Hepatitis C virus (HCV) infections in patients with hemophilia lead to the development of hepatocellular carcinoma (HCC) at a relatively younger age than that in patients without hemophilia. Although recent progress in direct-acting-antivirals has facilitated a high rate of sustained virological response (SVR), the clinical influence of HCV eradication in hemophilia patients remains unclear. This study aimed to compare the clinical outcomes of SVR against HCV in patients with and without hemophilia.
Patients and methodsThe study enrolled 699 patients who achieved SVR after HCV antiviral treatment. Patients were divided into two groups: 78 patients with hemophilia (H group) and 621 patients without hemophilia (NH group). We evaluated patient characteristics, clinical outcomes, and the cumulative incidence of HCC after SVR.
ResultsCompared with the NH group, patients in the H-group were significantly younger and had a lower hepatic fibrosis score. No difference was found in the incidence of liver-related disease or overall death between the two groups over a mean follow-up period of 7 years.
Four patients in the H group and 36 patients in the NH group were diagnosed with HCC after SVR. Multivariate analysis showed that male sex, age, and cirrhosis were significant risk factors for HCC incidence. There was no significant difference in the cumulative incidence of HCC after propensity-score matching adjusting for the risk factors of HCC between the two groups.
ConclusionHemophilia is not a significant risk factor for hepatocarcinogenesis after SVR against HCV.
Hemophilia is a rare congenital bleeding disorder associated with abnormalities in blood coagulation in approximately 1 in 10,000 individuals [1]. Clotting factor replacement is essential to treat patients with hemophilia. Before introducing testing for anti-HCV antibodies and heat inactivation, most patients with hemophilia were infected with hepatitis C virus (HCV) because of the use of clotting factor concentrates contaminated with HCV[ 2, 3]. In comparison with patients with chronic HCV infection without hemophilia, those with chronic HCV infection and hemophilia are known to show the following clinical differences: (1) the duration of infection can be estimated from blood transfusion records; (2) a greater proportion of cases show co-infection of hepatitis B virus (HBV) or human immunodeficiency virus (HIV); (3) a greater proportion of cases show mixed HCV genotype infection and (4) the patients may show a higher HCV viral load[ 4,5]. Elisabeth et al. reported that patients with hemophilia developed cirrhosis at an average age of 49 years and hepatocellular carcinoma (HCC) at an average age of 57 years [6], while patients who showed HCV infection without hemophilia are known to develop cirrhosis or HCC at a relatively older age [7]. Although recent advances in HCV treatment with the introduction of direct-acting antivirals (DAAs) have enabled a high rate of sustained virological response (SVR) [8–12], the clinical outcomes after SVR in patients with hemophilia remain unclear. The aim of this study was to clarify liver carcinogenesis and clinical outcomes after SVR in patients with hemophilia.
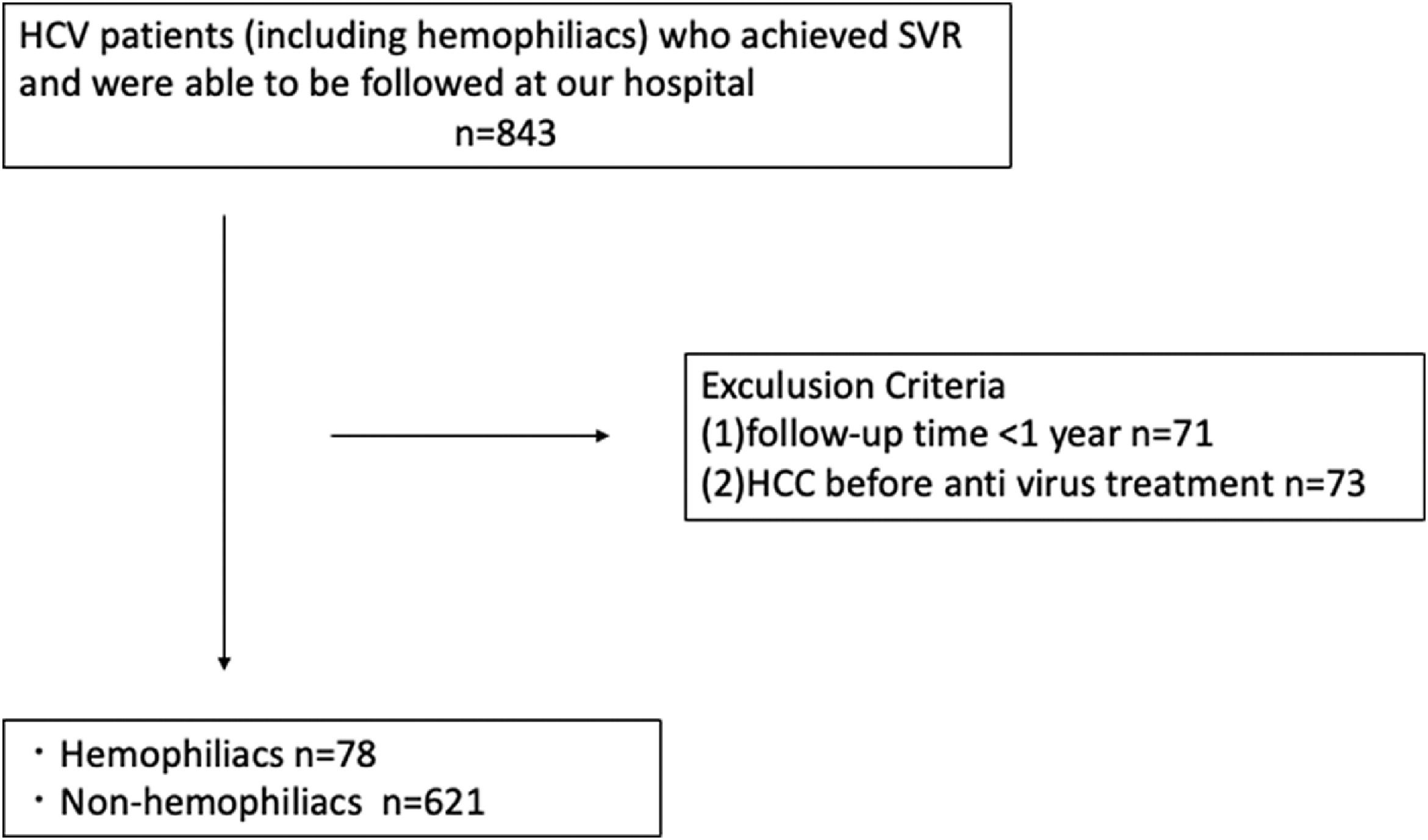
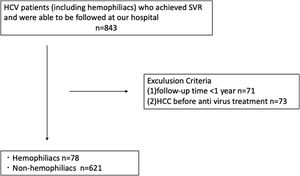
2Patients and methods2.1Study populationFrom January 2005 to December 2020, 850 patients with HCV who achieved SVR by antiviral therapy with interferon (IFN) or DAA were enrolled in this study. Patients were divided into two groups: those with hemophilia (H group) and those without hemophilia (NH group). Patients who met any of the following criteria were excluded from the study: (1) HCC before antiviral treatment and (2) less than one year of follow-up. Based on these criteria, 699 patients (78 hemophilia patients, including 59 hemophilia type A and 19 hemophilia type B patients, and 621 non-hemophilia patients) were analyzed retrospectively. The follow-up period was defined as the duration from the confirmation of SVR to HCC development or the last visit for HCC surveillance. SVR was defined as persistently negative results for serum HCV RNA at 6 months after treatment. This study was approved by the ethics committee of Nagoya University Hospital (2020-0479).
2.2Clinical and laboratory dataAge, sex, body mass index (BMI), hemophilia status (hemophilia A or B and severity: mild, moderate, or severe), alcohol consumption, smoking history, and blood data of eligible patients at the time of starting antiviral treatment were collected from the electronic medical record system. Liver fibrosis was assessed using the fibrosis index based on four factors (FIB-4 score): age (years) × aspartate aminotransferase (AST) level (IU/L)/platelet count (× 109/L) × (alanine aminotransferase (ALT) level (IU/L)1/2) [13]. In addition, the aspartate aminotransferase to platelet ratio index (APRI) was calculated as follows: 100 × (AST level/upper limit of normal)/platelet count (× 109 /L) [14]. Diabetes mellitus was diagnosed according to the American Diabetes Association criteria [15]: (1) blood glucose ≥ 200 mg/dL at any time, (2) fasting blood glucose ≥ 126 mg/dL, or (3) anti-diabetes medication. Dyslipidemia was diagnosed as triglyceride ≥ 150 mg/dL, high-density lipoprotein cholesterol < 40 mg/dL, low-density lipoprotein cholesterol ≥ 140 mg/dL, or antilipidemic medication. Alcohol consumption of more than 80 g/day was considered heavy drinking [16]. The diagnosis of cirrhosis was determined by liver biopsy, imaging, presence of varices, or fluid overload such as presence of edema or ascites.
2.3TreatmentA total of 327 patients treated before 2014 were mainly administered a combination therapy of peg-interferon and ribavirin-based regimen (peg-interferon and ribavirin: 271 patients; peg-interferon and ribavirin with protease inhibitor, 56 patients). The treatment regimen was decided according to the genotype of the virus and the viral load. After DAAs became available in 2014, 372 patients were treated with oral interferon-free DAA administration.
2.4Hepatocellular carcinoma surveillance after sustained virological responseAfter confirming SVR in the first 6 months, patients were followed up periodically at an interval of at least 6 months. The levels of tumor markers, α-fetoprotein (AFP), des-gamma-carboxyprothrombin (DCP), and liver enzymes were evaluated at every visit. Imaging examinations, such as abdominal ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) was performed every 6 months for HCC surveillance. When HCC was suspected, dynamic contrast-enhanced CT, dynamic contrast-enhanced MRI, and contrast-enhanced ultrasonography were performed to confirm the diagnosis. The diagnosis of HCC was mainly based on typical imaging features (presence of arterial enhancement and washout on portal phase).
2.5Statistical analysisContinuous variables were expressed as medians (interquartile range) and analyzed using the Mann–Whitney U-test. Categorical variables were analyzed using the chi-squared test or Fisher's exact test. The cumulative incidence of HCC was determined by the Kaplan–Meier method, and differences between the H and NH groups were assessed by the log-rank test. Cox proportional hazard models were used to determine the risk factors for HCC after SVR. First, we performed univariate analysis, followed by multivariate analysis including hemophilia and the following variables, which had P values less than 0.1 in the univariate analysis and were associated with HCC: age, male sex, liver cirrhosis, and use of DAAs. Propensity-score matching was performed to minimize the differences in baseline characteristics between the H and NH groups. The following variables were included in a multiple logistic regression to derive propensity scores: age, sex, alcohol consumption, HBV infection, use of DAAs, liver cirrhosis, APRI, and HCV genotype 1b. One-to-one propensity-score matching was performed by nearest-neighbor matching with a caliper width of 0.2, multiplied by the SD of the linearly transformed propensity scores. Statistical significance was set at P < 0.05.
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
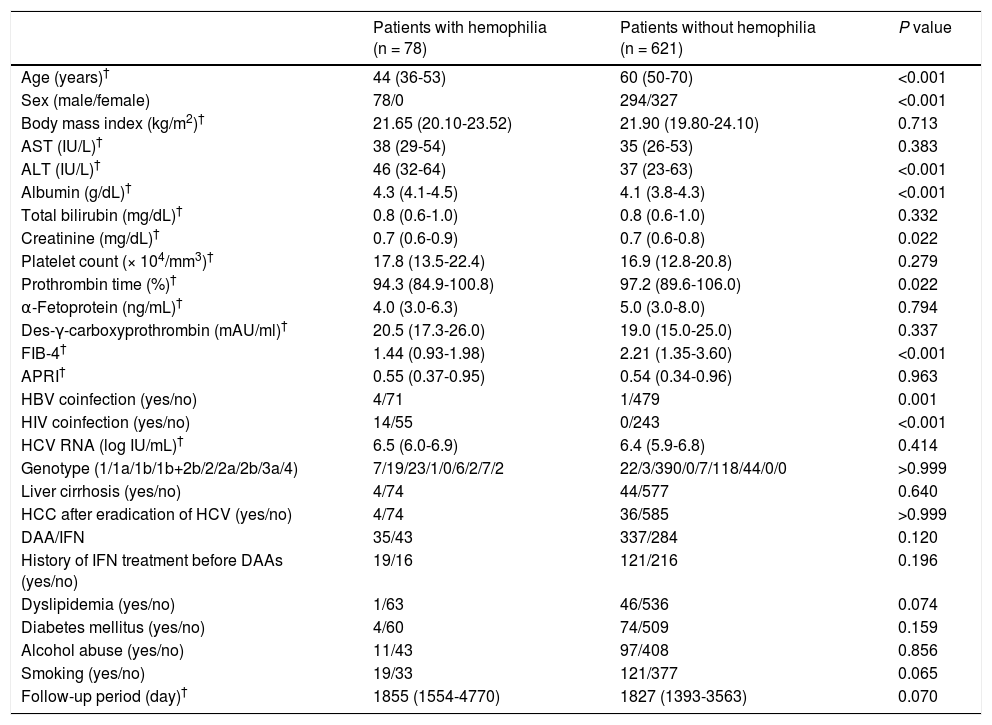
3ResultsThe study design is illustrated in Fig. 1. Analyses were performed on 78 patients in the H group and 621 patients in the NH group. A comparison of patient characteristics is shown in Table 1. All patients in the H group were men. The patients in the H group were significantly younger at the start of antiviral treatment, showed more co-infection with HIV and HBV, and had a lower FIB-4 score than those in the NH group; however, there was no intergroup difference in the rate of APRI or cirrhosis, suggesting that younger age determined lower FIB-4 score. IFN-based therapy was administered more frequently in the H group. At the start of antiviral treatment, the H group showed higher ALT levels, creatinine, albumin, and lower prothrombin time.
Patient characteristics
| Patients with hemophilia (n = 78) | Patients without hemophilia (n = 621) | P value | |
|---|---|---|---|
| Age (years)† | 44 (36-53) | 60 (50-70) | <0.001 |
| Sex (male/female) | 78/0 | 294/327 | <0.001 |
| Body mass index (kg/m2)† | 21.65 (20.10-23.52) | 21.90 (19.80-24.10) | 0.713 |
| AST (IU/L)† | 38 (29-54) | 35 (26-53) | 0.383 |
| ALT (IU/L)† | 46 (32-64) | 37 (23-63) | <0.001 |
| Albumin (g/dL)† | 4.3 (4.1-4.5) | 4.1 (3.8-4.3) | <0.001 |
| Total bilirubin (mg/dL)† | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) | 0.332 |
| Creatinine (mg/dL)† | 0.7 (0.6-0.9) | 0.7 (0.6-0.8) | 0.022 |
| Platelet count (× 104/mm3)† | 17.8 (13.5-22.4) | 16.9 (12.8-20.8) | 0.279 |
| Prothrombin time (%)† | 94.3 (84.9-100.8) | 97.2 (89.6-106.0) | 0.022 |
| α-Fetoprotein (ng/mL)† | 4.0 (3.0-6.3) | 5.0 (3.0-8.0) | 0.794 |
| Des-γ-carboxyprothrombin (mAU/ml)† | 20.5 (17.3-26.0) | 19.0 (15.0-25.0) | 0.337 |
| FIB-4† | 1.44 (0.93-1.98) | 2.21 (1.35-3.60) | <0.001 |
| APRI† | 0.55 (0.37-0.95) | 0.54 (0.34-0.96) | 0.963 |
| HBV coinfection (yes/no) | 4/71 | 1/479 | 0.001 |
| HIV coinfection (yes/no) | 14/55 | 0/243 | <0.001 |
| HCV RNA (log IU/mL)† | 6.5 (6.0-6.9) | 6.4 (5.9-6.8) | 0.414 |
| Genotype (1/1a/1b/1b+2b/2/2a/2b/3a/4) | 7/19/23/1/0/6/2/7/2 | 22/3/390/0/7/118/44/0/0 | >0.999 |
| Liver cirrhosis (yes/no) | 4/74 | 44/577 | 0.640 |
| HCC after eradication of HCV (yes/no) | 4/74 | 36/585 | >0.999 |
| DAA/IFN | 35/43 | 337/284 | 0.120 |
| History of IFN treatment before DAAs (yes/no) | 19/16 | 121/216 | 0.196 |
| Dyslipidemia (yes/no) | 1/63 | 46/536 | 0.074 |
| Diabetes mellitus (yes/no) | 4/60 | 74/509 | 0.159 |
| Alcohol abuse (yes/no) | 11/43 | 97/408 | 0.856 |
| Smoking (yes/no) | 19/33 | 121/377 | 0.065 |
| Follow-up period (day)† | 1855 (1554-4770) | 1827 (1393-3563) | 0.070 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; FIB-4, fibrosis-4 score; APRI, aspartate aminotransferase-to-platelet ratio index; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; DAA, direct-acting antiviral; IFN, interferon
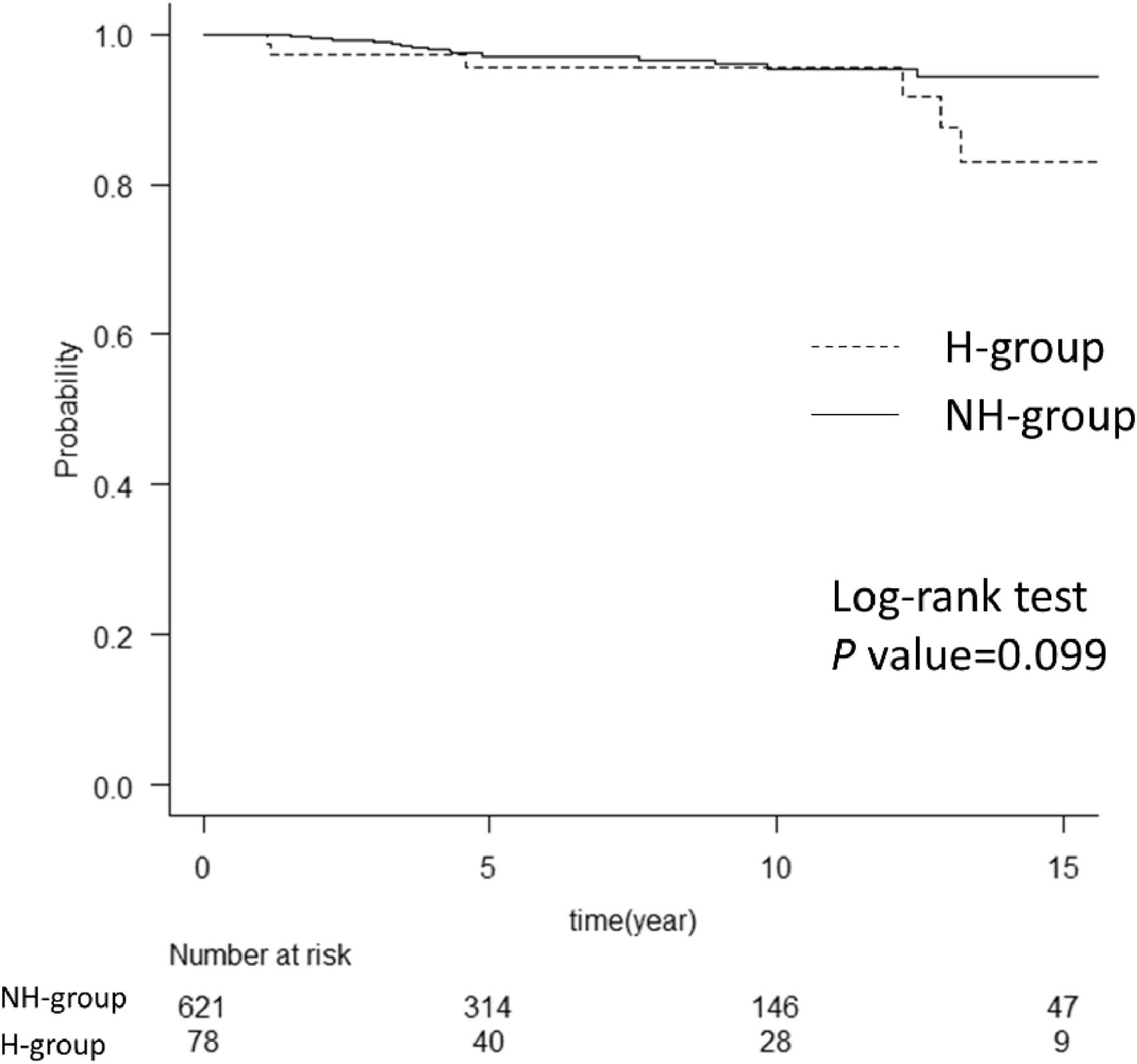
Although patients in the H group were 16 years younger than those in the NH group, there was no intergroup difference in overall survival after achieving SVR (Fig. 2). In the H group, the cause of death was liver failure in one case, cancer of other organs in two cases (one had colon cancer, the other had lung cancer), and bleeding events in three cases (one had cerebral hemorrhage and two had hemothorax). In the NH group, four patients died of HCC, five of cancer of other organs (two had intrahepatic cholangiocarcinoma, one had colon cancer, one had pancreatic cancer, and one had lung cancer), three had pneumonia, three of vascular events, and three of unknown causes.
Comparison of overall survival between the two groups. The graph shows a comparison of overall survival in hemophilia and non-hemophilia groups after achieving SVR. There was no difference between the two groups with the log-rank test. The numbers below show the number of patients at risk in each group. H group, hemophilia group; NH group, non-hemophilia group
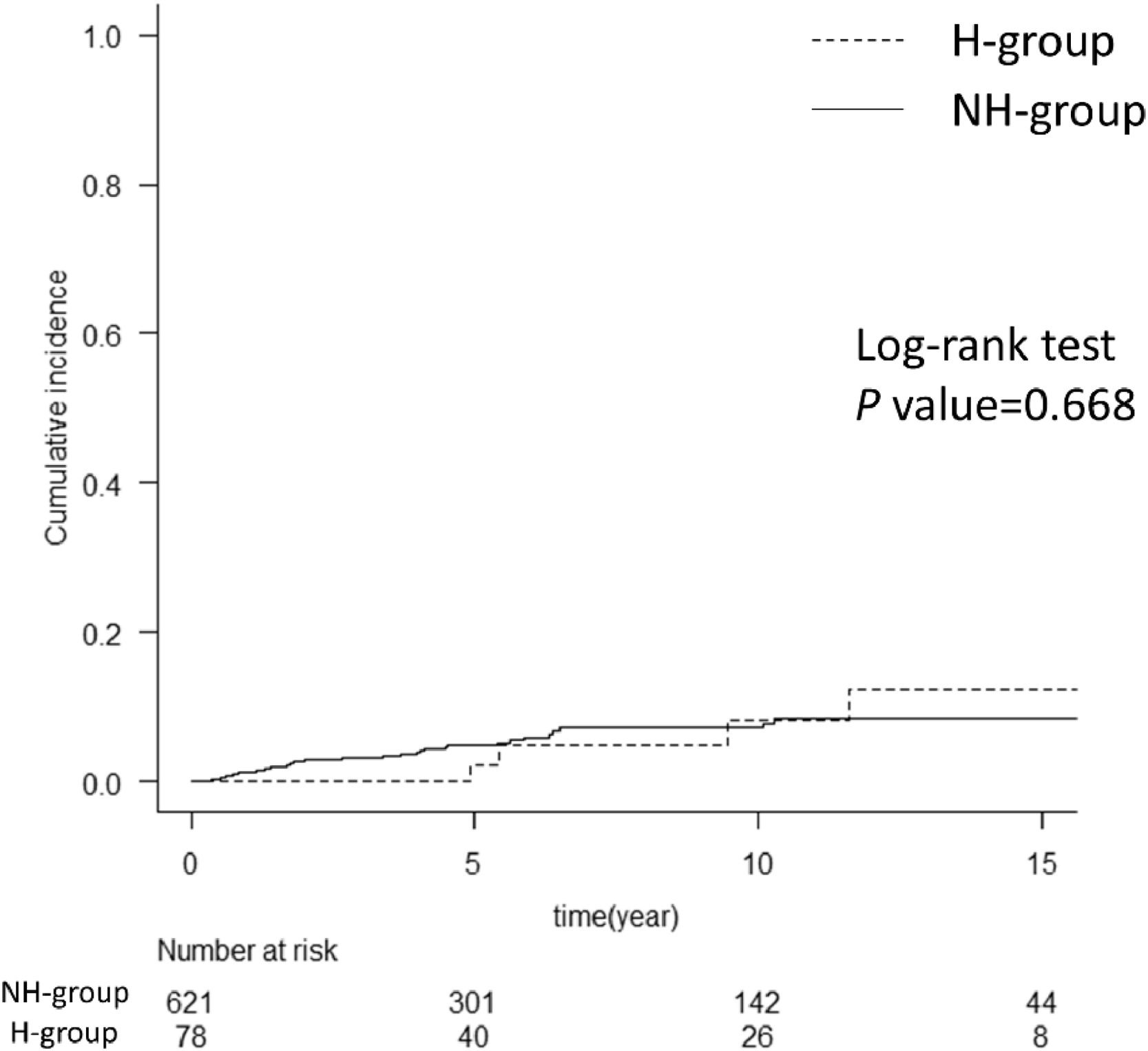
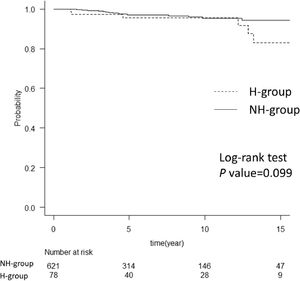
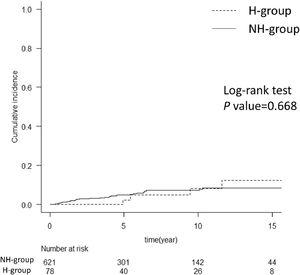
The cumulative incidence of HCC after SVR in the two groups is shown in Fig. 3. HCC developed within 10 years after SVR in 8% of patients in the H group and 6% in the NH group. Comparing the patient backgrounds at the time of starting antiviral treatment showed that the H group had a relatively younger age at the time of antiviral treatment, a lower FIB-4 score, and a significantly longer time to liver carcinogenesis than the NH group (Supplementary Table 1).
Comparison of cumulative incidence of HCC after achieving SVR between the two groups. The graph compares the cumulative incidence of HCC after achieving SVR in hemophilia and non-hemophilia groups. The two groups showed no difference with the log-rank test. The numbers below show the number of patients at risk in each group. HCC, hepatocellular carcinoma; SVR, sustained virological response; H group, hemophilia group; NH group, non-hemophilia group
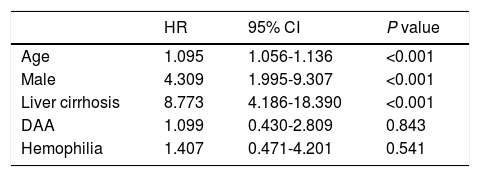
The Cox proportional hazard model was employed, adding variables with P values < 0.1 in univariate analysis and were associated with HCC (age, male sex, liver cirrhosis, and DAA) (Supplementary Table 2). Age, male sex, and liver cirrhosis were selected as risk factors (P < 0.05); however, the presence of hemophilia was not identified as a significant risk factor for HCC after SVR (Table 2). Regarding the genetic factors relating to HCC occurrence [17,18,19], host genetic variants in IL28B, TLL1, and PNPLA3 were measured in 226, 223, and 420 patients, respectively. However, these variants were not selected as independent risk factors for HCC development after SVR in our cohort (data not shown).
Multivariate analysis of risk factors for HCC after SVR in the Cox proportional hazard model
| HR | 95% CI | P value | |
|---|---|---|---|
| Age | 1.095 | 1.056-1.136 | <0.001 |
| Male | 4.309 | 1.995-9.307 | <0.001 |
| Liver cirrhosis | 8.773 | 4.186-18.390 | <0.001 |
| DAA | 1.099 | 0.430-2.809 | 0.843 |
| Hemophilia | 1.407 | 0.471-4.201 | 0.541 |
HCC, hepatocellular carcinoma; SVR, sustained virological response; HR, hazard ratio; CI, confidence interval; DAA, direct-acting antiviral
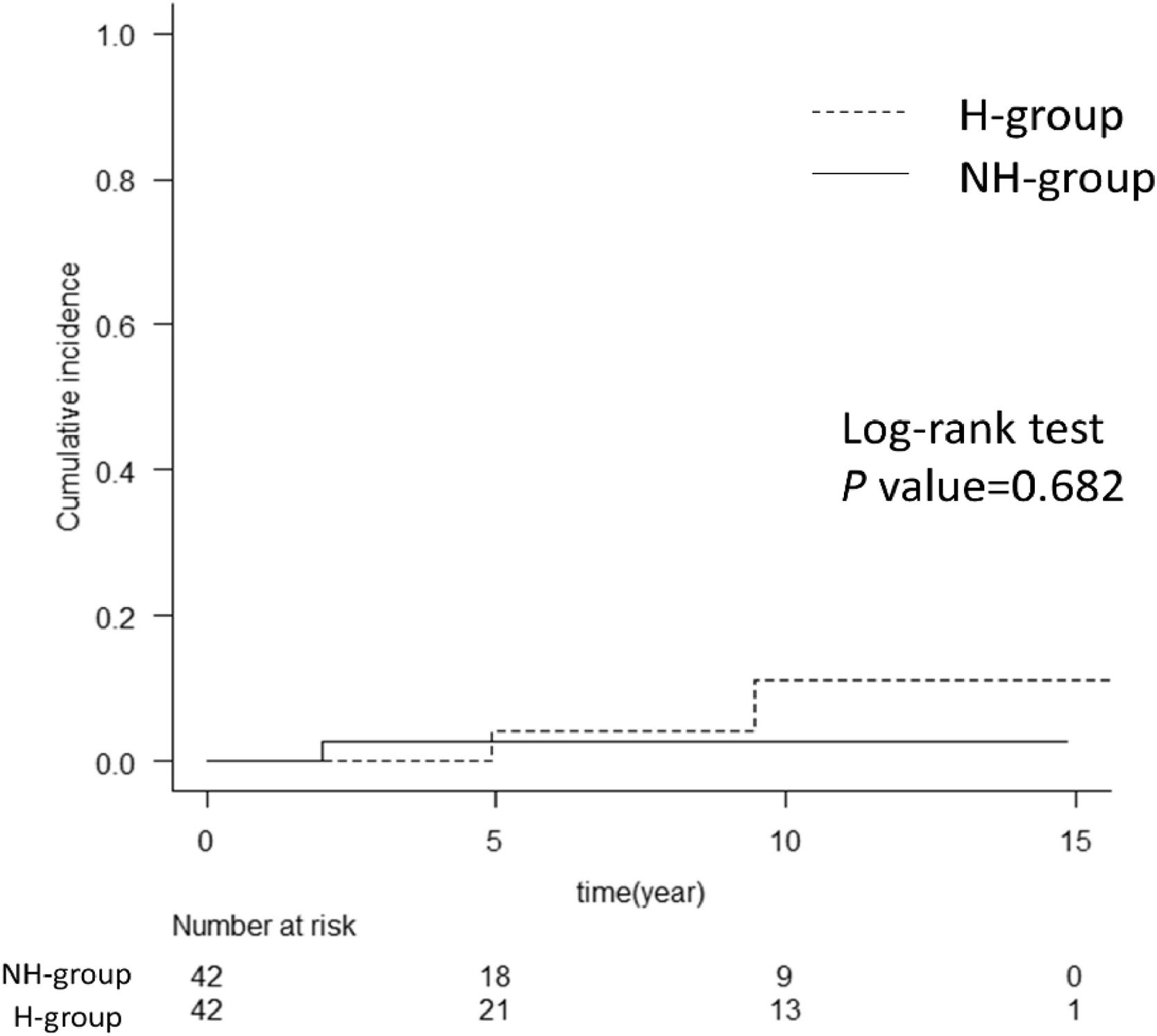
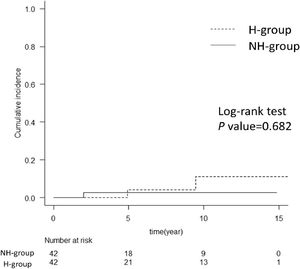
Since the H group in this study was significantly younger and all patients were male (Table 1), there are some possible biases in the two factors contributing to liver carcinogenesis. For a more detailed analysis of the risk of HCC after SVR in patients with hemophilia, we conducted propensity-score matching for the factors involved in liver carcinogenesis. We thereafter compared the cumulative incidence of HCC after SVR between the H and NH groups (Supplementary Table 3). Notably, there was no significant difference in the cumulative incidence of HCC after SVR between the two groups (Fig. 4).
Cumulative incidence of HCC after propensity-score matching. The graph shows a comparison of hemophilia and non-hemophilia groups for the cumulative incidence of HCC after propensity-score matching. There was no difference between the H and NH groups with the log-rank test. The numbers below show the number of patients at risk in each group. H-group, hemophilia group; NH group, non-hemophilia group; HCC, hepatocellular carcinoma.
This study investigated liver carcinogenesis and the long-term patients’ prognosis with and without hemophilia after HCV eradication. There was no difference in the overall survival after SVR or in the cumulative incidence of HCC in the H and NH groups. Thus, hemophilia was not an independent risk factor for HCC development after SVR.
HCC development in patients with HCV is strongly associated with an increased risk of advanced fibrosis and cirrhosis, with HCC occurring in 1%-2% of patients with mild fibrosis (F2), 5% of those with F3 fibrosis, and 7%-8% of those with cirrhosis (F4) per year [20].
Although lower hepatocarcinogenesis rates after HCV eradication have been widely reported, [21,22] liver carcinogenesis does occur after HCV eradication, albeit in a low proportion. Male sex, older age, alcohol abuse, HBV co-infection, cirrhosis, and HCV genotypes are known HCC occurrence risk factors [23,24,25]. Especially after HCV eradication, the risk factors for HCC development were reported as cirrhosis, alcohol use, older age, and HCV genotypes 3 [26]. In this study, the risk factors for HCC were male sex, older age, HBV infection, and cirrhosis.
Patients with hemophilia have been shown to develop cirrhosis and HCC at a relatively younger age than those without hemophilia [6,7,27]. A cohort study of 4,865 patients with hemophilia in the UK showed a 5.6-fold higher risk of HCC-related death and 16.7-fold higher risk of death due to liver disease than the general population [28]. Dirk et al. reported that HIV co-infection, older age at HCV infection, alcohol abuse, and the presence of genotype 1 were independent risk factors for progression to end-stage liver disease in patients with hemophilia [29]. These facts suggest that hemophilia may be a risk factor for liver carcinogenesis; however, the present results showed that hemophilia is not a risk factor for HCC carcinogenesis, at least after SVR.
In recent years, the use of DAAs has allowed the eradication of HCV with a high probability without causing severe adverse events [8–12]. Therefore, the management of cirrhosis and surveillance of HCC after SVR has become more important. Achievement of SVR has been widely recognized to improve the prognosis of non-hemophilic patients with HCV [30,31]. Among patients with hemophilia, the safety and efficacy of antiviral treatment for HCV by IFN or DAAs have also been reported [32]. However, Maor et al. reported that achievement of SVR with IFN therapy did not affect overall survival in patients with hemophilia [33]. For these reasons, the treatment course and clinical outcomes after SVR in hemophilia patients with HCV remain controversial.
In this study, we found no significant difference in the cumulative incidence of HCC after SVR or the long-term prognosis in patients with and without hemophilia, suggesting that SVR could reduce the rates of liver carcinogenesis and liver disease-related mortality in both groups of patients.
HCV–HIV co-infection is associated with increased rates of fibrosis and cirrhosis, [34–36] and this co-infection has a major adverse effect on the long-term prognosis of patients with hemophilia [37,38]. However, in our cohort, we found no incidence of HCC in patients with HCV-HIV co-infection. This might be because all patients with HIV co-infection were adequately treated for HIV and had undetectable levels of HIV.
In this study, the cumulative incidence of HCC after SVR with DAA treatment was significantly higher than that after IFN treatment (Supplementary Figure 1). This may have been due to the higher proportion of older patients and patients with liver cirrhosis in the DAA treatment group (Supplementary Table 4). Of the 372 patients treated with DAA, 140 had previously received IFN. HCC was observed in six patients treated with IFN and 15 patients without IFN treatment. There was no relationship between previous IFN treatment and HCC development in our DAA cohort. A greater proportion of patients with hemophilia received IFN, and the treatment course might have influenced a lower cumulative incidence of HCC in these patients; however, multivariate analysis in the Cox proportional hazard model showed that DAA treatment was not a significant risk factor for HCC (Table 2). Moreover, propensity-score matching showed no difference in the cumulative incidence of HCC in the H group (Fig. 4), indicating that hemophilia was not a risk factor for hepatocarcinogenesis. This may be partly due to the small number of cases in the H group and the small number of HCCs. Of note, four patients with HCC in the hemophilia group had no liver cirrhosis, which might suggest HCC development risk factors in hemophilia irrespective of liver cirrhosis. Due to the limited number of HCC patients in the hemophilia group, further study is needed to clarify this concern.
Although there were no differences in overall survival, deaths from liver failure (n = 1) and bleeding events (n = 3) were observed in patients with hemophilia during the study period. Since the age in the H group at the time of starting antiviral treatment was 16 years younger than that in the NH group, careful observation is needed in patients with hemophilia even after eradication of HCV.
5LimitationsThis study had some limitations. First, this was a single-center retrospective study. Second, only patients who achieved SVR were enrolled in this study, which might have led to a selection bias. Therefore, it remains unclear whether the eradication of HCV directly improves the prognosis of hemophilia patients. Third, viral and host factors were not considered.
6ConclusionHemophilia was not a risk factor for HCC after SVR. The eradication of HCV could decrease liver-related diseases, including HCC, in patients with and without hemophilia.
Author contributionsConceptualization: N.I. Data curation: N.I, Y.I, K.Y, T.I. Formal analysis: N.I, Y.I, Y.I, T.H. Methodology: N.I, S.O, T.K. Project administration: N.S, T.M, M.I, M.F. Visualization: Y.I, N.I. Writing – original draft: Y.I. Writing – review, and editing: N.I, Y.I. Approval of final manuscript: all authors.
The authors thank Dr. Satoshi Yasuda (Ogaki Municipal Hospital, Gifu, Japan) for assistance with data collection.