patients, but he also raised his concerns on the follow-up of PNALT patients. He found that among PNALT patients (n = 87), over 60% of them increased their ALT (> 40 UI/L) over a period of 5 years, and 13% had an increase in fibrosis score of ≥ 2 points between liver biopsies (indicative of fibrosis progression rather than potential sampling error). The rate of fibrosis progression was calculated in 0.35 ± 0.94 Ishak fibrosis points/year during a median interval between biopsies of 31 months, and similar to patients with elevated ALT. Shiffman, et al.,20 also showed that PNALT patients with had significantly lower histological inflammation and fibrosis than patients with abnormal ALT levels, but almost two-thirds had initial portal fibrosis and around 10% bridging fibrosis. During the 72-week follow-up period, ALT activity increased over normal in many (53%) of the untreated patients and none became HCV RNA undetectable. Overall, cross-sectional and longitudinal studies have shown a slower progression of liver fibrosis at approximately 50% of the rate of HVC patients with elevated ALT.12
The definition of PNALT probably still requires a consensus. Some authors (Italian Association for the study of the liver) propose that one should follow-up this subgroup of patients for more prolonged periods of time (≥ 18 months) before labeling them as “persistently normal ALT patients” which apparently would really have a benign course over time.24
Evaluation of Severity of Liver Disease in Pnalt PatientsAlthough the liver biopsy is still the most used and best procedure to identify the stage of liver fibrosis and inflammation; it is invasive and may be associated to severe complications.26
During the last decade, biochemical markers such as Fibrotest and Fibroscan,27-29 have been used to identify liver fibrosis stage as a reliable and nonin-vasive method in patients with chronic HCV infection. These noninvasive methods have been explored in patients with chronic HCV infection and PNALT.
Colletta, et al.,30 studied 40 HCV infected patients with PNALT with 2 liver biopsies with a median interval of 78 months and Fibroscan and FibroTest. Fibroscan had better correlation with liver biopsy than FibroTest, and was able to identify 14 out of the 14 patients (100%) eligible for antiviral therapy (with METAVIR fibrosis score F2 or greater), and FibroTest only 9 patients (64%). Thus, both sensibility and specificity of Fibroscan to identify patients with significant fibrosis was 100% in this study, using the previously reported cutoff values of 8.7 and 9.6 kPa.
Sebastini G, et al.,31, studied noninvasive markers for liver fibrosis (Forns index, AST-to-ALT ratio (AAR), FibroTest and the recently proposed Fibroindex, using liver histology as reference standard) and showed a good overall positive predictive value for significant fibrosis in PNALT patients, but none of the markers had a good negative predictive value to exclude significant fibrosis, resulting in a 30-40% misclassification rate. This study concluded that liver biopsy is still needed to correctly stage liver fibrosis and noninvasive methods and combination of markers needs to be validated in this subgroup of patients with PNALT levels.
Other authors32 have added another noninvasive parameter, the platelet count (< 150.000/uL), as a useful tool to include in the algorithm to decide whether to treat or not PNALT patients. Normal ALT patients with chronic HCV infection, with platelet count < 150.000 /uL had significantly higher rates of F2 and F3 fibrosis at baseline (32/68 patients; 47%) and during follow-up, than those with normal platelet count (33/141 patients; 23%) (p< 0.001), making easier the decision to start antiviral therapy in normal ALT thrombocytopenic patients without a liver biopsy.
Treatment of Chronic Hcv Patients With PnaltThe final decision whether to treat or not the patients with chronic HCV and PNALT needs to be individualized and should take into account different clinical and prognostic factors including: age, HCV genotype, liver histology, patient motivation, adherence, extrahepatic manifestations of HCV infection, potential contraindications for antiviral therapy, and comorbidity.33-35 In those patients infected with genotypes 2 or 3, and considering their excellent viral response rates, one should probably start antiviral therapy, unless any contraindications are present.
The first few old studies related to antiviral therapy in chronic HCV PNALT patients (with standard interferon monotherapy), found a low rate of response and raised concerns about worsening disease activity during and after antiviral therapy particularly in non-responders.21,33 Based on this studies and the apparent benign course of chronic HCV infection with PNALT levels, both the European Associaion for the Study of the Liver (EASL) and the National Institutes of Health (NIH) consensus conference stated initially that treatment in PNALT patients was not recommended.36,37 However these initial concerns have not been confirmed in more recent studies with combined antiviral therapy and thus treatment decisions should not be based only on the serum ALT levels.
There are only a few clinical studies with antiviral treatment in patients with chronic HCV infection with PNALT, because routinely they have been excluded from large randomized treatment trials and thus the efficacy and safety of combined therapy was not very clear.
Bini, et al.,38 treated a group of 46 chronic HCV PNALT patients with Interferon alpha-2b (3 million units subcutaneously three times per week) plus ri-bavirin (1000-1200 mg /day) for 24 weeks (Genotype 2/3; n = 10) or 48 weeks (Genotype 1; n = 36). They compared them with 92 matched HCV patients with abnormal ALT levels. Overall, a sustained virological response (SVR) was noted in 32% of PNALT patients and in 28% of those with abnormal ALT levels (P = 0.60). Patients with PNALT and genotype 1 had an SVR of 25%, and those with Genotype 2 or 3 had an SVR of 60%, very similar to the response of patients with abnormal ALT levels (20% and 50% respectively; p = NS). Three patients with PNALT had mild transient ALT elevations during therapy and antiviral treatment was associated with an overall improvement in the quality of life in both groups of patients.
The introduction of the new combination antiviral therapy with Peginterferon brought higher SVR rates in all HCV infected patients. In the largest multinational study so far on patients with PNALT, Zeuzem, et al.,39 randomized 491 chronic HCV infected PNALT patients to no therapy (69 patients) or to treatment with peginterferon alfa-2a 180 mug/wk plus ribavirin 800 mg/day for 24 weeks (212 patients) and 48 weeks (210 patients). All patients were monitored for 72 weeks. In the untreated group, no patient cleared HCV RNA and up to 52% had transient elevations of ALT above normal. Sustained virological response (SVR) rates were 30% and 52% in the 24-and 48-week treatment groups, respectively. Patients infected with genotype 1, had SVR rates of 13% and 40% after 24 and 48 weeks of treatment, respectively (P < .0001). Patients infected with genotypes 2 or 3 had SVR rates of 72% and 78% with 24 and 48 weeks of treatment, respectively (p= 0.452). No treatment-related flares of ALT were observed (Table 2). In this nice study, authors con-cluded that the efficacy and safety of antiviral combination therapy in PNALT patients was similar to that observed in patients with abnormal ALT levels, even though they used lower dose of ribavirin than currently recommended for genotype 1 (1000-1200 mg/day). The other interesting finding was that the median baseline ALT level of PNALT patients achieving SVR decreased from a median baseline ALT of 25 IU/L (normal) to 10-15 IU/L after SVR. This group also found a significant improvement in the quality of life and less fatigue in those patients with SVR after antiviral therapy.40
Effects of combined antiviral therapy with peginterferon plus ribavirin in patients with chronic HCV infection with PNALT.
| Study | n | Therapy | Duration of therapy | Results (SVR) |
|---|---|---|---|---|
| Zeuzem S (39) | 491 | PEG-IFN α-2a 180ug/week + RBV 800 mg/day | 24 weeks therapy (n=212) | Overall: 30% SVR Genotype 1: 13% SVR Genotype 2/3:72% SVR |
| 48 weeks therapy (n = 210) | Overall 52% SVR Genotype 1: 40% SVR Genotype 2/3: 78% SVR | |||
| No antiviral therapy (n=69) | 0% SVR | |||
| Puoti C (41) | 88 | PEG-IFN α-2a 180ug/week + RBV 800-1200 mg/day | Genotype 1 (n=32): 48 weeks PEG-lFN plus RBV 1000-1200 mg/d | 62% SVR |
| Genotype 2/3 (n=56): 24 weeks PEG-lFN plus RBV 800 mg /d | 87% SVR |
n: number of patients. PEG-lFN: Peg-inteferon. RBV: Ribavirin. SVR: sustained virological response.
More recently, Puoti, et al.,41 evaluated the efficacy and safety of combined antiviral therapy with pe-ginterferon in 88 patients with chronic HCV infection and PNALT. Patients received peginterfe-ron alfa-2a 180 mug/wk plus ribavirin 800 mg/day for 24 weeks (genotypes 2; n = 46 and genotype 3; n = 10) or 1000-1200 mg/day for 48 weeks (Genotype 1; n = 32). Overall SVR was obtained in 78% of patients (69/88). Patients with genotype 1 had an SVR of 62% and those with genotype 2 and 3 had an SVR of 89% and 80% respectively (Table 2). Younger and leaner patients and those with genotype non-1 and lower baseline HCV RNA were more likely to achieve an SVR. The overall SVR rate was 88% in patients with rapid virological response (RVR) (58/66) and 50% in those without RVR. Their conclusion was that patients with PNALT have comparable or even higher SVR rates than HCV patients with abnormal ALT levels. They, thus suggest that in selected cases immediate therapy might be preferred to the ‘wait-and-see’ common policy.
The controversy on the decision whether to treat or not this subgroup of patients with PNALT is well exemplified by a recent survey performed by the New England Journal of Medicine in 2009, where a case vignette of a healthy 25-year-old black woman with asymptomatic hepatitis C with normal ALT was presented, and her case was followed by 3 different management approaches defended by expert hepatologists in that issue of the Journal.42 Readers were invited to participate voting for one of the treatment options. A total of 3216 votes were then evaluated. The most popular treatment option (1400 votes; 44%) was to perform a liver biopsy and to base further treatment on the findings of the biopsy. The second option (1086 votes; 34%) was to do an expectant management with periodic assessment of liver function, and finally 22% (708 votes) decided to commence immediately HCV therapy with peginterferon and ribavirin.43 The 3216 voters were from 115 different countries and identified themselves mainly as physicians (72%). Most voters were from United States (45%), followed by Italy (5%), United Kingdom (4%), and Brazil (3%).
The addition of new antiviral agents (HCV poly-merase or protease inhibitors) to the current combination therapy, especially for genotype 1 patients, has improved SVR rates in chronic HCV patients. In a recent study, McHutchison, et al.,44 randomized 250 patients with chronic HCV genotype 1 infection, to different arms of treatment including three groups with telaprevir (protease inhibitor) based therapy and a control group with peginterferon alfa-2a (180 μg per week) plus ribavirin (1000 or 1200 mg per day, according to body weight) for 48 weeks. Twenty-two percent (22%) of randomized patients had normal ALT levels at baseline. The SVR was 41% (31/75 patients) in the 48 week control combination therapy (peginterferon alfa-2a plus ribavirin) and 67% (53/79 patients) in the telaprevir 12 weeks associated with combination therapy for 48 weeks (p = 0.002), although the discontinuation rate because of adverse effects was higher in the telaprevir arms (21% v/s 11% in the non-telaprevir arm).
Current Recommendations on the Management of Chronic Hcv Patients With PnaltThe recently published 2009 AASLD guidelines for the management of chronic HCV infection3 recommend that:
- •
“Regardless of the serum ALT level, the decision to initiate therapy with pegylated interferon and ribavirin, should be individualized based on the severity of liver disease by liver biopsy, the potential for serious side effects, the likelihood of response, and the presence of comorbid conditions” (Evidence: Class I, Level B).
- •
“The treatment regimen for HCV-infected persons with normal ALT levels should be the same as that used for persons with elevated serum ALT levels” (Evidence: Class I, Level B)”.
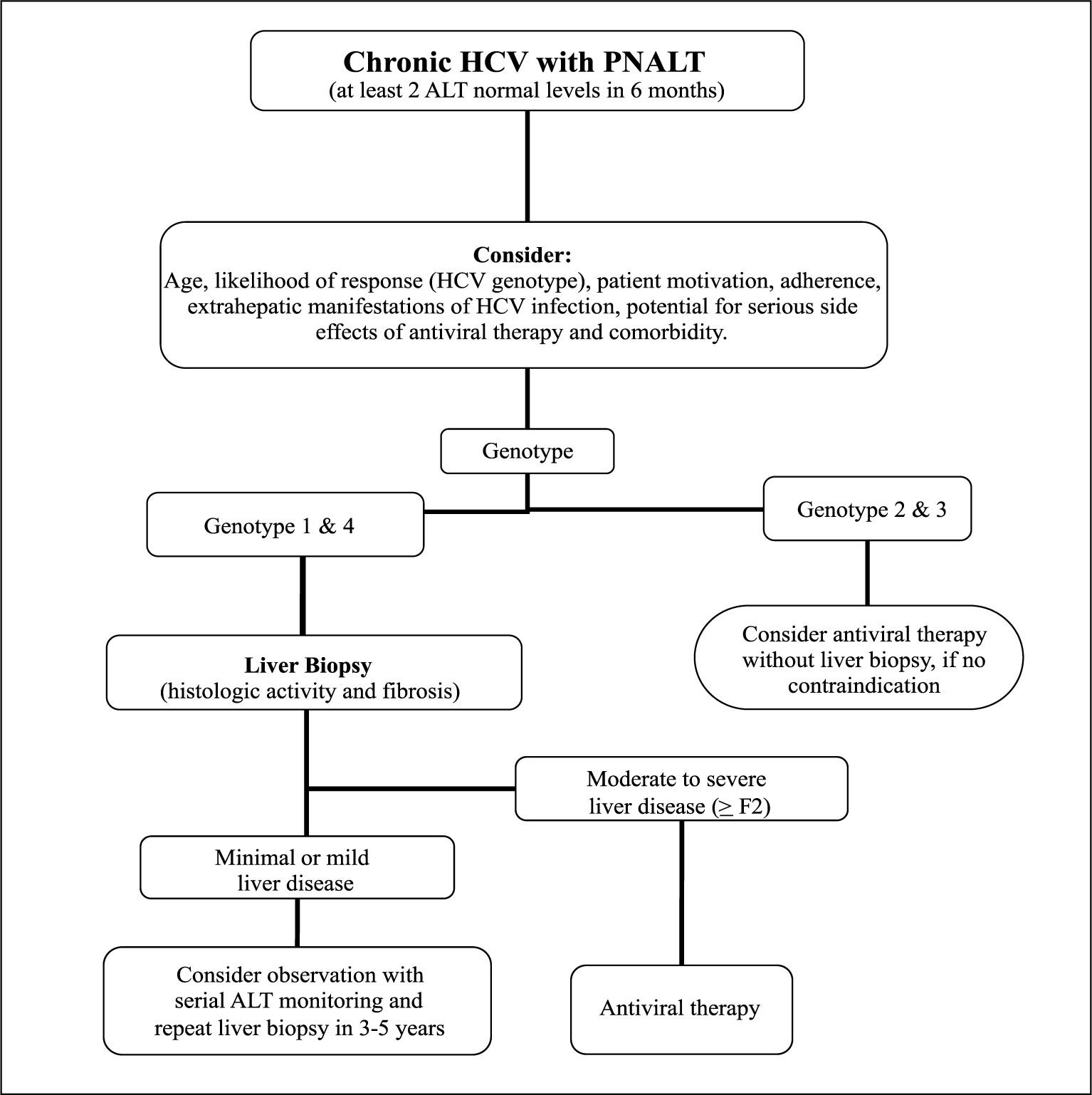
As we move towards a better understanding of the natural history of chronic HCV patients with PNALT, by improving the SVR rates of HCV patients (especially for genotype 1 infections) with new antiviral agents, and with the possibility of having accurate noninvasive methods of evaluation of severity of liver disease, the management algorithm of this subgroup of patients with PNALT (Figure 1), may change in the near future,45 probably towards treating more patients, and with the possibility of potentially decreasing the overall morbidity and mortality of HCV patients.46
In Summary- •
About 30% of patients with chronic HCV infection show persistently normal alanine amino-transferase levels (PNALT) over time (> 6 months follow-up).
- •
Liver histology in this subgroup of patients is usually benign, with minimal or mild inflammation and absent fibrosis.
- •
Over time, 20-30% of this subgroup of patients may show slow progression of liver disease on histology, with development of fibrosis, thus requiring antiviral therapy.
- •
Chronic HCV patients with PNALT should be evaluated in a similar manner as patients with elevated ALT levels because they are at risk for developing significant liver disease.
- •
The indication for treatment of hepatitis C should be evaluated independently from baseline ALT activity.
- •
The final decision whether or not to treat initially this subgroup of patients must be individualized considering: histology, likelihood of response (genotype), patient motivation, symptoms, co-morbidity, potential for serious side effects, and the age of the patient.
- •
The genotype and the severity of liver disease by liver biopsy are the main factors when considering antiviral therapy in this subgroup of patients.
- •
PNALT patients with severe extrahepatic manifestations of HCV infection, such as cryoglobuli-nemia with vasculitis or gomerulonephritis should be considered for antiviral therapy.
- •
Patients with PNALT, in whom the decision was not to treat based on the above considerations (normal or near normal histology, genotype 1, women, with no comorbidity), should receive counseling concerning the potential transmission of the infection to others (they persist viremic) and avoid excessive alcohol consumption. They also need to be monitored with ALT determinations every 6 to 12 months and consider the possibility of serial liver biopsies in the future to evaluate the progression of liver disease.
- •
More recently, noninvasive methods for evaluation of liver fibrosis, like Fibroscan and/or Fibro-Test have been studied in this selected subgroup of PNALT patients, showing that Fibroscan may be useful to select patients with significant fibro-sis for antiviral therapy, with excellent accuracy, and eventually without the need of a liver biopsy.
- •
The antiviral treatment regimen for chronic HCV patients with PNALT should be the same as the one for patients with abnormal ALT levels.
- •
The sustained virological response to antiviral combined treatment (pegylated interferon and ri-bavirin) for chronic HCV patients with PNALT is considered similar to the one observed in patients with abnormal ALT levels.