Daclatasvir and asunaprevir dual therapy is approved for the treatment of HCV genotype 1b infection in several countries.
AimTo evaluate the efficacy and safety of daclatasvir and asunaprevir dual therapy in Turkish patients.
Material and MethodsSixty-one patients with HCV genotype 1b were enrolled in the Turkish early access program. Most of the patients were in difficult-to-treat category. Patients were visited at each 4 week throughout the follow-up period. Laboratory findings and adverse events were recorded at each visit.
ResultsFifty-seven of 61 enrolled patients completed 24 weeks of treatment. Two patients died as a result of underlying diseases at 12-14th weeks of treatment. Two patients stopped the treatment early as a consequence of virological breakthrough, and 2 patients had viral relapse at the post-treatment follow-up. Overall SVR12 rates were 90% (55/61) and 93.2% (55/59) according to intention-to-treat (ITT) and per protocol (PP) analysis respectively. In ITT analysis, SVR12 was achieved by 93% (13/14) in relapsers, 80% (12/15) in interferon-ineligible patients and 91% (20/22) in previous nonresponder patients. SVR12 rates were 86.5% and 91.4% in patients with cirrhosis according to ITT and PP analysis respectively. SVR12 was 95.8% in non-cirrhosis group in both analysis. Patients with previous protease inhibitor experience had an SVR12 of 87.5%. Common adverse events developed in 28.8% of patients. There were no treatment related severe adverse event or grade-4 laboratory abnormality.
ConclusionsDaclatasvir and asunaprevir dual therapy is found to be effective and safe in difficult-to-treat Turkish patients with HCV genotype 1b infection.
Currently, treatment of chronic hepatitis C virus(HCV) infection includes interferon free regimens. Directacting antiviral drugs are taken orally and consists of three major subgroups including protease and polymerase inhibitors, and the multifunctional non-structural protein (NS5A).1
Asunaprevir is a non-structural NS3/4A complex protease inhibitor and daclatasvir is a pangenotypic NS5A inhibitor of HCV replication complex. Combination of asunaprevir and daclatasvir has potent activity against genotype 1b HCV infection.2
Daclatasvir plus asunaprevir combination has been first approved for the treatment of genoype 1b subgroup in Japan. Recently, Korea, Taiwan, Canada and Turkey have also approved that dual therapy with similar indications.
Phase II and III studies showed potent antiviral affects using daclatasvir and asunaprevir combination in patients infected with HCV genotype 1b.3-6 In the Japan phase 3 study, SVR24 in Japanese patients were 91% and 84% in cir-rhotics and non-cirrhotics respectively.7,8 A recent pooled analysis have reported that SVR12 was 84% in patients with cirrhosis and 85% in patients without cirrhosis.8
Preliminary data demonstrated the efficacy and safety of dual therapy even in hard to treat patients including patients with cirrhosis, liver transplantation and chronic renal failure.9-11 However, efficacy and safety data of daclatasvir and asunaprevir combination therapy is scarce other than Japanese studies.3 Herein, we evaluated the antiviral activity and safety of daclatasvir and asunaprevir combination therapy in Turkish patients infected with HCV genotype 1b.
Material and MethodsStudy designThe study includes the results of patients enrolled in early access program of BMS in Turkey. Inclusion criteria allowed only the patients who were considered to have a serious or life-threatening condition that was impacting life expectancy within 12 months. Patients having Child B or C cirrhosis, or having liver-transplantation history were not allowed to be enrolled into the program. Request forms filled by healthcare professionals were submitted for both BMS company and health authority approval. All patients signed the informed consent forms before the treatment. The program started at February 2014 and ended in August 2014.
TreatmentDual therapy was provided before commercialization in early access program. All patients received daclatasvir 60 mg tablets once daily and asunaprevir 100 mg softgel capsules twice daily for 24 weeks unless a viral breakthrough developed. All patients were visited at each 4 week during the treatment period and at the 12th week after treatment. Biochemical tests, complete blood count, coagulation tests and HCV RNA were tested at each visit. HCV RNA was tested once more when it became positive suggesting viral breakthrough or recurrence.
- •
HCV RNA assessment. Hepatitis C virus RNA was quantified using the Roche COBAS® TaqMan® assay with an LLOQ of 15 IU/mL.
- •
Resistance analysis. Direct sequencing from baseline samples was performed in a group of patients from one center (23 patients). Only NS5A resistance was analysed in that group.
- •
Efficacy. Sustained virologic response (SVR12) at post-treatment week 12.
- •
Safety assessments. Based on incidence of adverse events (AE), serious adverse events (SAE), discontinuations because of adverse events, and grade 3 or 4 laboratory abnormalities. Adverse events were recorded at each 4-week visit during study follow-up.
- •
Cost effectivity. Markov simulation model was used to estimate the cost-effectiveness of dual therapy, and the effectivity was compared with available treatment regimens paid by social security institution in Turkey (pegylated interferon+ribavirin ± telaprevir or bo- ceprevir).
Continuous variables were analyzed using the Mann-Whitney U-test, and categorical variables were analyzed using the paired sample test. All statistical analyses were performed using the SPSS software package (version 16.0 for Windows, SPSS Inc., Chicago, IL, USA), with p < 0.05 denoting statistical significance.
ResultsPatient characteristics before daclatasvir plus asunaprevir treatmentFifty-seven patients completed 24 weeks of dual treatment. Two patients died at 12-14th week of treatment as a consequence of miliary tuberculosis in one and systemic vasculitis in the other. Both were HCV RNA negative at week 8 and 12. Ten were treatment-naïve, 15 were in the interferon-ineligible/intolerant group, 14 were relapser and 22 were in the non-responder group (null responders and partial responders).
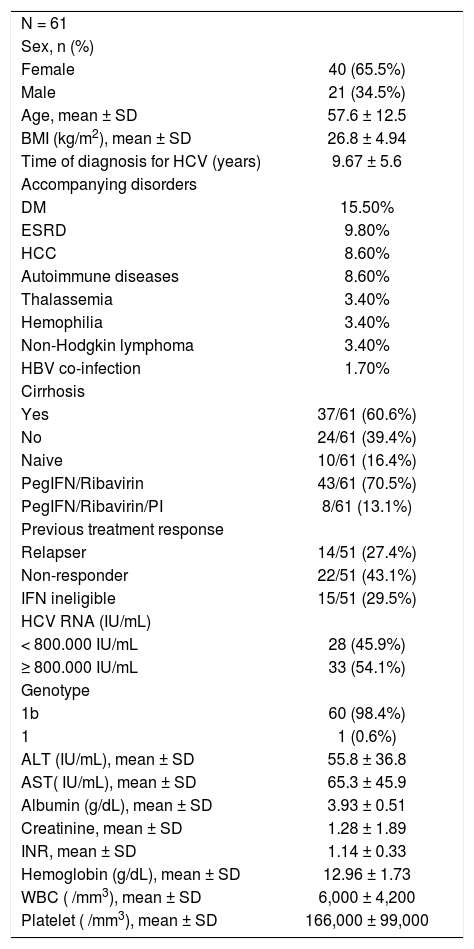
Demographic baseline and laboratory characteristics of patients are shown in table 1. Two thirds of the patients were female. Most of the patients had cirrhosis, high baseline serum HCV RNA levels (> 800,000 iu/mL) and co-morbid diseases including diabetes mellitus, end stage renal disease, hepatocellular carcinoma, autoimmune diseases, non-Hodgkin lymphoma, thalassemia, hemophilia and hepatitis B co-infection with decreasing order of frequency (Table 1). Eight patients were unresponsive to pegylated in-terferon/ribavirin plus telaprevir or boceprevir previously.
Patient demographics and laboratory findings.
| N = 61 | |
| Sex, n (%) | |
| Female | 40 (65.5%) |
| Male | 21 (34.5%) |
| Age, mean ± SD | 57.6 ± 12.5 |
| BMI (kg/m2), mean ± SD | 26.8 ± 4.94 |
| Time of diagnosis for HCV (years) | 9.67 ± 5.6 |
| Accompanying disorders | |
| DM | 15.50% |
| ESRD | 9.80% |
| HCC | 8.60% |
| Autoimmune diseases | 8.60% |
| Thalassemia | 3.40% |
| Hemophilia | 3.40% |
| Non-Hodgkin lymphoma | 3.40% |
| HBV co-infection | 1.70% |
| Cirrhosis | |
| Yes | 37/61 (60.6%) |
| No | 24/61 (39.4%) |
| Naive | 10/61 (16.4%) |
| PegIFN/Ribavirin | 43/61 (70.5%) |
| PegIFN/Ribavirin/PI | 8/61 (13.1%) |
| Previous treatment response | |
| Relapser | 14/51 (27.4%) |
| Non-responder | 22/51 (43.1%) |
| IFN ineligible | 15/51 (29.5%) |
| HCV RNA (IU/mL) | |
| < 800.000 IU/mL | 28 (45.9%) |
| ≥ 800.000 IU/mL | 33 (54.1%) |
| Genotype | |
| 1b | 60 (98.4%) |
| 1 | 1 (0.6%) |
| ALT (IU/mL), mean ± SD | 55.8 ± 36.8 |
| AST( IU/mL), mean ± SD | 65.3 ± 45.9 |
| Albumin (g/dL), mean ± SD | 3.93 ± 0.51 |
| Creatinine, mean ± SD | 1.28 ± 1.89 |
| INR, mean ± SD | 1.14 ± 0.33 |
| Hemoglobin (g/dL), mean ± SD | 12.96 ± 1.73 |
| WBC ( /mm3), mean ± SD | 6,000 ± 4,200 |
| Platelet ( /mm3), mean ± SD | 166,000 ± 99,000 |
At week 4, HCV RNA levels became undetectable in 82% of the patients. Overall, 59/61 patients (96.7%) had undetectable HCV RNA at week 12 during treatment and 57/59 patients (96.6%) had undetectable HCV RNA at week 24 on treatment.
- •
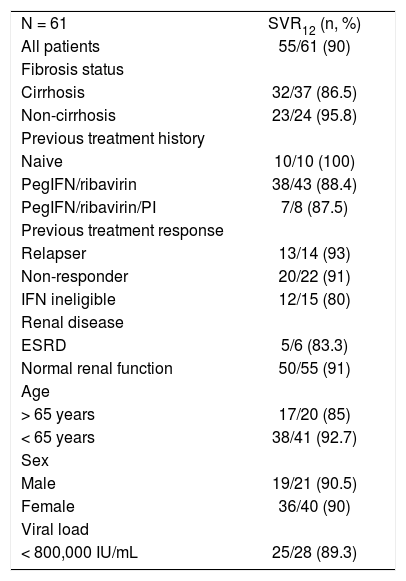
ITT analysis. SVR12 rates were 80% (12/15), 91% (20/22) and 93% (13/14) in interferon-ineligible/intolerant, non-responder and relapsed patients respectively (Table 2). Among cirrhotic patients, SVR12 rate was 86.5%.
Table 2.SVR12 rates according to previous treatment history and cirrhosis status (ITT analysis).
N = 61 SVR12 (n, %) All patients 55/61 (90) Fibrosis status Cirrhosis 32/37 (86.5) Non-cirrhosis 23/24 (95.8) Previous treatment history Naive 10/10 (100) PegIFN/ribavirin 38/43 (88.4) PegIFN/ribavirin/PI 7/8 (87.5) Previous treatment response Relapser 13/14 (93) Non-responder 20/22 (91) IFN ineligible 12/15 (80) Renal disease ESRD 5/6 (83.3) Normal renal function 50/55 (91) Age > 65 years 17/20 (85) < 65 years 38/41 (92.7) Sex Male 19/21 (90.5) Female 36/40 (90) Viral load < 800,000 IU/mL 25/28 (89.3) - •
PP analysis. At 12th week after the end of treatment period, 12 (80%) interferon-ineligible/intolerant, 20 (95.2%) non-responder and 13 (100%) relapsed patients had achieved SVR12. Patients with cirrhosis also achieved high rates of SVR12 (32/35, 91.4%).
SVR12 rates were 83.3% (5/6) and 87.5 (7/8) in patients with end stage renal disease and previous protease inhibitor experience, respectively. SVR was not associated with sex, age or baseline HCV RNA levels.
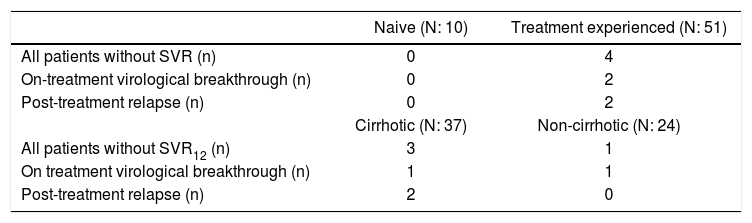
Virologic failureFour patients in treatment-experienced group had vi-rologic failures. Three of 4 were cirrhotic whereas 1 was non-cirrhotic. Two patients with undetectable HCV RNA at the 4th week of treatment had virologic breakthrough whereas 2 patients experienced viral relapse during post-treatment follow-up (Table 3). One of the non-responder had obesity and the other had insulin resistance. Among the remaining, one patient had chronic renal failure, whereas the other had no any accompanying diseases.
Reasons for not achieving SVR according to previous treatment and cirrhosis.
| Naive (N: 10) | Treatment experienced (N: 51) | |
|---|---|---|
| All patients without SVR (n) | 0 | 4 |
| On-treatment virological breakthrough (n) | 0 | 2 |
| Post-treatment relapse (n) | 0 | 2 |
| Cirrhotic (N: 37) | Non-cirrhotic (N: 24) | |
| All patients without SVR12 (n) | 3 | 1 |
| On treatment virological breakthrough (n) | 1 | 1 |
| Post-treatment relapse (n) | 2 | 0 |
We could investigate pretreatment resistance-associated variants in 23 patients from one center in this study. Only one of those 23 patients had pretreatment Y93H polymorphism (HCV RNA levels were negative at 4th and 12th weeks of treatment, but the patient died at the 13th week of treatment). Of the 22 patients without baseline polymorphism one had virologic breakthrough and the other had post-treatment viral relapse.
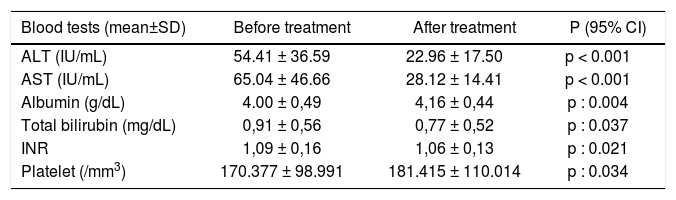
Improvement in liver biochemical testsTwelve weeks after treatment, all liver tests including ALT, AST, albumin, bilirubin and INR, and platelet counts improved significantly in the patients (Table 4). These results suggest that viral eradication by interferon-free daclatasvir and asunaprevir dual therapy also leads improvement in liver biochemical parameters.
Liver tests before and after treatment.
| Blood tests (mean±SD) | Before treatment | After treatment | P (95% CI) |
|---|---|---|---|
| ALT (IU/mL) | 54.41 ± 36.59 | 22.96 ± 17.50 | p < 0.001 |
| AST (IU/mL) | 65.04 ± 46.66 | 28.12 ± 14.41 | p < 0.001 |
| Albumin (g/dL) | 4.00 ± 0,49 | 4,16 ± 0,44 | p : 0.004 |
| Total bilirubin (mg/dL) | 0,91 ± 0,56 | 0,77 ± 0,52 | p : 0.037 |
| INR | 1,09 ± 0,16 | 1,06 ± 0,13 | p : 0.021 |
| Platelet (/mm3) | 170.377 ± 98.991 | 181.415 ± 110.014 | p : 0.034 |
A total of 57 patients completed 24 weeks of therapy. Two patients died during the study period. Both deaths occurred at the 12-14th weeks of treatment as a result of underlying diseases including tuberculous meningitis in one and systemic vasculitis in the other. Both had unde-tectable HCV RNA at week 8 and 12 on treatment.
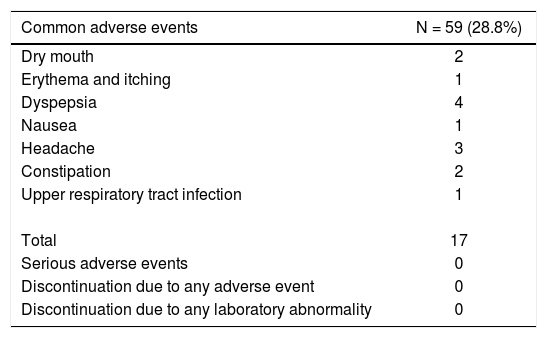
None of the patients discontinued treatment due to any adverse events. The most common adverse event was dyspepsia (Table 5). There was no serious adverse event or any grade-4 laboratory abnormality.
Safety profile of dual therapy in the early access pro¬gramme.
| Common adverse events | N = 59 (28.8%) |
|---|---|
| Dry mouth | 2 |
| Erythema and itching | 1 |
| Dyspepsia | 4 |
| Nausea | 1 |
| Headache | 3 |
| Constipation | 2 |
| Upper respiratory tract infection | 1 |
| Total | 17 |
| Serious adverse events | 0 |
| Discontinuation due to any adverse event | 0 |
| Discontinuation due to any laboratory abnormality | 0 |
Currently, total cost of 24-week daclatasvir and asuna-previr dual therapy in Turkey is about 13.500 US dollars (present in the market but not paid by social security institution yet). Treatment of genotype 1b HCV infection with daclatasvir and asunaprevir dual therapy provided cost saving and health gain compared to the available treatment regimens.
In treatment-naïve patients, for life years gained (LYG), dual therapy was more cost-effective than pegylated interferon + ribavirin (-1.100 US dollars), boceprevir + pegylated interferon + ribavirin (-4.764 US dollars), telaprevir + pegylated interferon + ribavirin (-7.843 US dollars) and without-treatment (-1.000 US dollars).
In treatment-experienced patients, for LYG, dual therapy was also superior to pegylated interferon + ribavirin (-1.300 US dollars), boceprevir + pegylated interferon + ribavirin (-5.000 US dollars), telaprevir + pegylated interferon + ribavirin (-3.500 US dollars) and without-treatment (-400 US dollars).
Quality adjusted life years (QALYs) analysis for dual therapy was also better than all other available alternatives.
DiscussionIn this study, we demonstrated that daclatasvir-asuna-previr combination therapy is highly effective and safe in Turkish patients with chronic HCV. Daclatasvir-asuna-previr dual therapy seems to be successfull even in hard-to-treat patients with chronic renal failure under hemodialysis and protease inhibitor experience.
As initial enrollment criteria, most of our patients had problematic baseline characteristics such as prior treatment, high serum HCV RNA, cirrhosis and accompanying diseases. However, SVR was more than 90% irrespective of any poor prognostic signs for HCV treatment.
Response to dual therapy is affected mainly by daclatasvir resistance associated polymorphism12,13 SVR12 in patients without baseline NS5A (daclatasvir) resistance associated polymorphism has been reported as 90-100%, wheras it decreases dramatically to less than 40% in the presence of such polymorphism at baseline.12 Fortunately, frequency of NS5A polymorphism seems to be less than 20% in Japanese and non-Japanese population with HCV infection.12 However that knowledge needs further validation especially in non-Japanese patients. In our study we could analyze baseline NS5A resistance associated polymorphism in 23 patients. There was only one polymorphism and it was in the dead patient who had undetectable serum HCV RNA at 4th and 12th weeks of treatment.
One of the main advantage of daclatasvir and asunapre-vir dual therapy is absence of contraindication in patients with chronic renal failure. There are few data regarding safety of oral antiviral drugs in those patients. Ombitasvir-paritaprevir-ritonavir-dasabuvir and graziprevir-elbasvir combinations seem to be safe, whereas there is lack of evidence of sofosbuvir safety in chronic renal disease pa-tients.14-18 Reports demonstrated that daclatasvir and asunaprevir dual therapy is also safe in those patients. According to a recent study, 20 of 21 (95.5%) chronic hemo-dialysis patients using daclatasvir plus asunaprevir developed sustained virologic response.9 Our data is also supporting both efficacy and safety of dual therapy in patients with chronic renal failure. Five of 6 patients had SVR and none had any adverse effects during the 24 week treatment duration.
There is limited data about the efficacy of oral antiviral agents in patients with previous protease inhibitor experience. The underlying logic is that previous protease inhibitor (NS3/4) use would increase the risk of resistance. Although the frequency of NS3/4 resistance is very low and the resistance may disappear with time, the sponsors of registration clinical trials do not want to enroll those patient groups. Actually several reports of oral antiviral combinations including sofosbuvir-simeprevir combination have depicted that previous protease inhibitor experience did not have significant importance in respect to resistance and virological response.19 Although study protocol did not allow enrollment of patients who were unresponsive to previous telaprevir or boceprevir treatment, 8 included patients had such a history and 7 of 8 patients had SVR in the present study.
Daclatasvir and asunaprevir have a good safety profile.20,21 Headache, fatigue, diarrhea, nausea and asthenia are the most frequently reported symptoms that occur in less than one fourth of the patients. A transient increment of liver transaminases and bilirubin attributed to asunaprevir have been reported in about 5-29% of patients.21 However, those rare adverse events did not cause to stop treatment in most of those patients. Interestingly none of our patients had transaminase elevations. Also, none of the patients had to stop the dual therapy as a result of any adverse events.
Although some pretreatment variables such as viral load, presence of cirrhosis, age and insulin resistance had negative effect on SVR with interferon-ribavirin treatment of HCV,22,23 those factors seem to have less affect SVR with recent antiviral therapies. Since the number of the patients without SVR was too low in the present study, it is hard to speculate the pretreatment variables associated with viral failure.
In conclusion, successful viral eradication by interfer-on-free daclatasvir and asunaprevir dual therapy leads to high sustained viral response in difficult to treat patient population. No serious adverse events or any adverse event resulting in discontinuation of therapy is reported.