Introduction and aim. In recent decades, Italy has become a land of immigration from countries suffering a socio-economic crisis. The aim of this study was to perform an organized screening to identify and offer care to immigrants with HCV infection.
Material and methods. The screening, performed from 2012 to 2015, involved 1,727 immigrants in the Campania and Apulia regions in southern Italy. Results. Screening was accepted by 1,727 (85%) out of 2,032 immigrants interviewed; 70 (4.1%) of the 1,727 were anti-HCV-positive, all unaware of their serological condition, 31 (44.3%) of whom were HCV-RNA-positive and 39 negative. The 31 HCV-RNA-positive immigrants were further investigated at a third-level clinic of infectious diseases. The HCV viral load was 2.6 × 107 ± 7.7 ×107 IU/mL, and 35.5% showed HCV-genotype 1a or 1b, 23.8% genotype 2 and 22.6% genotype 3. Two immigrants had liver cirrhosis and, in accordance with the Italian Healthcare Authority guidelines, received an interferon-free regimen and achieved a sustained virological response (SVR); 18 had chronic hepatitis, 6 of whom with a high risk of progression and received interferon-based therapy, with SVR in 4, whereas 12 at low risk were put on a waiting list for future interferon-free treatment, once licensed. The remaining 11 HCV-RNA-positive immigrants were considered HCV inactive chronic carriers and were included in a long-term observational program.
Conclusion. The screening program can be considered successful since it was accepted by 85% of the subjects interviewed and identified 70 anti-HCV-positive immigrants, all unaware of their clinical and virological condition.
One hundred and seventy million people worldwide live with chronic hepatitis C virus (HCV) infection1–6 and nearly 350,000 people per year die of an end-stage liver disease associated with decompensated liver cirrhosis or hepatocellular carcinoma (HCC).1–4,7,8 Different levels of HCV endemicity have been described in different geographical areas. Countries with high endemicity are those in northern and central Africa, with an HCV infection rate ≥ 3%, the highest rate registered in Egypt, where a rate of 10% has been reported due to extensive unsafe re-use of glass syringes and needles to treat schistosomiasis with tartar emetic from the 1960’s to 1980’s.9 An intermediate rate of anti-HCV positivity, 2-2.9%, has been detected in most countries in eastern and southern Asia, including those of the former Soviet Union, and in sub-equatorial and southern Africa. An anti-HCV-positivity rate of 0.5 to 1.9% has been observed in most countries in western Europe and in North and South America.10
In recent decades, due to the socio-economic and political crises in northern and sub-Saharan Africa, eastern Europe and central and eastern Asia, western countries have become lands of immigration from these subcontinents, with a consequently increasing incidence of HCV infection.11 At present, nearly 5.5 million immigrants live in Italy, mostly born in northern or sub-Saharan Africa, Eastern Europe or the Indo-Pakistani subcontinent.12,13
A recent meta-regression study of 50 articles considered 38,635 migrants with an anti-HCV prevalence ranging from 2.2% to 5.6% who came from sub-Saharan Africa, Asia and eastern Europe to geographical areas with low endemicity like Australia, Canada, western Europe, Israel and the United States.14,15 Similarly, in our recent study, we described the screening of 882 immigrants from countries with intermediate/high endemicity, 4.1% of whom were anti-HCV-positive.16
Early detection of HCV infection is critical since the treatments available lead to viral eradication in nearly all patients treated, prevent the progression of liver disease and decrease all-cause and liver-related mortality.17–23 The literature data on HCV infection in immigrant populations is scanty since they are rarely investigated for this infection in their country of origin and in the host countries.24–26
This paper describes a screening program performed to identify HCV infection in 1,727 undocumented immigrants or low-income refugees, evaluate their demographic, epidemiological, laboratory and clinical characteristics and defined follow-up and treatment procedures where necessary.
Material and MethodsPatientsThis multicentre prospective study involved 5 first-level clinical centers in southern Italy, two in Naples and two in Caserta, in the Campania Region, and one in the surroundings of Foggia in the Apulia Region, and three tertiary clinics of infectious diseases, one located in Naples, one in Caserta and one in Foggia, cities giving hospitality to a large population of low-income refugees and undocumented immigrants mostly from Africa, Asia and eastern Europe: nearly 38,000 in the city of Naples, 3,000 in Caserta and 3,000 in Foggia. The study is based on a screening program started in January 2012 and still in progress involving all undocumented immigrants and low-income refugees consecutively observed for a clinical consultation at one of the 5 first-level units. In Italy, the juridical definition of refugee refers to immigrants fleeing their country of origin because of war or political, religious or racial discrimination, whereas undocumented immigrants are subjects who have come to Italy without a legal permit in search of work or better living conditions. The refugees have access to all the healthcare facilities of the National Healthcare System, whereas for undocumented immigrants access is limited to minors, pregnant women and patients with serious pathological conditions or transmissible diseases. Due to the reduced economic resources of Italy in the last decade, undocumented immigrants and refugees have poor living conditions, with a low income mostly from casual day-today work. In addition, they are prevalently young, without family ties and with language, cultural and social barriers.
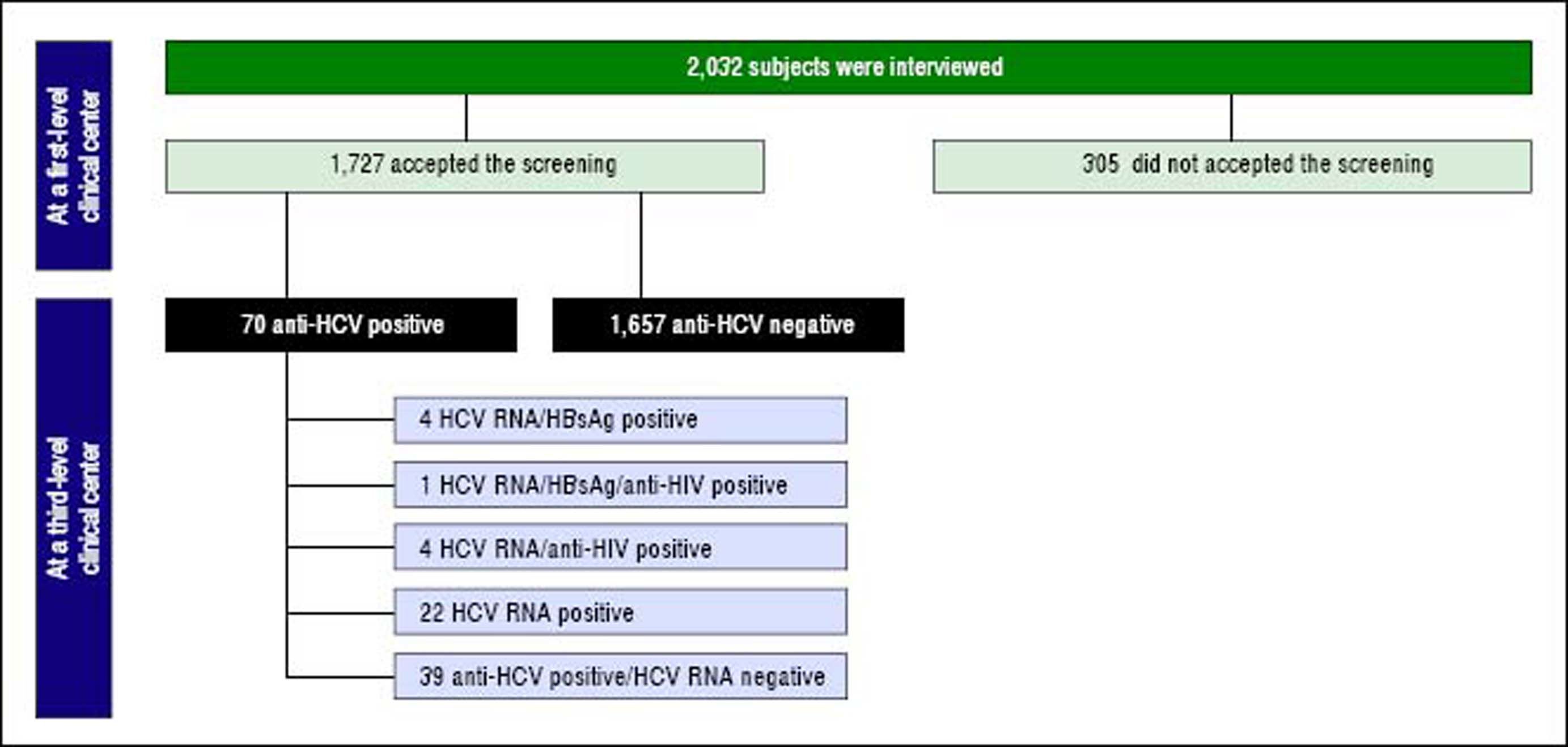
The above-mentioned first-level clinical centers are out-patient clinics of general medicine consulted by low-income refugees and undocumented immigrants mostly for lumbago, headache, pruritus, cough, high blood pressure and allergy symptoms. During the clinical consultations, a physician and a cultural mediator explained to the immigrants the importance of testing for HBV, HCV and HIV serum markers and offered them free screening in anonymity. Adhesion to the screening and a signed informed consent, written in the immigrant’s own language, was obtained on a voluntary basis from 1,727 (85%) of the 2,032 undocumented immigrants or low-income refugees interviewed. The flow-chart offers a more rapid understanding of the main steps in the patients’ selection, screening and follow-up (Figure 1).
Information on the demographics, socioeconomic status and environmental and clinical data, as well as the risk factors for acquiring HBV, HCV and HIV infections, were collected in an anonymous questionnaire for the 1,727 subjects who accepted the screening. A serum sample obtained at the time of the clinical consultation was tested for anti-HCV and markers of other blood-borne/sexually-transmitted infections. All undocumented immigrants and refugees received the results of their serological screening and full instructions on the transmission and prevention of blood-borne and sexually-transmitted diseases.
All anti-HCV-positive refugees or undocumented immigrants were referred to the nearest tertiary unit of infectious diseases for further investigation, monitoring and treatment if necessary. The three tertiary units of infectious diseases have been co-operating for over 15 years in several clinical investigations on HIV, HBV and HCV infections using the same clinical approach and the same laboratory methods.16,27,28
The 70 anti-HCV-positive immigrants were considered asymptomatic chronic carriers if, in the absence of clinical, biochemical and ultrasound signs of chronic hepatitis or liver cirrhosis, their alanine aminotransferase (ALT) values remained persistently normal over an observation period of at least 12 months. Chronic hepatitis was diagnosed based on liver histology or, if not performed, on abnormal ALT values lasting 6 months or more and on the absence of clinical, biochemical and U.S. signs characterizing liver cirrhosis.29 Liver cirrhosis was diagnosed on the basis of liver histology or, if not available, from the presence of unequivocal clinical, biochemical and ultrasound signs.29 HCV-related HCC was diagnosed on the basis of histological and/or imaging findings and on α-fetoprotein serum levels.30
The study was approved by the Ethics Committee of the Azienda Ospedaliera Universitaria of the Second University of Naples.
MethodsSerum samples were tested for HBsAg, anti-HCV, anti-HIV, total anti-HBc and anti-HBs by commercial immune-enzymatic assays (Abbott Laboratories, North Chicago, IL, USA): AxSYM HBsAg (V.2) M/S for HBsAg, AXSYM HCV 3.0 for anti-HCV, AXSYM HIV 1/2 COMBO for HIV, AXSYM core for total anti-HBc and AXSYM AUSAB for anti-HBs). Anti-HIV reactivity was always confirmed by a Western blot assay (Genelabs Diagnostics, Science Park Drive, Singapore), which identifies both HIV-1 and HIV-2 strains.
HCV RNA was determined on serum of anti-HCV-positive patients by a real-time PCR method with a lower sensibility of 40 IU/mL. Briefly, HCV RNA was extracted with MinElute Virus Spin Kit (Qiagen) and quantified with real-time PCR in a Light cycler 1.5 (Roche Diagnostics, Branchburg, NJ, USA). The amplified sample by nested PCR with a specific primer for the NS5B region was then sequenced (Genetic Analyzer ABI 3100) using the Big Dye Terminator v. 1.1. Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA). The sequenced samples obtained were compared with other samples by BioEdit software (North Carolina State University). The HCV genotypes were determined (MEGA 5.2).
Statistical analysisContinuous variables were summarized as means and standard deviations and categorical variables as absolute and relative frequencies; differences in the means or proportions were evaluated by the Student t-test or by the χ2 test with the Yates correction, respectively. A p value < 0.05 was considered to be statistically significant. The following variables were analyzed: age, sex, legal status, country of origin, duration of stay in Italy and risk factors for acquiring HCV infections.
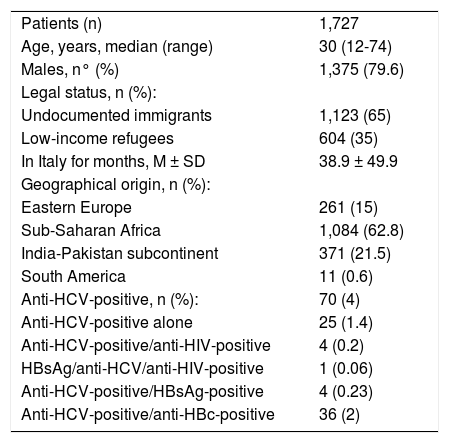
ResultsA total of 1,727 subjects, 1,375 males and 352 females, were screened. They had been living in Italy for a mean period of 39 months, were aged 12-74 (median 30 years), were prevalently undocumented immigrants (65%) and of sub-Saharan origin (63%) (Table 1).
Demographic and initial characteristics of the 1,727 immigrants.
| Patients (n) | 1,727 |
| Age, years, median (range) | 30 (12-74) |
| Males, n° (%) | 1,375 (79.6) |
| Legal status, n (%): | |
| Undocumented immigrants | 1,123 (65) |
| Low-income refugees | 604 (35) |
| In Italy for months, M ± SD | 38.9 ± 49.9 |
| Geographical origin, n (%): | |
| Eastern Europe | 261 (15) |
| Sub-Saharan Africa | 1,084 (62.8) |
| India-Pakistan subcontinent | 371 (21.5) |
| South America | 11 (0.6) |
| Anti-HCV-positive, n (%): | 70 (4) |
| Anti-HCV-positive alone | 25 (1.4) |
| Anti-HCV-positive/anti-HIV-positive | 4 (0.2) |
| HBsAg/anti-HCV/anti-HIV-positive | 1 (0.06) |
| Anti-HCV-positive/HBsAg-positive | 4 (0.23) |
| Anti-HCV-positive/anti-HBc-positive | 36 (2) |
Seventy (4.1%) of the 1,727 subjects screened were anti-HCV-positive, more precisely, 4 anti-HCV/anti-HIV-positive, 4 anti-HCV/HBsAg-positive, 1 anti-HCV/anti-HIV/ HBsAg-positive, 36 anti-HCV/anti-HBc-positive and the remaining 25 anti-HCV-positive.
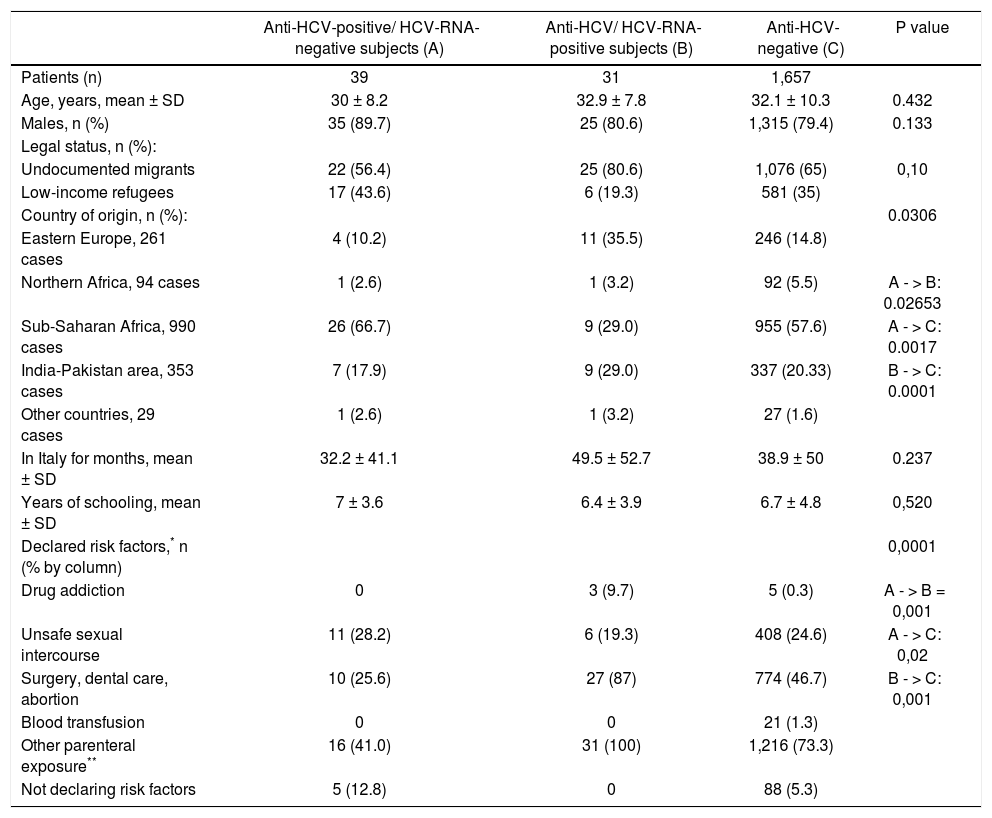
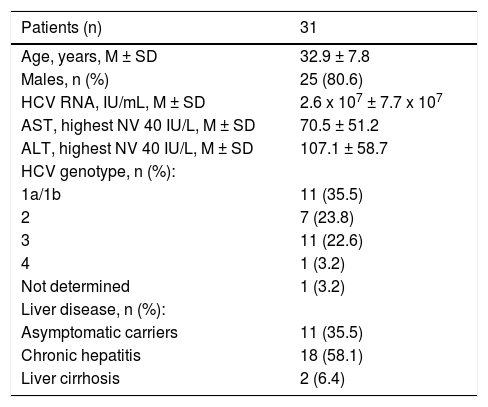
The 70 anti-HCV-positive immigrants were investigated for HCV RNA, 39 (55.7%) of whom resulted positive and 31 (44.3%) negative. The demographic and epidemiological characteristics of 3 subgroups of patients established based on the presence or absence of serum anti-HCV and HCV RNA (namely, anti-HCV-positive/HCV-RNA-negative, anti-HCV-positive/HCV-RNA-positive, anti-HCV-negative) are compared in table 2. Differences in the patients’ ages, gender and legal status between these three subgroups were not significant to the statistical analysis, whereas differences in the country of origin and the declared risk factors reached statistical significance. More precisely, patients in the anti-HCV/HCV-RNA-positive subgroup compared with those in the other two subgroups more frequently came from Northern Africa and (p = 0.0306) and more frequently declared risk factors associated with parenteral transmission of HCV infection (p = 0.0001) (Table 3). The clinical and virological characteristics of the 31 immigrants with detectable HCV viremia are shown in table 3. The HCV viral load was 2.6 × 107 ± 7.7 x 107 IU/mL, 35.5% of cases showed HBV-genotype 1a or 1b, 23.8% genotype 2 and 22.6% genotype 3 (Table 3). Eleven (35.5%) HCV-RNA-positive cases were HCV inactive chronic carriers, 18 (58.1%) had chronic hepatitis and 2 (6.4%) liver cirrhosis.
Demographic and initial characteristics of the 1,727 immigrants according to HCV serology and viremia.
| Anti-HCV-positive/ HCV-RNA-negative subjects (A) | Anti-HCV/ HCV-RNA-positive subjects (B) | Anti-HCV-negative (C) | P value | |
|---|---|---|---|---|
| Patients (n) | 39 | 31 | 1,657 | |
| Age, years, mean ± SD | 30 ± 8.2 | 32.9 ± 7.8 | 32.1 ± 10.3 | 0.432 |
| Males, n (%) | 35 (89.7) | 25 (80.6) | 1,315 (79.4) | 0.133 |
| Legal status, n (%): | ||||
| Undocumented migrants | 22 (56.4) | 25 (80.6) | 1,076 (65) | 0,10 |
| Low-income refugees | 17 (43.6) | 6 (19.3) | 581 (35) | |
| Country of origin, n (%): | 0.0306 | |||
| Eastern Europe, 261 cases | 4 (10.2) | 11 (35.5) | 246 (14.8) | |
| Northern Africa, 94 cases | 1 (2.6) | 1 (3.2) | 92 (5.5) | A - > B: 0.02653 |
| Sub-Saharan Africa, 990 cases | 26 (66.7) | 9 (29.0) | 955 (57.6) | A - > C: 0.0017 |
| India-Pakistan area, 353 cases | 7 (17.9) | 9 (29.0) | 337 (20.33) | B - > C: 0.0001 |
| Other countries, 29 cases | 1 (2.6) | 1 (3.2) | 27 (1.6) | |
| In Italy for months, mean ± SD | 32.2 ± 41.1 | 49.5 ± 52.7 | 38.9 ± 50 | 0.237 |
| Years of schooling, mean ± SD | 7 ± 3.6 | 6.4 ± 3.9 | 6.7 ± 4.8 | 0,520 |
| Declared risk factors,* n (% by column) | 0,0001 | |||
| Drug addiction | 0 | 3 (9.7) | 5 (0.3) | A - > B = 0,001 |
| Unsafe sexual intercourse | 11 (28.2) | 6 (19.3) | 408 (24.6) | A - > C: 0,02 |
| Surgery, dental care, abortion | 10 (25.6) | 27 (87) | 774 (46.7) | B - > C: 0,001 |
| Blood transfusion | 0 | 0 | 21 (1.3) | |
| Other parenteral exposure** | 16 (41.0) | 31 (100) | 1,216 (73.3) | |
| Not declaring risk factors | 5 (12.8) | 0 | 88 (5.3) | |
Clinical and virological characteristics according to the clinical classification of the 31 anti-HCV/HCV-RNA-positive subjects.
| Patients (n) | 31 |
|---|---|
| Age, years, M ± SD | 32.9 ± 7.8 |
| Males, n (%) | 25 (80.6) |
| HCV RNA, IU/mL, M ± SD | 2.6 x 107 ± 7.7 x 107 |
| AST, highest NV 40 IU/L, M ± SD | 70.5 ± 51.2 |
| ALT, highest NV 40 IU/L, M ± SD | 107.1 ± 58.7 |
| HCV genotype, n (%): | |
| 1a/1b | 11 (35.5) |
| 2 | 7 (23.8) |
| 3 | 11 (22.6) |
| 4 | 1 (3.2) |
| Not determined | 1 (3.2) |
| Liver disease, n (%): | |
| Asymptomatic carriers | 11 (35.5) |
| Chronic hepatitis | 18 (58.1) |
| Liver cirrhosis | 2 (6.4) |
The 11 HCV inactive chronic carriers were included in a long-term observational follow-up program, whereas the patients with chronic hepatitis or cirrhosis received treatment or remained untreated according to their clinical condition. Only two patients showed liver cirrhosis, one with genotype 1a and one with genotype 3a. One of them received an interferon-free treatment with sofosbuvir + ledipasvir + ribavirin for 3 months and the other with sofosbuvir + daclatasvir + ribavirin for 6 months and both showed SVR with a clearance of HCV infection. In compliance with the guidelines of the National Healthcare Authority, of the 18 patients with chronic hepatitis, 6 were at a high risk of progression and were treated with Peg-IFNα2a (180 µg once-a-week) or Peg-IFNα2b (1.5 µg once-a-week), 3 with genotype 2 for 6 months, and 2 with genotype 3 and 1 with genotype 1b for 12 months. HCV clearance was observed in 3 patients with genotype 2 and in one with genotype 3. The remaining 12 subjects with chronic hepatitis at a low risk of progression were included in a waiting list for interferon-free therapy. Out of the 8 HCV-RNA-positive patients treated for HCV infection, 4 were coinfected with HBV, but the low HBV-DNA serum levels allowed the detection of the HBV genotype only in one (genotype HBV D1), who received Peg-Interferon treatment. HIV infection was detected in 4 of the 8 patients mentioned above (one with triple HCV/HBV/ HIV infection and undetectable HBV DNA) and in one of the 23 HCV-RNA- positive patients who did not receive anti-HCV treatment. These 5 anti-HIV-positive patients were treated for HIV infection or left untreated according to the current Italian guidelines.31,32
DiscussionThe social and economic crises in Italy and some other western Mediterranean countries in the last decade have not favored the integration of people fleeing from cruel wars and/or extreme need.30 Proper healthcare assistance for this fragile population requires economic support, dedicated structures and well-trained physicians and cultural mediators. The 5 first-level clinical centers operating in this study have had documented long-lasting experience in the clinical, psychological and legal management of vulnerable populations. We underscore the essential role played by the cultural mediators during the clinical consultations, since they were able to explain the importance of screening, prevention and the treatment of transmittable diseases to immigrants in their own languages. Cultural mediators are also of great help to immigrants to overcome the numerous bureaucratic obstacles present in the healthcare procedures. A good quality of life and adequate medical assistance are essential to ensure the health and welfare of low-income refugees and undocumented immigrants.33
In this study, we interviewed 2,032 immigrants, a group of subjects that can be considered fairly representative of the nearly 44,000 immigrants living in the cities (Naples, Caserta and Foggia) where the screening was carried out. Adhesion to the screening was obtained on a voluntary basis for 85% of the 2,032 immigrants, a high percentage that emphasizes the success of the screening procedures adopted and the importance of the assistance and care given by the physicians and cultural mediators operating at the 5 first-level clinical centers. The prevalence of anti-HCV positivity in the immigrant population is clearly higher than that estimated for the Italian population (4.1% vs. 1.2%). However, only 1.8% of the immigrant population showed active HCV replication and, consequently, after screening, the burden of further diagnostic and therapeutic intervention was limited. The diagnosis was possible for all 31 HCV-RNA-positive immigrants, one-third of whom were HCV inactive carriers and two-thirds had a progressive disease. In particular, liver cirrhosis was observed only in two cases, both treated with an interferonfree regimen in accordance with the National Healthcare Authority guidelines which dictate a progressive application of this expensive treatment. SVR was achieved in both cases. Six of the 18 patients with chronic hepatitis were considered at a high risk of progression and were treated with an interferon-based therapy according to their HCV genotype,34–37 with SVR in two-thirds. Treatment choices were not affected by co-infection with HBV since only one the 5 patients with dual HCV/HBV infection showed HBV-DNA serum levels requiring treatment and in this case Peg-Interferon was indicated to treat both infections.
Twelve patients with chronic hepatitis at a low risk of progression were included in a follow-up observational program, a sort of waiting list for interferon-free therapy. We confide in a more extensive application of these regimens in the near future. The 11 inactive HCV chronic carriers were included in a long-term observational program.
No patient showed HCV-related extrahepatic manifestations in the present study probably because most patients were young adults and, consequently, we cannot comment adequately on these complications which, however, may involve severe immunological reactions.38–40
In conclusion, to our best knowledge, this is the first organized screening program performed to identify and give medical care to HCV-infected immigrants. The screening program can be considered successful since it allowed us to identify out of the 1,727 subjects tested 70 anti-HCV-positive immigrants, all unaware of their serological and clinical condition. Of these 70, only 31 were HCV-RNA-positive and required further investigation to define follow-up and possible treatment procedures.
Author ContributionsNC, LA, CS, ES, reviewed the literature, contributed to drafting the article, critically reviewed the manuscript and approved the final version; LG, MP, GDC,LO MM, GS collected the data; CM and MS performed laboratory analyses.
Conflict of Interest StatementAll the authors of the manuscript declare they have no conflict of interest in connection with this paper.