Introduction and aim. Neonatal cholestasis constitutes for 19 to 33% of all chronic liver disease in India. Cholestasis leads to fibrosis of liver and ultimately cirrhosis. There are various methods of diagnosis of fibrosis of liver like fibroscan, aspartate transaminase to platelet ratio index (APRI) index, FIB-4, fibro index, forns index, heap score, magnetic elastography. Here we are comparing APRI index with METAVIR index in patients with neonatal cholestasis without biliary atresia and determining whether APRI index can be used as a tool to determine fibrosis in these patients.
Material and methods. Patients with neonatal cholestasis without biliary atresia were included in the study. This retrospective analysis was done between 2009 and 2015. All patients underwent a liver biopsy and METAVIR index was calculated. APRI at the time of liver biopsy was determined.
Results. Forty-eight patients were included in this study with mean age of 3.5 ± 2.8 months with a male: female ratio of 35:13. Metavir Index F0 was seen in was 32 (66.67%) patients, F1 in 6(12.5%), F2 in 4(8.33%), F3 in 0 and F4 in 6(12.5%) patients respectively. Mean APRI for F0-F3 was 1.38 and for F4 was 3.74 respectively. With an APRI of 1.38, the sensitivity and specificity to detect fibrosis/cirrhosis was 100% and 21.43% respectively.
Conclusion. APRI is not an effective tool to measure fibrosis or cirrhosis in patients with non-BA neonatal cholestasis in Indian children.
Neonatal cholestasis (NC) is defined as conjugated hyperbilirubinemia occurring in the newborn. In India, it constitutes 19% to 33% of all chronic liver diseases in children reporting to tertiary care hospitals.1 Three studies from Indian subcontinent had noted neonatal hepatitis to be the major cause for NCS2–4 suggestive that infective causes tend to predominate and thus could lead to high incidence of NCS in the Indian subcontinent. Neonatal cholestasis comprises two major groups, extrahepatic biliary atresia, and neonatal hepatitis. Early detection is essential to facilitate timely intervention and minimize adverse outcomes in several conditions, including biliary atresia (BA), hypothyroidism, and galactosemia. Giant cell transformation of hepatocytes occurs commonly in infants with cholestasis and can occur in any form of neonatal liver injury. It is more common and more severe in intrahepatic forms of cholestasis. Fibrosis can be seen in zone 1. This is reversible if the cholestasis is relieved. The zone 1 fibrosis extends to meet bands from adjacent zones so that eventually zone 3 is enclosed by a ring of connective tissue. Biliary cirrhosis follows prolonged cholestasis.5’6 Though cirrhosis is considered common endpoint of progressive fibrinogenesis, the pattern of fibrotic development differs according to the aetiology of liver disease causing fibrosis.7,8
There are various methods to look for fibrosis, invasive and noninvasive. Liver biopsy is the gold standard for diagnosis of liver fibrosis. However, liver biopsy can have life-threatening complications in both adults and children. It is therefore difficult to use as a follow-up tool. Moreover, the accuracy of liver biopsy has also been questioned because of sampling errors and intra-observer and inter-observer variability, which lead to an overstaking or understanding of fibrosis.9 Thus, there is a need for accurate non-invasive methods of measuring the degree of liver fibrosis and to evaluate regression or progression of liver fibrosis. There are some non-invasive methods like fibroscan, FIB-4, fibro index, forns index, heap score, magnetic elastography, Aspartate transaminase to platelet ratio index (APRI).10
Wai, et al. validated APRI in patients with hepatitis C.11 Sang Yong Kim, et al. found that APRI may be used as tool for assessing liver fibrosis without additional risks in patients with BA during postoperative follow-up care.10 We undertook this study to validate use of APRI for determining liver fibrosis and cirrhosis in patients with non-BA neonatal cholestasis and correlate with Metavir Index on liver histology.
Material and MethodsThis retrospective study was conducted in the Pediatric Liver Clinic, B. J. Wadia hospital for children, Parel, Mumbai after approval of the ethics committee. All infants who had presented with neonatal cholestasis from 20092015 and had undergone a liver biopsy were included in the study. Patient who were diagnosed to have BA on liver biopsy or intra-operative cholangiogram were excluded from the study. Patients with conditions such as muscle disorders, haematological disorders, sepsis and congenital bone disorders which may affect the aspartate transaminase (AST) or platelet counts were excluded from the study. Patients whose records do not have simultaneous platelet count or AST at the time of liver biopsy were also excluded from the study. Liver function tests and hemogram at the time of liver biopsy were analyzed for APRI index. Liver biopsy specimens were reviewed by one experienced pathologist. The pathologist scored the liver fibrosis by METAVIR score as F0- no fibrosis; F1- portal fibrosis without septa; F2- portal fibrosis with few septa; F3- numerous septa without cirrhosis; and F4- cirrhosis.2 For all of the subjects, demographic information included sex and age in months at the time of liver biopsy were noted. The biochemical parameters included serum AST, alanine aminotransferase, albumin, total bilirubin, direct bilirubin, gamma-glutamyl transpeptidase, alkaline phosphatase, platelet count, and prothrombin time. The APRI was calculated as:8 APRI = AST level/Upper normal limit for AST Platelet count (103/L) X 100. Normal value of AST ranged from 5 to 40 U/L.
Statistical AnalysisDistributed data were presented as the mean (SD); factors associated were analyzed by the t-test and proportions by the χ2 test. The diagnostic performance of the APRI was assessed using receiver operating characteristics (ROC) curves. All of the cut-off values are associated with the probability of a true positive (sensitivity) and a true negative (specificity). The ROC curve is a plot of sensitivity versus 1-specificity for all of the possible cut-off values. The most commonly used accuracy index is the area under the ROC curve; values near 1.0 indicate high diagnostic accuracy. ROC curves were thus constructed for the detection of subjects with Metavir fibrosis stage 3 or greater and cirrhosis. Optimal cut-off values for APRI were chosen to obtain 95% sensitivity, or to obtain 95% specificity according to the diagnostic question. All statistical analyses were performed using the MedCalc software package version 7.2 for Windows® (MedCalc). A p-value < 0.05 (2-tailed) was considered statistically significant.
We plotted Box and whisker diagram of the APRI in relation to the Metavir fibrosis score. The box represents the interquartile range, the whiskers indicate the highest and lowest values, and the asterisks represent the outliers. The line across the box indicates the median value. This box was plotted for fibrosis versus nonfibrosis that is F0, F1, F2 with F3, F4 and cirrhosis versus noncirrhosis this is F0, F1, F2, F3 with F4.
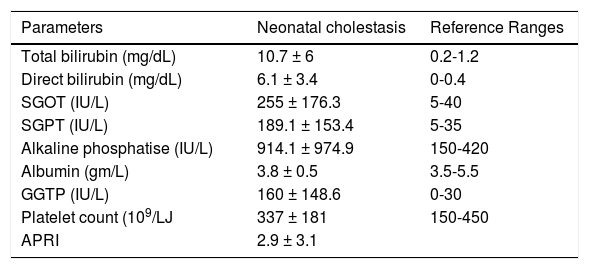
ResultsForty-eight patients who underwent liver biopsy were diagnosed as non-BA neonatal cholestasis. Mean age of presentation was 3.5 ± 2.7 months. Male: female ratio was 35:13. The distribution of Metavir Index for F0, F1, F2, F3, F4 was 32 (66.67%), 6(12.5%), 4(8.33%), 0, 6 (12.5%) respectively. Liver function tests and APRI index are depicted in table 1.
Liver function tests and APRI index.
| Parameters | Neonatal cholestasis | Reference Ranges |
|---|---|---|
| Total bilirubin (mg/dL) | 10.7 ± 6 | 0.2-1.2 |
| Direct bilirubin (mg/dL) | 6.1 ± 3.4 | 0-0.4 |
| SGOT (IU/L) | 255 ± 176.3 | 5-40 |
| SGPT (IU/L) | 189.1 ± 153.4 | 5-35 |
| Alkaline phosphatise (IU/L) | 914.1 ± 974.9 | 150-420 |
| Albumin (gm/L) | 3.8 ± 0.5 | 3.5-5.5 |
| GGTP (IU/L) | 160 ± 148.6 | 0-30 |
| Platelet count (109/LJ | 337 ± 181 | 150-450 |
| APRI | 2.9 ± 3.1 |
SGOT: serum glutamic oxaloacetic transaminase. SGPT: serum glutamic-pyruvic transaminase. GGTP: gamma-glutamyl transpeptidase. APRI: aspartate aminotransferase to platetet ratio index.
Box and whisker were plotted between F0-F3 with F4 (Figure 1). Mean APRI for F0-F3 was 2.76 ± 3.21 and for F4 was 3.71 ± 1.40 and median was 1.38 and 3.74 respectively. With an APRI of 1.38, the sensitivity and specificity to detect fibrosis/cirrhosis was 100% and 21.43% respectively. The ROC for the same is depicted in figure 2. Area under curve was 0.746.
DiscussionThe prognosis of chronic cholestatic diseases depends, on the extent of fibrosis in the liver.12,13 Although liver biopsy is the gold standard method to demonstrate liver fibrosis, it can result in life-threatening complications in children.14–16
The distribution of fibrosis within the liver parenchyma is heterogeneous. Bedossa, et al.17 recently reported that by using the Metavir scoring system only 75% of 25-mm biopsy specimens were classified correctly in terms of stage of fibrosis. Using the Batts and Ludwig classification,18 Regev, et al.19 showed a difference of at least 1 stage of fibrosis between the right and left lobes in 33% of 124 patients, whereas Siddique, et al.20found that 45% of patients had a difference of at least 1 stage fibrosis between 2 specimens obtained from the same puncture site. In this study, we took scoring system of METAVIR index as it is universal.
As non-invasive index of hepatic fibrosis and cirrhosis, the APRI was developed as an alternative in patients with chronic hepatitis C. The classic cut-off values of the APRI are well validated for both ordinary and special patients with chronic hepatitis C, but not for patients with neonatal cholestasis. Moreover, hepatic fibrosis and cirrhosis in chronic hepatitis C may differ from fibrosis in neonatal cholestasis. De Le’dinghen, et al.21 showed that the APRI significantly correlated to Metavir fibrosis scores in children with chronic liver diseases, including BA. Sang Yong Kim, et al.10 determined the accuracy and reliability of the APRI in assessing fibrosis and histopathologic stage in patients with BA at the time of the Kasai portoenterostomy (KP). Study results showed a significant positive correlation between the APRI and fibrosis stages in Metavir F3 fibrosis or greater cirrhosis. Significant areas under the ROC curves for F3 and F4 were 0.92 and 0.91, respectively. In our study, no patient were in F3 group and ROC curve for F4 was 0.746 which was way below the expected of 1 suggestive that APRI in non-BA neonatal cholestasis is not a good tool for diagnosis of fibrosis in these patients. This may be due to various reasons. It may be due to ethnic variation as this is the first study done in Indian population. Very few patients with neonatal cholestasis had fibrosis or cirrhosis (only 6 patients had cirrhosis and none had fibrosis out of 48 patients). Thus the group in F4 were very few to compare. More studies with a larger sample size may be needed to estimate APRI and fibrosis in non-BA neonatal cholestasis. Our study was a one-time cross-sectional analysis. We have not repeated liver biopsies again in these patients nor estimated APRI to comment on the correlation over a period of time. Thus, reliability of APRI in older children with non- BA neonatal cholestasis may be different since fibrosis develops over a period of time and we have estimated the APRI at an average age of 3% months. Metavir Index though used universally is known to have variation of staging in various studies22 and hence co-relation with APRI may not be up to the mark. In such a scenario, correlation with other non-invasive methods to analyze fibrosis may need to be analyzed.
ConclusionAPRI is not an effective tool to measure fibrosis or cirrhosis in patients with non-BA neonatal cholestasis in Indian children.
Abbreviations- •
APRI: aspartate transaminase to platelet ratio index.
- •
AST: aspartate transaminase.
- •
BA: biliary atresia.
- •
GGTP: gamma-glutamyl transpeptidase.
- •
KP: Kasai portoenterostomy.
- •
NC: neonatal cholestasis.
- •
ROC: receiver operating characteristics.
- •
SGOT: serum glutamic oxaloacetic transaminase.
- •
SGPT: serum glutamic-pyruvic transaminase.