Sustained virologic response (SVR) is achieved in most cases of C-type liver disease after direct-acting antiviral (DAA) therapy. Although liver fibrosis improves, the degree of improvement is different. This study aimed to analyze the factors involved in improving liver fibrosis using the fibrosis 4 (FIB-4) index.
Material and methodsPatients were monitored for >3 years after SVR. At the start of therapy (SOT), liver fibrosis was categorized as either mild (<1.45 n = 28), moderate (1.45–3.25 n = 139), or advanced (>3.25 n = 236) based on the FIB-4 index. The FIB-4 index in the advanced group decreased significantly compared to that of the other two, so we selected the advanced group as the analysis target. SOT and end of therapy (EOT) factors that contributed to the FIB-4 index ≤3.25 at 3 years after therapy were examined using a multivariate analysis.
ResultsAmong the SOT factors, age (<72 years old), absence of liver cirrhosis (LC), alanine transferase (ALT) (≥50 U/L), platelet (PLT) (≥10.2 × 104/mm3), and total bilirubin (T.Bil) (<0.8 mg/dl) were the significant factors contributing to the improvement of the FIB-4 index. Among the EOT factors, age (<72 years), PLT (≥12.0 × 104/mm3), and hemoglobin (Hb) (≥12.1 g/dl) were the significant factors contributing to the improvement of FIB-4 index.
ConclusionsFactors involved in the improvement of liver fibrosis after SVR were young age, absence of LC, low T.Bil., high ALT, high PLT, and high Hb levels. The levels of T.Bil, PLT, and Hb were considered to be related to portal hypertension. Aging strongly impaired the improvement in liver fibrosis.
Hepatitis C virus (HCV) is a major public health concern, affecting approximately 180 million people worldwide [1]. The progression of chronic HCV infection leads to liver cirrhosis and cancer. HCV infection is associated with both liver-related and non-liver-related deaths [2]. Total eradication of HCV with antivirals inhibits hepatocarcinogenesis [3] and reduces both liver-related and non-liver-related deaths [4]. After approval in the early 1990s, interferon (IFN) therapy was successful in eradicating HCV in some HCV-associated liver diseases. However, these IFN therapies benefited only a subset of HCV patients [5,6], they were especially limited in elderly patients owing to their low tolerability and low antiviral effect. DAAs directly inhibit HCV proliferation. Since the advent of IFN-free therapy in 2014, which consists of only DAAs without any IFN, HCV treatment using DAA has seen considerable success owing to its high SVR rate and superior tolerability. It allows for the treatment of patients who cannot receive IFN-based therapy due to comorbidities (e.g., diabetes mellitus, renal dysfunction, and anemia) and old age; many of these patients have achieved SVR [7]. Hepatic reserve and hepatic fibrosis improve in cases where SVR has been achieved; however, the degree of improvement varies depending on the case [8]. In addition, subsequent liver carcinogenesis is suppressed in cases where liver fibrosis improves after SVR [9]. The objective of this study was to determine the factors affecting the improvement of hepatic fibrosis in patients with advanced fibrosis.
2Methods2.1Study designThis retrospective study included patients who received DAA therapy at Sapporo Kosei General Hospital. A total of 642 patients with HCV-associated chronic liver disease started DAA therapy between September 2014 and October 2016. Among them, 403 patients that could be followed up for 3 years or longer after achieving SVR (male, 164; female, 239; genotype 1:299, genotype 2, 104; median age, 69 years) were included. Daclatasvir/asunaprevir (DCV/ASV) was administered in 214, sofosbuvir/ribavirin (SOF/RBV) in 104, sofosbuvir/ledipasvir (SOF/LDV) in 66, and ombitasvir/paritaprevir/ritonavir (OBV/PTV/r) in 19 patients [10–13]. The treatment duration was 24 weeks for DCV/ASV and 12 weeks for the other drug combinations. Sixty-five patients (16.1%) had previously been treated for hepatocellular carcinoma (HCC). In these patients, the absence of recurrent HCC was confirmed by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) before the start of DAA therapy.
The diagnosis of LC was based on morphology, either through liver histology, ultrasonography (US), CT, or MRI. Patients with decompensated LC were excluded because DAA therapy is currently not approved for this condition in Japan at the time of drafting this article.
The study conformed to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Sapporo Kosei General Hospital. Informed consent for the study was obtained from the Sapporo Kosei General Hospital website in the form of an opt-out form. The number of ethical approvals was 422 (12/9/2017).
2.2Patient classificationBiochemical tests were performed periodically at the SOT, EOT, and 1, 2, and 3 years after the therapy to evaluate the changes in hepatic fibrosis. Assessment of hepatic fibrosis was based on the FIB-4 index, calculated from the serum ALT level, AST level, PLT count, and age [14,15]. The equation is as follows: FIB-4 index = [age (years) × AST (U/L)] / [PLT (109/L) × ALT (U/L)1/2].
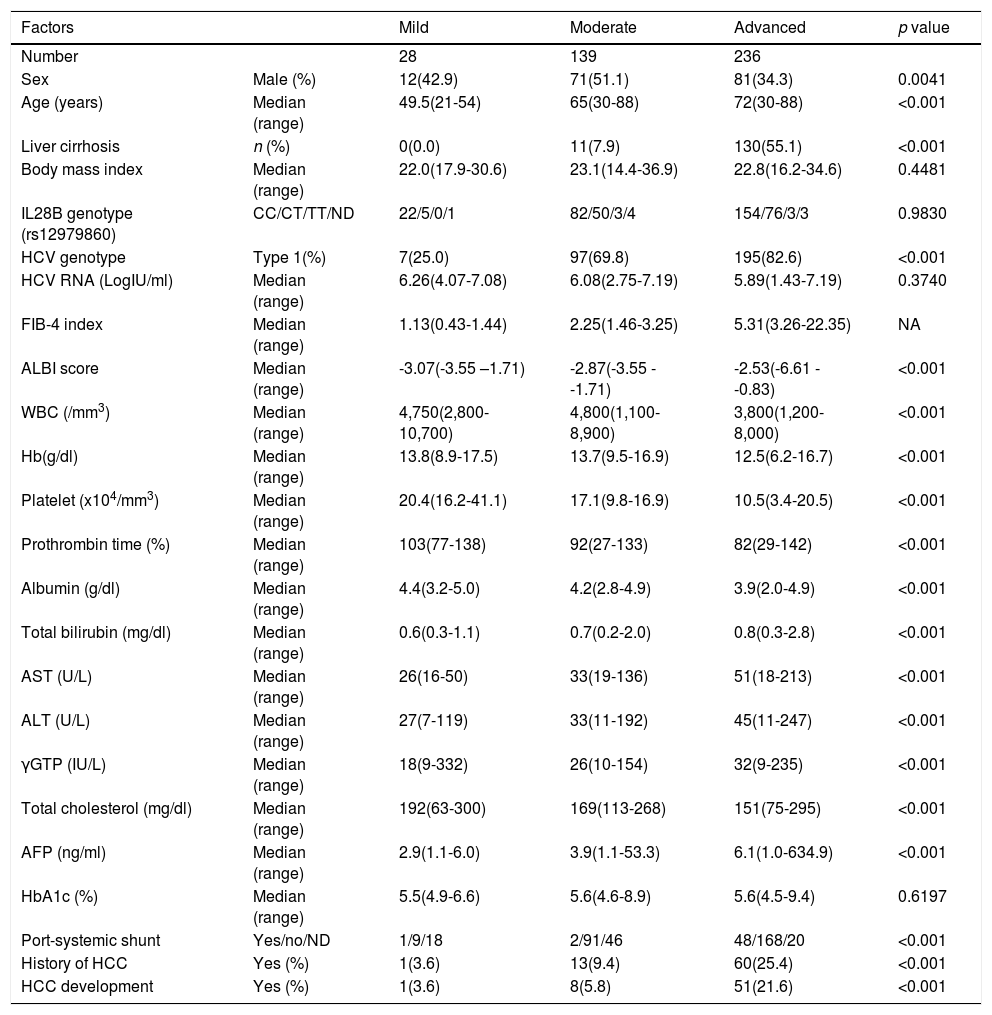
Subjects were divided into three groups based on the FIB-4 index at the SOT following the classification report by Sterling et al. [14], mild (FIB-4 index < 1.45, n = 28), moderate (FIB-4 index 1.45-3.25 n = 139), and advanced (FIB-4 index > 3.25 n = 236). The background factors of each group are listed in Table 1. The changes in the FIB-4 index of the three groups were examined at five points for the SOT, EOT, and 1, 2, and 3 years after the end of DAA therapy.
Background of the fibrosis groups.
| Factors | Mild | Moderate | Advanced | p value | |
|---|---|---|---|---|---|
| Number | 28 | 139 | 236 | ||
| Sex | Male (%) | 12(42.9) | 71(51.1) | 81(34.3) | 0.0041 |
| Age (years) | Median (range) | 49.5(21-54) | 65(30-88) | 72(30-88) | <0.001 |
| Liver cirrhosis | n (%) | 0(0.0) | 11(7.9) | 130(55.1) | <0.001 |
| Body mass index | Median (range) | 22.0(17.9-30.6) | 23.1(14.4-36.9) | 22.8(16.2-34.6) | 0.4481 |
| IL28B genotype (rs12979860) | CC/CT/TT/ND | 22/5/0/1 | 82/50/3/4 | 154/76/3/3 | 0.9830 |
| HCV genotype | Type 1(%) | 7(25.0) | 97(69.8) | 195(82.6) | <0.001 |
| HCV RNA (LogIU/ml) | Median (range) | 6.26(4.07-7.08) | 6.08(2.75-7.19) | 5.89(1.43-7.19) | 0.3740 |
| FIB-4 index | Median (range) | 1.13(0.43-1.44) | 2.25(1.46-3.25) | 5.31(3.26-22.35) | NA |
| ALBI score | Median (range) | -3.07(-3.55 –1.71) | -2.87(-3.55 - -1.71) | -2.53(-6.61 - -0.83) | <0.001 |
| WBC (/mm3) | Median (range) | 4,750(2,800-10,700) | 4,800(1,100-8,900) | 3,800(1,200-8,000) | <0.001 |
| Hb(g/dl) | Median (range) | 13.8(8.9-17.5) | 13.7(9.5-16.9) | 12.5(6.2-16.7) | <0.001 |
| Platelet (x104/mm3) | Median (range) | 20.4(16.2-41.1) | 17.1(9.8-16.9) | 10.5(3.4-20.5) | <0.001 |
| Prothrombin time (%) | Median (range) | 103(77-138) | 92(27-133) | 82(29-142) | <0.001 |
| Albumin (g/dl) | Median (range) | 4.4(3.2-5.0) | 4.2(2.8-4.9) | 3.9(2.0-4.9) | <0.001 |
| Total bilirubin (mg/dl) | Median (range) | 0.6(0.3-1.1) | 0.7(0.2-2.0) | 0.8(0.3-2.8) | <0.001 |
| AST (U/L) | Median (range) | 26(16-50) | 33(19-136) | 51(18-213) | <0.001 |
| ALT (U/L) | Median (range) | 27(7-119) | 33(11-192) | 45(11-247) | <0.001 |
| γGTP (IU/L) | Median (range) | 18(9-332) | 26(10-154) | 32(9-235) | <0.001 |
| Total cholesterol (mg/dl) | Median (range) | 192(63-300) | 169(113-268) | 151(75-295) | <0.001 |
| AFP (ng/ml) | Median (range) | 2.9(1.1-6.0) | 3.9(1.1-53.3) | 6.1(1.0-634.9) | <0.001 |
| HbA1c (%) | Median (range) | 5.5(4.9-6.6) | 5.6(4.6-8.9) | 5.6(4.5-9.4) | 0.6197 |
| Port-systemic shunt | Yes/no/ND | 1/9/18 | 2/91/46 | 48/168/20 | <0.001 |
| History of HCC | Yes (%) | 1(3.6) | 13(9.4) | 60(25.4) | <0.001 |
| HCC development | Yes (%) | 1(3.6) | 8(5.8) | 51(21.6) | <0.001 |
FIB-4 index, fibrosis-4 index; SOT, start of therapy; EOT, end of therapy; NA, not applicable; IL28B, interleukin 28B; CC, wild type allele; CT, heterozygotic polymorphism; TT, homozygotic polymorphism; ND, not determined; HCV, hepatitis C virus; ALBI score, ALBI score, albumin-bilirubin score; WBC, white blood cell; Hb, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGTP, gamma-glutamyl transpeptidase; AFP, alpha-fetoprotein; HbA1c, hemoglobin A1c; HCC, hepatocellular carcinoma
The factors involved in lowering the FIB-4 index to <3.25 after 3 years in 230 advanced cases were investigated. The following parameters were evaluated to identify the factors involved in the improvement of advanced fibrosis to moderate fibrosis: patient background factors (age, sex, interleukin 28 B genotype, presence or absence of previous HCC therapy, presence or absence of LC, and body mass index), biochemical test results at SOT (white blood cell [WBC] count, hemoglobin [Hb], PLT count, albumin [ALB], T.Bil., ALT, AST, gamma-glutamyl transpeptidase [γ-GTP], total cholesterol [TC], prothrombin time [PT], hemoglobin A1c [HbA1c], alpha-fetoprotein [AFP] level, presence or absence of HCC occurrence during follow-up, and the presence or absence of a portosystemic shunt within 1 year from the SOT). The portosystemic shunt has a hepatofugal flow (flow directed away from the liver). In this study, we mainly focused on the gastro-renal shunt, splenorenal shunt, paraumbilical vein, and intrahepatic portal vein shunt. If the presence of the aforementioned far-hepatic vessels was confirmed by a radiologist with contrast CT, we considered it a portosystemic shunt.
Similarly, the factors involved in the FIB-4 index <3.25 after 3 years were examined using the EOT factors.
Since the relationship between age and improvement of the FIB-4 index was derived from the aforementioned examination, we assessed the improvement of the FIB-4 index by age group over time. Following the definition of elderly people by the working group in the Japan Gerontological Society and the Japan Geriatrics Society [16], the patients were classified as follows: non-old patients (≤64 years), pre-old patients (65–74 years), and old patients (≥75 years).
Finally, the correlation between hepatic reserve and hepatic fibrosis, before and after SVR, was examined. The hepatic reserve was evaluated using the albumin-bilirubin (ALBI) score [17], which was calculated from the blood ALB and T.Bil levels (the lower the score, the better the hepatic reserve).
2.3Statistical AnalysisThe Kruskal–Wallis and Cochran–Armitage tests were used to assess the differences in the distribution of background factors between the liver fibrosis groups. The Mann–Whitney U test was used to assess the degree of liver fibrosis among the three groups. Receiver operating characteristic (ROC) analysis and binomial logistic regression analysis were used to assess the relationship between the transition of liver fibrosis after SVR and the background factors. The correlation matrix was used to determine the correlation between hepatic fibrosis (FIB-4 index) and hepatic reserve (ALBI score). The Smirnov–Grubb test was used to determine and remove outliers. All statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). All tests of significance were two-tailed, and p < 0.05 was considered to be statistically significant.
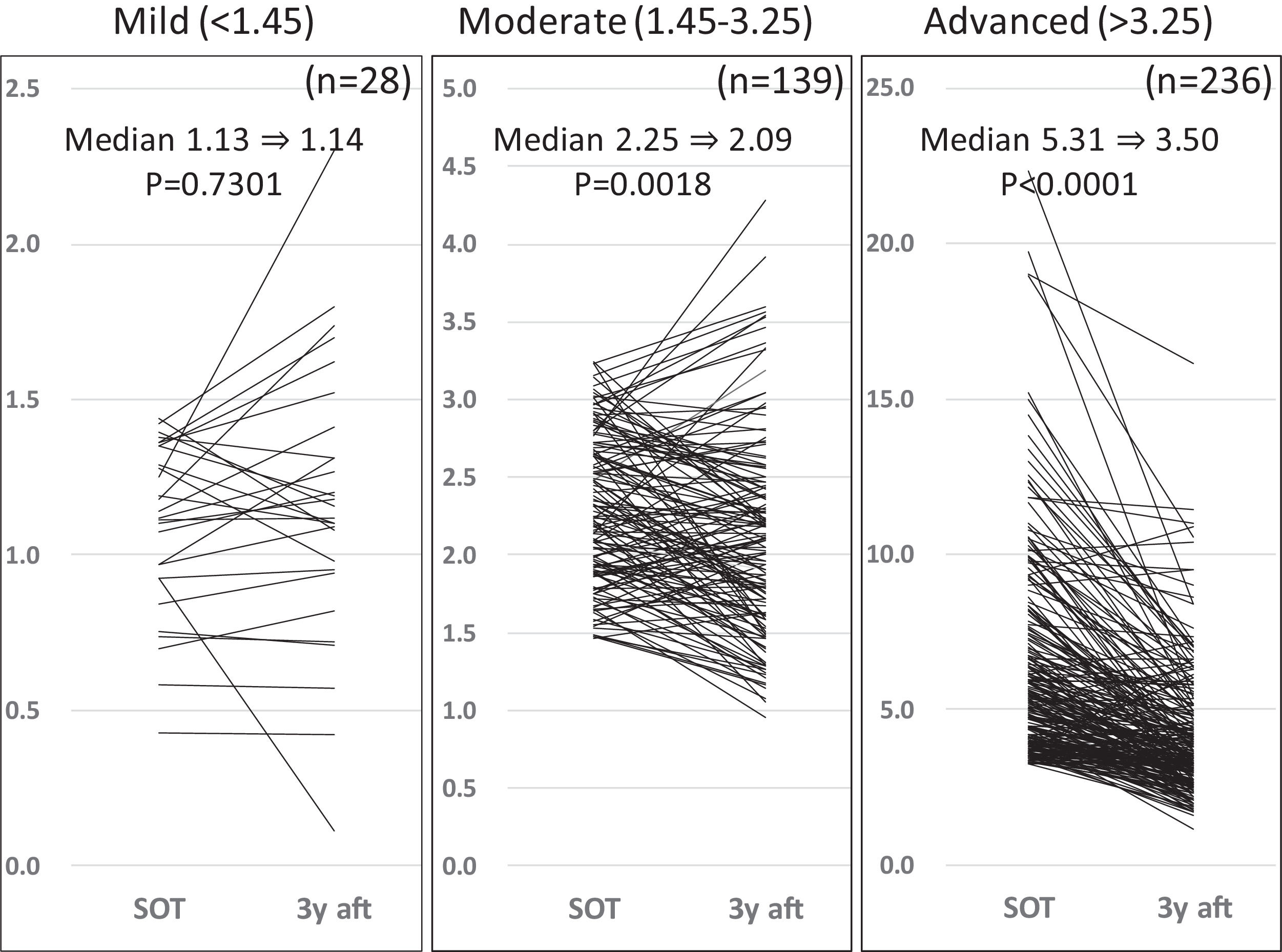
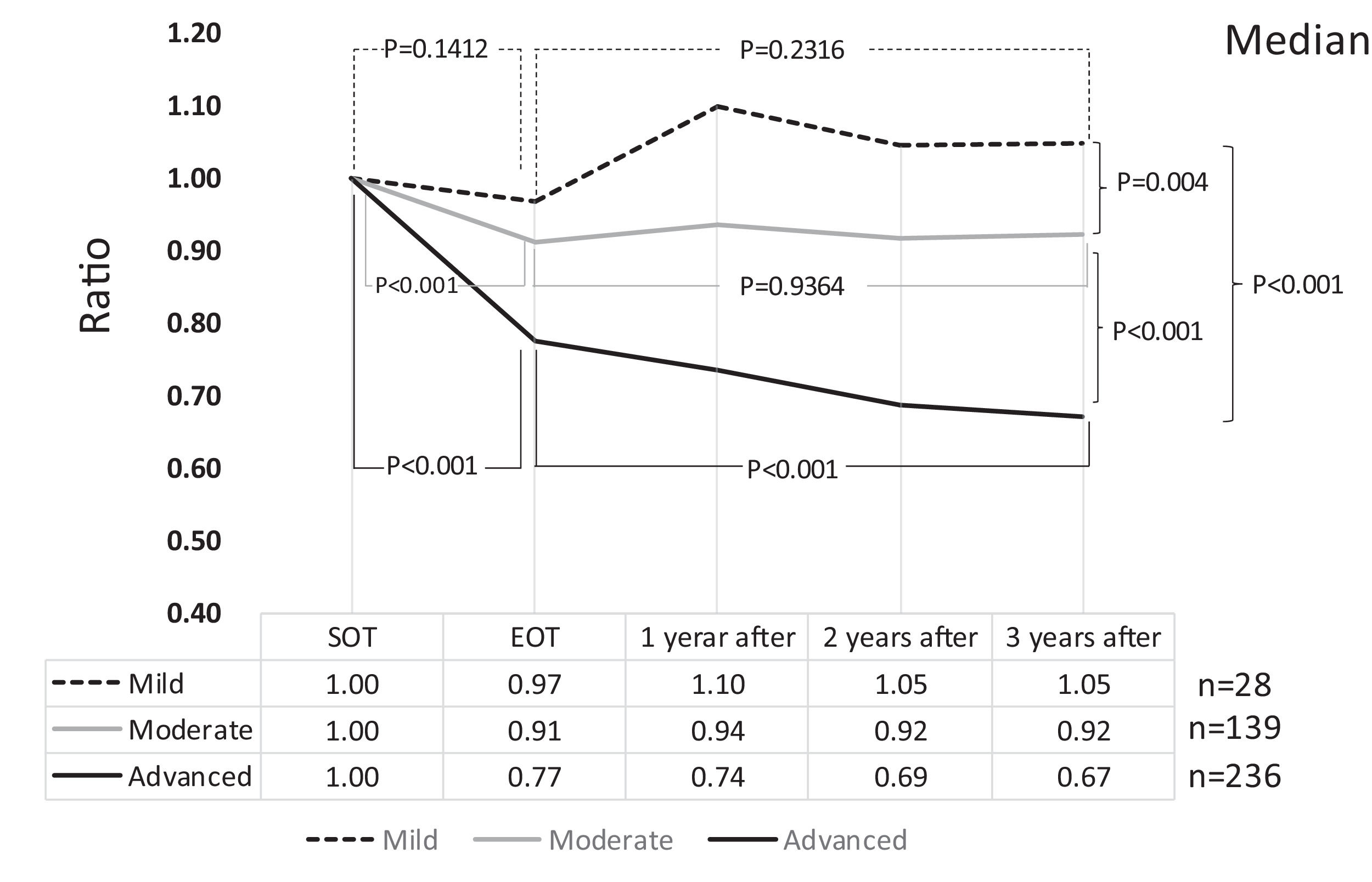
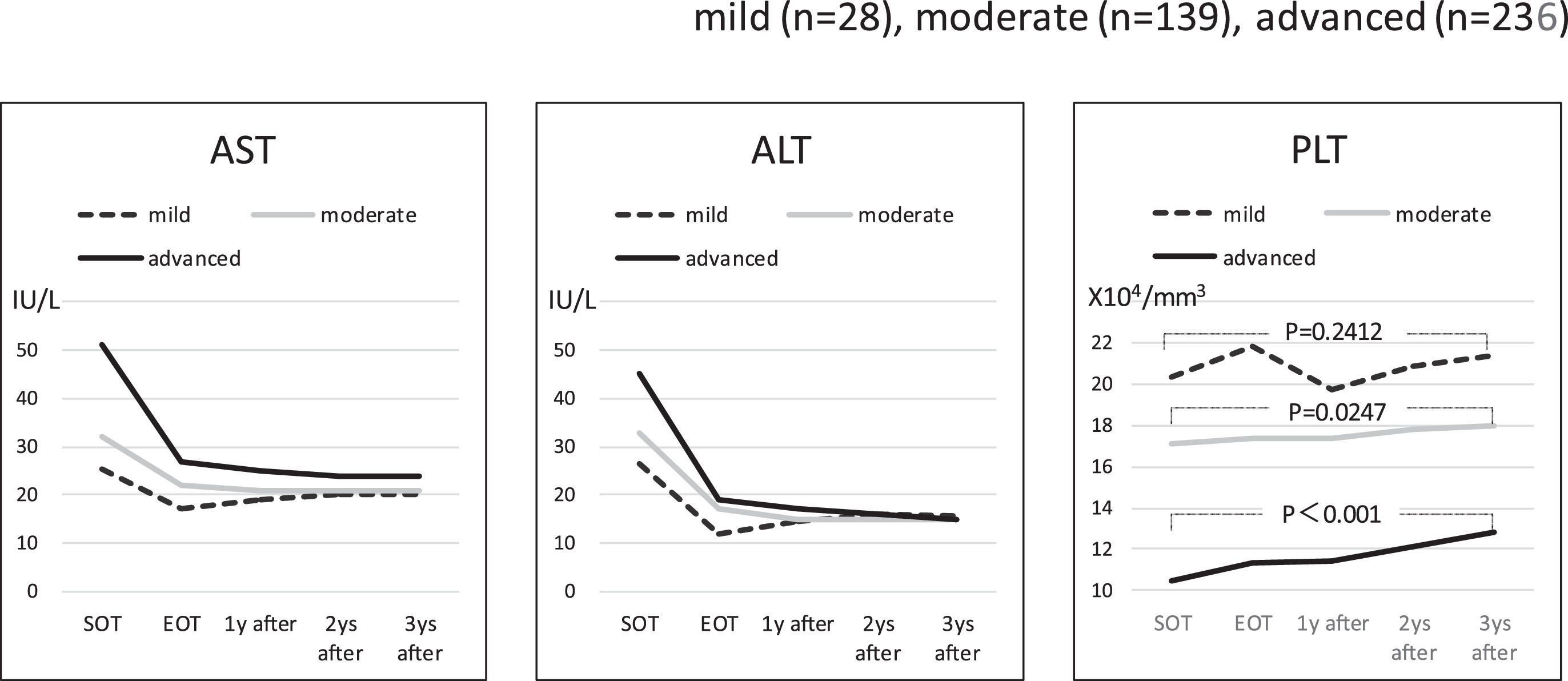
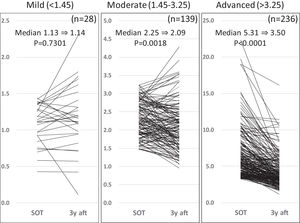
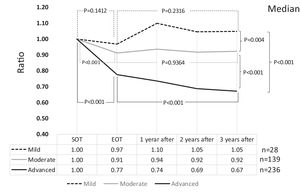
3Results3.1Liver fibrosis after achieving SVRFrom the FIB-4 index of SOT, 403 patients were divided as follows: 28 in the mild group (<1.45), 139 in the moderate group (1.45–3.25), and 236 patients as the advanced group (>3.25), according to the degree of liver fibrosis. Table 1 presents the background of each fibrosis group. In the advanced group, there were significantly more women, elderly patients, cirrhotic patients, patients with decreased hepatic reserve, patients with HCC, and patients with a portosystemic shunt. Changes were observed in the FIB-4 index between the SOT and 3 years after the treatment for each fibrotic group: mild group (1.13 to 1.14, p = 0.7301), moderate group (2.25 to 2.09, p = 0.0018), and advanced group (5.31 to 3.50, p < 0.0001). No significant decrease was observed in the mild group (Fig. 1). Examination of the attenuation rate (ratio to the value in the SOT) of the FIB-4 index value over time showed no significant changes throughout the course for the mild group. In contrast, in the moderate group, significant attenuation was observed in the EOT, but then progressed to plateau, and in the advanced group, strong attenuation was observed in the EOT, followed by continuous attenuation. Only the advanced group showed significant attenuation from EOT to 3 years after EOT (p < 0.001) (Fig. 2). The components of the FIB-4 index, AST level, and ALT level declined rapidly between the SOT and EOT in all groups, followed by a plateau. In contrast, PLT increased moderately and continuously during this period in the moderate and advanced groups (Fig. 3).
Changes in FIB-4 index for three fibrosis grade groups at the start of therapy, and after 3 years of direct-acting antivirals therapy After 3 years, the FIB-4 index showed a significant decrease in the moderate and advanced groups but not in the mild group. SOT, start o therapy; 3y aft, 3 years after end of therapy.
Changes in FIB-4 index by the grade of FIB-4 index (ratio with the start of therapy). A comparison of the FIB-4 index between the start of therapy and end of therapy showed a significant decrease in the moderate and advanced groups. Compared to the end of therapy after 3 years, only the advanced group showed a significant decrease. SOT, start o therapy; EOT, end of therapy; 1 year after, 1 year after EOT; 2 years after, 2years after EOT; 3 years after, 3years after EOT.
Components of the FIB-4 index Changes in the serum AST level, ALT level, and PLT count (the components of FIB-4 index) around direct-acting antiviral therapy in the three fibrosis grade groups. AST and ALT decreased rapidly at end of therapy in all three groups, but there were no remarkable changes thereafter. PLT had a gradual increase throughout the period in the moderate and advanced groups, but it did not show a significant change in the mild group. SOT, start o therapy; EOT, end of therapy; 1 year after, 1 year after EOT; 2 years after, 2years after EOT; 3 years after, 3years after EOT.
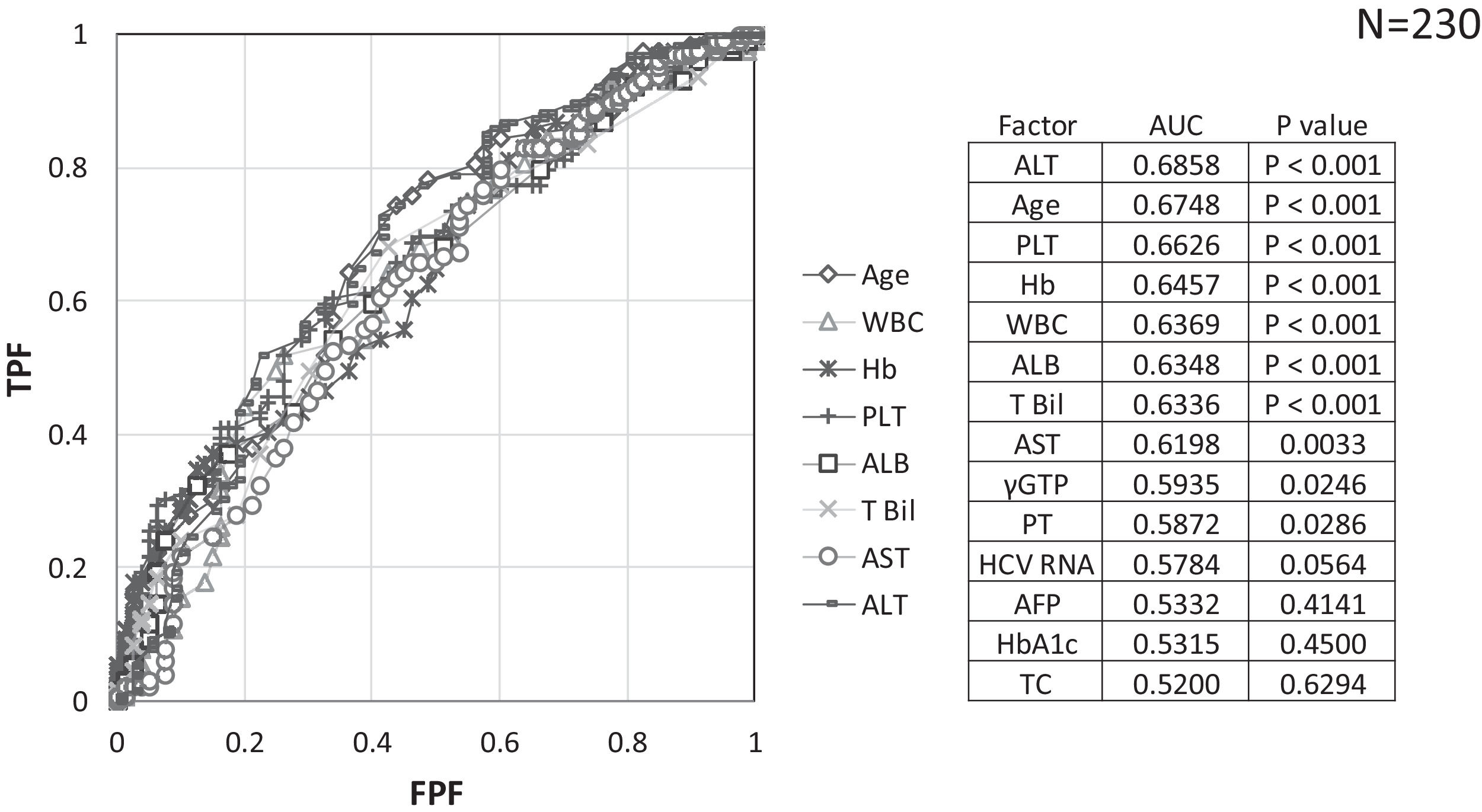
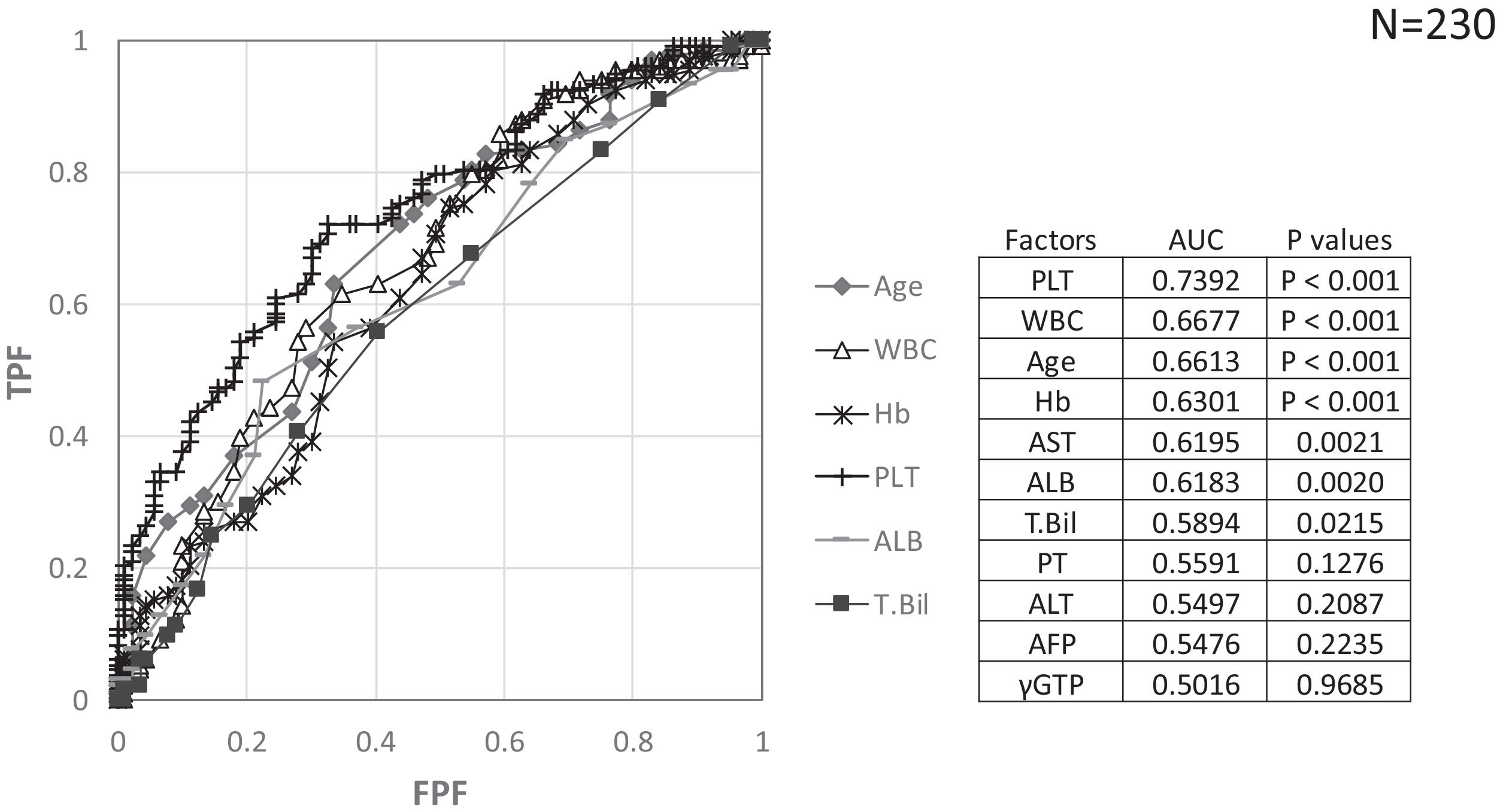
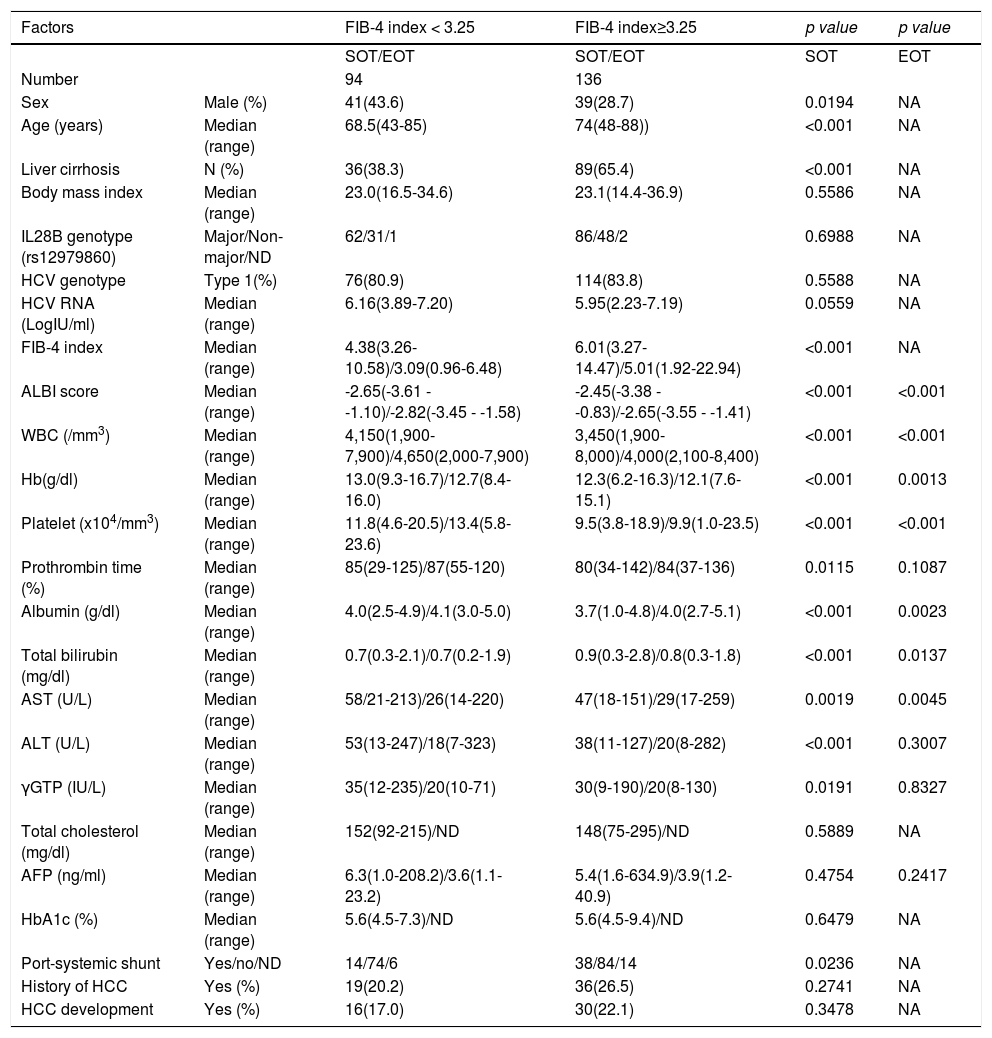
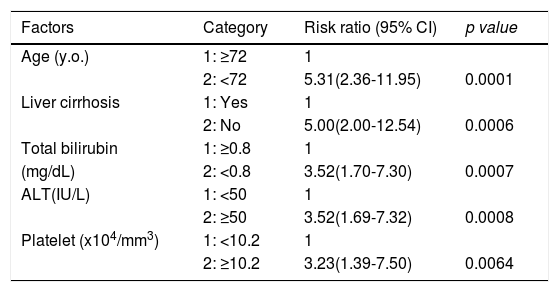
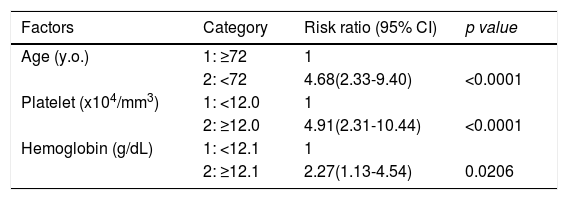
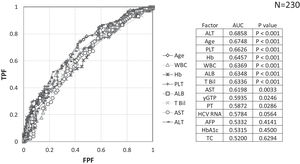
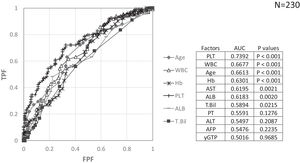
Factors involved in the improvement from the advanced stage (FIB-4 index > 3.25) over 3 years were examined in 236 advanced cases (six cases were excluded as outliers). In 94 of 230 patients (40.9%), an FIB-4 index of <3.25 was achieved 3 years after EOT. Table 2 shows the background factors and the results of the blood biochemical tests at the SOT and EOT of the improved (FIB-4 index < 3.25) and non-improved (FIB-4 index ≥ 3.25) cases. Three factors were significant for the categorical data: sex, presence or absence of LC, and presence or absence of portosystemic shunt. The numerical data that were significant in the ROC analysis were ALT level, age, PLT count, Hb level, WBC count, ALB level, T.Bil level, AST level, γGTP level, and PT for SOT (Fig. 4). In the EOT, there were seven significant factors, including PLT count, WBC count, age, Hb, AST, ALB, and T.Bil levels (Fig. 5). The FIB-4 index values were excluded because age, AST level, ALT level, and PLT count were included in the ROC analysis. Binomial logistic regression analysis was performed using the SOT, EOT, and categorical data, using the cut-off values obtained from the ROC curve. Five significant factors contributed to the improvement of the FIB-4 index <3.25, including age (<72 years, odds ratio (OR): 5.31), LC (absent, OR: 5.00), T.Bil level (<0.8 mg/dL, OR: 3.52), ALT level (≥50 IU/L, OR: 3.0), and PLT count (≥102,000/mm3, OR: 3.23) for SOT (Table 3). For EOT, there were three factors, including age (<72 years, OR: 4.68), PLT count (>120,000/mm3, OR: 4.91), and Hb level (>12.1 g/dl, OR: 2.27) (Table 4).
Background factors and blood biochemical tests at the start of therapy and end of therapy for FIB-4 index < 3.25 and FIB-4 index≥3.25 cases, 3 years after the direct acting antiviral treatment.
| Factors | FIB-4 index < 3.25 | FIB-4 index≥3.25 | p value | p value | |
|---|---|---|---|---|---|
| SOT/EOT | SOT/EOT | SOT | EOT | ||
| Number | 94 | 136 | |||
| Sex | Male (%) | 41(43.6) | 39(28.7) | 0.0194 | NA |
| Age (years) | Median (range) | 68.5(43-85) | 74(48-88)) | <0.001 | NA |
| Liver cirrhosis | N (%) | 36(38.3) | 89(65.4) | <0.001 | NA |
| Body mass index | Median (range) | 23.0(16.5-34.6) | 23.1(14.4-36.9) | 0.5586 | NA |
| IL28B genotype (rs12979860) | Major/Non-major/ND | 62/31/1 | 86/48/2 | 0.6988 | NA |
| HCV genotype | Type 1(%) | 76(80.9) | 114(83.8) | 0.5588 | NA |
| HCV RNA (LogIU/ml) | Median (range) | 6.16(3.89-7.20) | 5.95(2.23-7.19) | 0.0559 | NA |
| FIB-4 index | Median (range) | 4.38(3.26-10.58)/3.09(0.96-6.48) | 6.01(3.27-14.47)/5.01(1.92-22.94) | <0.001 | NA |
| ALBI score | Median (range) | -2.65(-3.61 - -1.10)/-2.82(-3.45 - -1.58) | -2.45(-3.38 - -0.83)/-2.65(-3.55 - -1.41) | <0.001 | <0.001 |
| WBC (/mm3) | Median (range) | 4,150(1,900-7,900)/4,650(2,000-7,900) | 3,450(1,900-8,000)/4,000(2,100-8,400) | <0.001 | <0.001 |
| Hb(g/dl) | Median (range) | 13.0(9.3-16.7)/12.7(8.4-16.0) | 12.3(6.2-16.3)/12.1(7.6-15.1) | <0.001 | 0.0013 |
| Platelet (x104/mm3) | Median (range) | 11.8(4.6-20.5)/13.4(5.8-23.6) | 9.5(3.8-18.9)/9.9(1.0-23.5) | <0.001 | <0.001 |
| Prothrombin time (%) | Median (range) | 85(29-125)/87(55-120) | 80(34-142)/84(37-136) | 0.0115 | 0.1087 |
| Albumin (g/dl) | Median (range) | 4.0(2.5-4.9)/4.1(3.0-5.0) | 3.7(1.0-4.8)/4.0(2.7-5.1) | <0.001 | 0.0023 |
| Total bilirubin (mg/dl) | Median (range) | 0.7(0.3-2.1)/0.7(0.2-1.9) | 0.9(0.3-2.8)/0.8(0.3-1.8) | <0.001 | 0.0137 |
| AST (U/L) | Median (range) | 58/21-213)/26(14-220) | 47(18-151)/29(17-259) | 0.0019 | 0.0045 |
| ALT (U/L) | Median (range) | 53(13-247)/18(7-323) | 38(11-127)/20(8-282) | <0.001 | 0.3007 |
| γGTP (IU/L) | Median (range) | 35(12-235)/20(10-71) | 30(9-190)/20(8-130) | 0.0191 | 0.8327 |
| Total cholesterol (mg/dl) | Median (range) | 152(92-215)/ND | 148(75-295)/ND | 0.5889 | NA |
| AFP (ng/ml) | Median (range) | 6.3(1.0-208.2)/3.6(1.1-23.2) | 5.4(1.6-634.9)/3.9(1.2-40.9) | 0.4754 | 0.2417 |
| HbA1c (%) | Median (range) | 5.6(4.5-7.3)/ND | 5.6(4.5-9.4)/ND | 0.6479 | NA |
| Port-systemic shunt | Yes/no/ND | 14/74/6 | 38/84/14 | 0.0236 | NA |
| History of HCC | Yes (%) | 19(20.2) | 36(26.5) | 0.2741 | NA |
| HCC development | Yes (%) | 16(17.0) | 30(22.1) | 0.3478 | NA |
FIB-4 index, fibrosis-4 index; SOT, start of therapy; EOT, end of therapy; NA, not applicable; IL28B, interleukin 28B; major, major allele; minor, minor allele; ND, not determined; HCV, hepatitis C virus; ALBI score, ALBI score, albumin-bilirubin score; WBC, white blood cell; Hb, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGTP, gamma-glutamyl transpeptidase; AFP, alpha-fetoprotein; HbA1c, hemoglobin A1c; HCC, hepatocellular carcinoma
Binominal logistic regression analysis of factors involved in the improvement of FIB-4 score to less than 3.25, 3 years after direct acting antiviral treatment.
| Factors | Category | Risk ratio (95% CI) | p value |
|---|---|---|---|
| Age (y.o.) | 1: ≥72 | 1 | |
| 2: <72 | 5.31(2.36-11.95) | 0.0001 | |
| Liver cirrhosis | 1: Yes | 1 | |
| 2: No | 5.00(2.00-12.54) | 0.0006 | |
| Total bilirubin | 1: ≥0.8 | 1 | |
| (mg/dL) | 2: <0.8 | 3.52(1.70-7.30) | 0.0007 |
| ALT(IU/L) | 1: <50 | 1 | |
| 2: ≥50 | 3.52(1.69-7.32) | 0.0008 | |
| Platelet (x104/mm3) | 1: <10.2 | 1 | |
| 2: ≥10.2 | 3.23(1.39-7.50) | 0.0064 |
CI, confidence interval; ALT, alanine aminotransferase
Binominal logistic regression analysis of factors involved in the improvement of FIB-4 score to less than 3.25, 3 years of direct acting antiviral treatment
| Factors | Category | Risk ratio (95% CI) | p value |
|---|---|---|---|
| Age (y.o.) | 1: ≥72 | 1 | |
| 2: <72 | 4.68(2.33-9.40) | <0.0001 | |
| Platelet (x104/mm3) | 1: <12.0 | 1 | |
| 2: ≥12.0 | 4.91(2.31-10.44) | <0.0001 | |
| Hemoglobin (g/dL) | 1: <12.1 | 1 | |
| 2: ≥12.1 | 2.27(1.13-4.54) | 0.0206 |
CI, confidence interval.
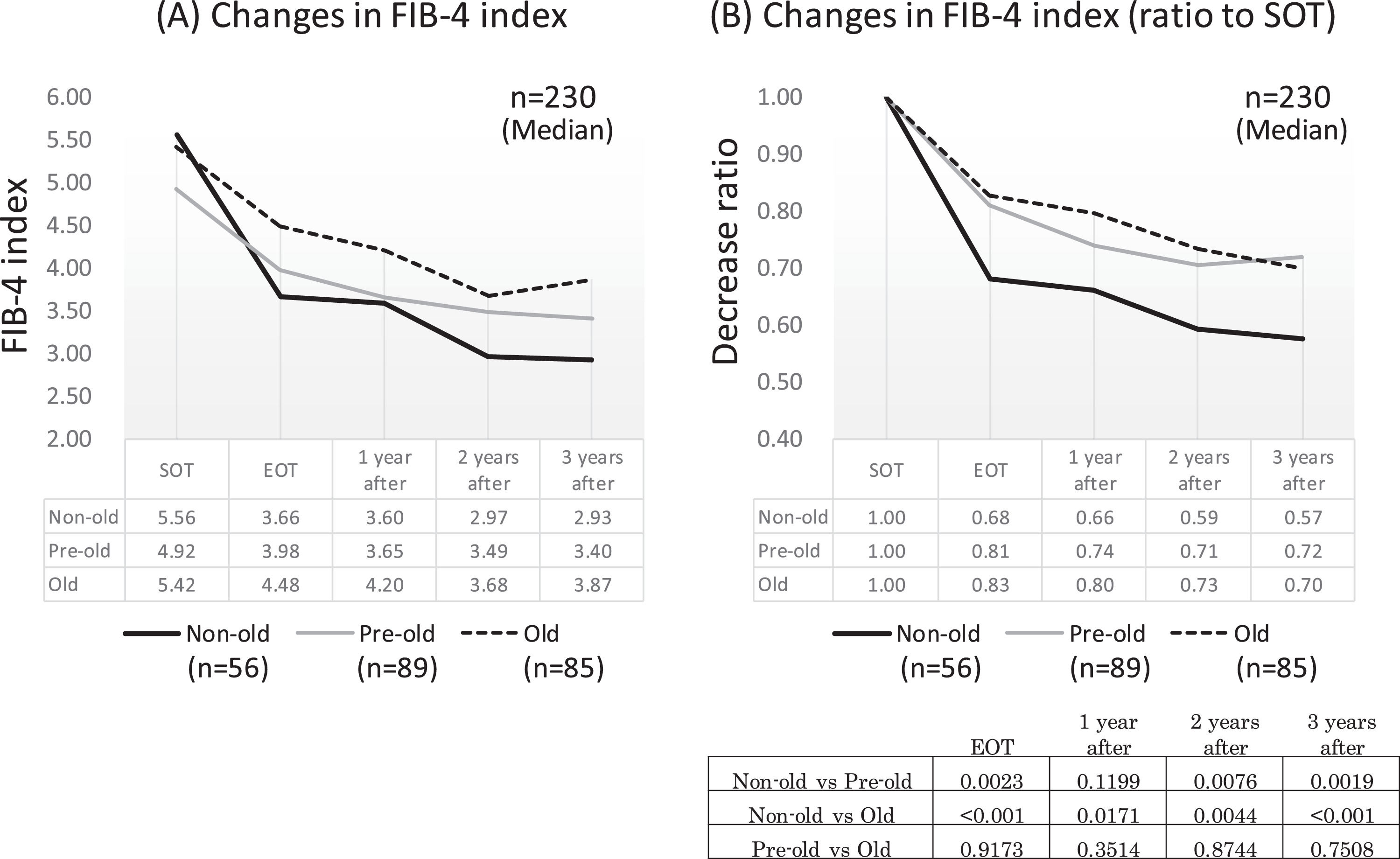
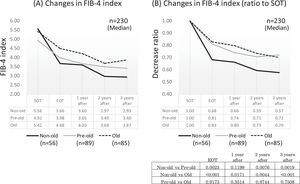
Since age was a significant factor for improving the FIB-4 index, we compared the time-dependent decline in fibrosis by age in the advanced group. Of the 230 patients in the advanced group (excluding six outlier cases), 56 were non-old (<65 years old), 89 were pre-old (65–74 years old), and 85 were old (≥75 years old). In each group, the FIB-4 index showed a significant decrease in EOT, followed by a decrease over time. A comparison of the decay over time between the age groups showed that the non-old group had a significantly stronger attenuation than the pre-old and old groups, except for the point after 1 year (p < 0.01). However, there was no difference in the attenuation between the pre-old and old groups (Figs. 6A and 6 B).
Changes in FIB-4 index around DAA therapy in each age group. All three age groups, non-older (less than 65 years old), pre-older (65-74 years old), and old (more than 75 years old) showed a rapid decrease of FIB-4 index at end of therapy, which then decreased gradually. At end of therapy, and 2 and 3 years after therapy, the non-older group showed a significantly stronger attenuation of the FIB-4 index than pre-older and old groups. No difference in the attenuation of the FIB-4 index after treatment between pre-older and old groups SOT, start o therapy; EOT, end of therapy; 1 year after, 1 year after EOT; 2 years after, 2years after EOT; 3 years after, 3years after EOT.
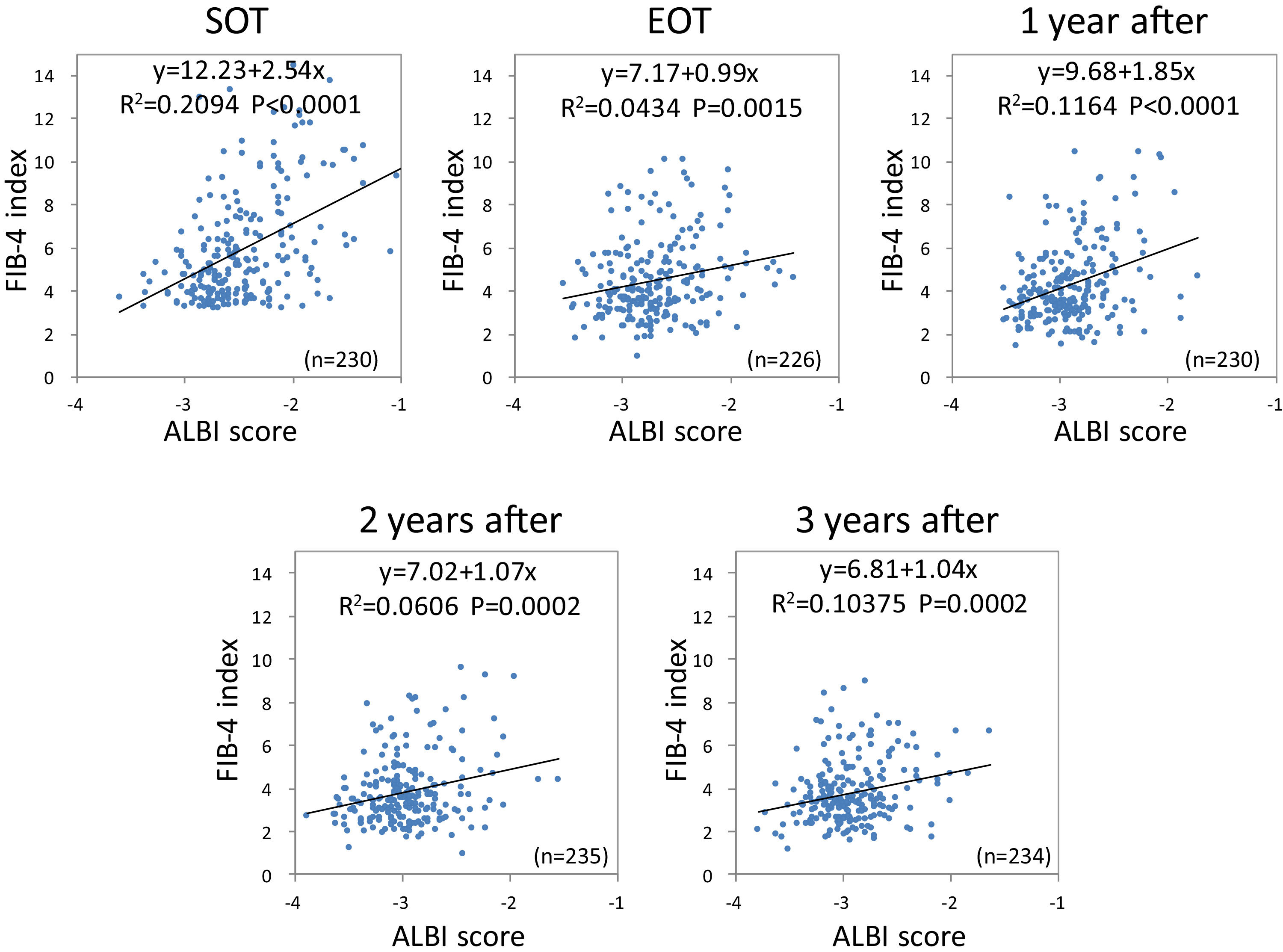
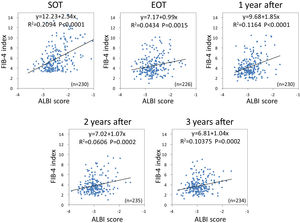
Using the FIB-4 index and ALBI score, we investigated the changes in the relationship between liver fibrosis and hepatic reserve over time after SVR. Outliers at each point were excluded. Based on Pearson's correlation analysis, the FIB-4 index was moderately related to the ALBI score (r = 0.4576, p < 0.0001) at the SOT. After DAA therapy, the correlation between the two variables decreased (r = 0.2083, p = 0.0015 at EOT; r = 0.3412, p < 0.0001 at 1 year after; r = 0.2462, p = 0.002 at 2 years after; and r = 0.3221, p = 0.0002 at 3 years after) (Fig. 7).
Correlation between liver fibrosis (FIB-4 index) and hepatic reserve (ALBI score) for direct-acting antivirals therapy FIB-4 index was moderately related to the ALBI score at the start of therapy. After direct-acting antivirals therapy, the correlation between the two variables decreased. FIB-4, fibrosis-4; ALBI, albumin-bilirubin.
In this study, we focused on the improvement of liver fibrosis in the elderly after SVR, which has not been discussed so far. We determined the factors affecting the improvement of hepatic fibrosis in patients with advanced fibrosis. In Japan, as of 2005, 54.2% of the patients who tested positive for HCV antibody were aged ≥65 years, and this positive rate increased with age [18]. These patients would have aged further by 2014 when DAA therapy was finally approved for health insurance reimbursement in Japan. When IFN therapy was more common, antiviral therapy was not aggressively prescribed to elderly patients owing to the low tolerability and low efficacy of the therapy [19,20]. However, with the advent of DAA therapy, antiviral therapy is more commonly prescribed to elderly patients, resulting in a high SVR rate [10,11,12,13]. After SVR, improvement in hepatic reserve and liver fibrosis was observed in most of the patients, with varying degrees. In our previous study, the factors involved in the improvement of the hepatic reserve (ALBI grade) after SVR were the presence or absence of liver carcinogenesis after SVR, sex, the presence or absence of portosystemic shunt, Hb level, and ALT value at the start of DAA [8]. Currently, there are no drugs that directly improve liver fibrosis, and by eliminating HCV, hepatitis is suppressed, and fibrosis indirectly improves. Recent studies have reported that a non-invasive measurement method predicts the degree of fibrosis in liver biopsy after achieving SVR, and the improvement of fibrosis tends to be remarkable, especially in cases with advanced fibrosis [21,22]. In our study, the fluctuation of liver fibrosis after SVR was examined using the FIB-4 index, which combines the standard biochemical values (PLT, AST, and ALT) and age as a surrogate marker [14,15].
As reported in other papers [21,22,23,24], the liver fibrosis value measured by transient elastography, and serum fibrosis markers decreased significantly at the end of DAA therapy (EOT). These measurements reflect both liver fibrosis and inflammation, and are thought to mainly reflect fibrosis as HCV is eliminated and inflammation improves at EOT. Moreover, from the results of liver biopsy, Huang et al. [21] reported that most of the cutoff values of noninvasive measurements for advanced fibrosis and cirrhosis decreased significantly after SVR by DAA, maybe because of the inflammation improvement. In other words, after SVR it is considered that the serum fibrosis marker reflects true liver fibrosis excluding liver inflammation.
The degree of liver fibrosis at the SOT was divided into mild (<1.45), moderate (1.45–3.25), and advanced (>3.25) stages based on the FIB-4 index value. The advanced and moderate groups showed a significant decrease; in contrast, the mild group did not show a significant decrease. In the moderate and advanced groups, the FIB-4 index was strongly attenuated between the SOT and EOT. In the time course examination of factors constituting the FIB-4 index, AST and ALT levels decreased rapidly from the SOT to EOT, then plateaued. In contrast, PLT increased gradually throughout the course, and this increase was particularly remarkable in the advanced group. It is speculated that the decrease in the FIB-4 index in the EOT is mainly related to the decrease in AST and ALT levels (improvement of inflammation) [21,23,24]. The PLT count increased moderately after the EOT, resulting an improvement in the FIB-4 index. Subsequent studies were conducted since the attenuation of the FIB-4 index during the period was significantly higher in the advanced group. Although the risk of liver carcinogenesis remains for a long time after SVR [25,26,27], the risk of carcinogenesis decreases by approximately 50% in cases where the FIB-4 index has decreased to ≤3.25 after SVR [9]. Therefore, we investigated the factors involved in the decrease of FIB-4 index to ≤3.25, 3 years after the EOT in the advanced group.
Through binomial logistic regression analysis, five significant factors contributing to the improvement of FIB-4 index to ≤3.25 were identified, including age, LC, T.Bil level, ALT level, and PLT count for the SOT. Three significant factors for EOT included age, PLT count, and Hb level. Of these factors, age, ALT level, and PLT count were included in the FIB-4 index formula. Regarding ALT, the lower its level before treatment, the worse the improvement in liver fibrosis. Singh et al. reported a greater magnitude of decline in liver stiffness measured by transient elastography after achieving SVR in patients with higher baseline ALT levels. They explained that this may be a reflection of the higher hepatic inflammatory burden, which responds rapidly to effective antiviral therapy, causing a larger magnitude of change in stiffness [28]. Regarding PLT, portal hypertension or the presence of splenomegaly is likely associated with improved fibrosis. Regarding T.Bil, is a factor included in both the Child Pugh score [29] and the ALBI score [17], and is strongly involved in hepatic reserve. Age is involved in improving the FIB-4 index in both the SOT and EOT analyses. Furthermore, it is a factor in many fibrosis indexes [30,31,32], and according to this formula, fibrosis progresses with aging. In this study, the improvement in liver fibrosis after SVR was impaired with aging. However, even in the elderly, liver fibrosis improved over time, albeit moderately, and the risk of liver carcinogenesis seemed to gradually decrease in the long term. Liver cirrhosis, diagnosed by biopsy or diagnostic imaging, inhibited the improvement of fibrosis. More intense fibrosis, which is a morphologically nodular liver, may be present in the liver. In addition to age and platelets, low Hb levels also impaired the improvement of liver fibrosis in the EOT. Hb is also associated with portal hypertension or splenomegaly.
In our report aging impaired the improvement of fibrosis-4 index after achieving SVR. However, the contradictory result was that the advanced group, which showed the strongest attenuation of FIB-4 index, was significantly older. Liver tissue fibrosis is thought to be based on a balance between fiber synthesis and degradation. In the advanced group, we assumed that both the signal that promotes fibrosis and the signal that eliminates fibrosis are strongly expressed simultaneously. It is thought that the activity of hepatic stellate cells and Kupffer cells is weakened by the rapid improvement of liver inflammation by DAA therapy, while the attenuation of FIB-4 index is increased because the action of the factor that improves fibrosis continues. And it is possible that the factors for improving fibrosis are not strongly expressed in the elderly and cases with mild fibrosis.
In our previous study, aging was not a factor that impaired the improvement of hepatic reserve [8], but it was a negative factor in the improvement of fibrosis. Therefore, we investigated the correlation between liver fibrosis and hepatic reserve before and after SVR using the FIB-4 index and ALBI score in advanced cases. In the SOT, both demonstrated a moderate correlation with r = 0.4576. After achieving SVR, the correlation was milder (r = 0.2084 to r = 0.3412). During the period of active hepatitis, both the hepatic reserve and liver fibrosis gradually worsened; however, the degree of improvement after SVR may differ between the two indexes. After achieving SVR, it was found that aging was not involved in the improvement of hepatic reserve, but it was an inhibitory factor for improving liver fibrosis. This may be one of the reasons why the correlation is impaired.
There are some cases in which fibrosis is exacerbated despite the achievement of SVR. Seko et al. reported that the presence of varices is a factor involved in the exacerbation of the FIB-4 index >1.0 [33]. In our cohort, only one patient showed an increase in FIB-4 index of >1.0 out of the 42 patients who underwent upper gastrointestinal endoscopy before and after achieving SVR. There was no difference in the improvement of the FIB-4 index between the exacerbated varices cases (14 cases) and the unchanged or improved varices cases (28 cases). In 10 out of 14 cases, exacerbation of varices was observed, despite a decrease of >1.0 in FIB-4. It is possible that the varicose veins worsened because the portal vein blood continued to flow in the direction of the shunt with low resistance despite the improvement of liver fibrosis. The relationship between the presence of varices and improvement of liver fibrosis may need to be examined in a larger number of cases.
A limitation of our study is that it did not include patients with decompensated LC, because DAA therapy was not approved for decompensated LC in Japan until sofosbuvir 400 mg/velpatasvir 100 mg tablets were marketed in January 2019 [34]. Further studies investigating long-term changes in liver fibrosis and therapeutic benefits in patients with decompensated LC are therefore crucial. In cases of decompensated LC, several outcomes such as improvement of the hepatic reserve, liver transplantation, and death from liver failure even after achieving SVR could be observed [35–37]. Treatment benefits need to be considered, including changes in the long-term hepatic reserve and liver fibrosis. In addition, this study did not include information on lifestyle habits before and after SVR, such as drinking and eating habits, and the relationship between the changes in the hepatic reserve, liver fibrosis, and lifestyle habits is unclear. These issues require further investigation in future studies.
5ConclusionsThe factors involved in the improvement of liver fibrosis after achieving SVR were LC, T.Bil level, PLT level, Hb level, ALT level, and age. The levels of T.Bil, PLT, and Hb were considered to be related to portal hypertension. Aging strongly impaired the improvement in liver fibrosis after achieving SVR.
Availability of data and materialThe data that support the findings of this study are available from the corresponding author, upon reasonable request.