Background and aim: Outcome of hepatocellular carcinoma (HCC) depends mainly on its early diagnosis. The performance of traditional biomarkers is not satisfactory. Osteopontin (OPN) is of potential importance. This study aim to assess the diagnostic value of plasma OPN compared with alpha-fetoprotein (AFP) for the diagnosis of HCV- related HCC.
Methods: We recruited 113 HCC patients compared with 120 matched cirrhotic patients and 120 Controls. The plasma level of OPN and serum AFP for all participants were assessed.
Results: The median plasma OPN level was significantly higher in the HCC group than in the cirrhotic patient group or in the normal control group (p-value < 0.001), while OPN levels were not differed significantly in correlation with the degree of liver function deterioration in terms of advanced Child-Pugh class (p-value < 0.9). The diagnostic efficacy of OPN were superior to AFP in terms of AUC, sensitivity, specificity, PPV and NPV either in diagnosis of early or late stages of HCC (0.88 vs. 0.56; P = 0.0001, 0.991 vs. 0.899; p = 0.01; respectively).
Conclusion: Plasma OPN level is a potential diagnostic marker for HCC, especially among high-risk group of patients. These values extend beyond the traditional tumor biomarkers as AFP, as it possesses good prognostic value.
Hepatocellular carcinoma (HCC) is the fifth most common tumor and the third most common cause of cancer-related deaths worldwide.1,2HCC incidence has increased sharply over recent decades and this has been partially attributed to chronic HCV infection.3–6In Egypt, the growing incidence of HCC, which is nearly doubled over the last decade7–10is parallel with that Egypt are plugged with highest prevalence of HCV in the world, ranging from 6 to 28%.11-13The prevelance of serological markers of HCV infection in patients with HCC is nearly 80%.14
HCC represents the leading cause of death from all other cancer sites.10
The incidence and mortality rates for HCC are virtually identical, reflecting the overall poor survival of patients with this tumor. Most effective therapies exist for HCC if diagnosed early.15
Currently, α-fetoprotein (AFP) is the most validated serological marker for HCC. Even though AFP’s performance in early stage HCC are deficient,16the sensitivity and specificity of plasma AFP detection (> 20 ng/mL) in screening tests were set at about 50% and 85%, respectively.17According to the new update of the American Association for the Study of Liver Diseases (AASLD) practice guideline for HCC, AFP alone should not be used for HCC screening unless ultrasound is not available.15Recent systematic reviews of the literature show that the quality of evidence supporting the use of AFP as a diagnostic and screening test for HCV-related HCC is limited.18,19In addition; this is traditional biomarkers don’t reflect biological features of the tumor, don’t provide information about HCC behavior and thus don’t allow the physician to predict outcome of HCC patients accurately.20With the emerging of the new era of molecular targeted therapy of HCC, the evaluation of these novel agents will also require novel tools, the well-established concepts in oncology may be no longer valid, which indicate that there is much room for improvement in both the efficacy of the AFP test itself as well as in the development of other serological markers.21
Consequently, a series of additional biomarkers for HCC screening has been suggested. Osteopontin (OPN) is an integrin-binding glycophosphoprotein that is expressed by several cell types especially by transformed malignant epithelial cells and believed to be involved in many physiological cellular functions especially in regulation of migration, invasion and thus metastasis as well as survival of tumor cells.22Although the elevated expression of OPN at mRNA levels and its association with prognosis of the patients with HCC has been reported,23,24the plasma level of OPN in HCV-related HCC, and its diagnostic significance of the patients were not fully elucidated. The potential value of plasma OPN has been recently suggested in the surveillance of Egyptian patients with HCV infection.25However, this study was small compared to our current study.
The aim of this study was to determine:
- •
Plasma OPN levels in the patients with HCV-related HCC in comparison with the levels in the patients with HCV-related chronic liver diseases or healthy control people.
- •
To evaluate the clinical utility of OPN as HCC plasma biomarker.
This prospective case controlled hospital based study recruiting three groups of individuals attending the Internal Medicine Department of Minia University Hospital between February 2009 and January 2010. A series of patients with HCC with chronic hepatitis C (CHC) was compared with two different groups: one consisted of patients with liver cirrhosis (LC) and the other one, the controls, included individuals who were treated in our hospital for a wide spectrum of acute conditions, other than liver diseases as the primary diagnosis of hospital admission.
Hepatocellular carcinoma group (group 1)This group consisted of 113 (97 [85.8%] patients were males and 16 [14.1%] patients were females) consecutive patients with HCC, 98 patient (86.7%) diagnosed by means of cytological or histological examination of hepatic focal lesions, while the remaining 15 patients (13.2%) were diagnosed by the appropriate imaging characteristics as defined by accepted guidelines.25CHC diagnosis was based on anti-HCV positive by ELISA and by detectable viraemia by PCR.
Liver cirrhosis (LC) group (group 2)This group comprised 120 patients (84 (70%) patients were males, 36 (30%) patients were females) with HCV related LC, by selecting from 250 cirrhotic patients of our department, matched according to age (±5 years), gender, Patients with LC were admitted to our hospital for diagnosis, staging or therapy of LC. The presence of cirrhosis was defined by histology or non-histologically by evidence of portal hypertension in the presence of chronic liver disease (CLD). Evidence of portal hypertension included:
- •
A cirrhotic-appearing liver on ultrasound, CT or MRI examinations with splenomegaly and no vascular thrombosis.
- •
Thrombocytopenia with a platelet count < 120 mm-3, and/or
- •
Presence of esophagogastric varices on endoscopic examination.
Controls must have an ultrasound, CT or MRI showing no evidence of hepatic mass within 6 months prior to enrollment. Patients with an elevated AFP (> 20 ng/mL) at enrollment were excluded.
Control group (group 3)The control group comprised 120 control subjects < were females). To evaluate and compare the data of patients who already had the HCC (HCC group) and of cases with LC (LC group), who were health-check examinees of the same hospital and who showed no abnormality in laboratory examinations were included. Those who were younger than 18 years of age, admitted for malignancies, prior cancer therapy, history of previous resection of HCC alcohol-related disorders, hepatic viral infection rather than HCV, patients with previous history of interferon based therapy for HCV were excluded from our study.
Baseline dataDemographic and clinical data on the etiology of CLD or HCC, the presence of LC, status of liver function in terms of Child-Pugh class, tumor-nodes-metastases (TNM) stage of HCC determined by The American Joint Committee on Cancer/United International Consensus Committee (AJCC/UICC) staging system for HCC (6th edition),26Okuda stage, and the Cancer of the Liver Italian Program (CLIP) stage were obtained by reviewing the medical records and radiological studies. Staging was determined by the Barcelona Clinic Liver Cancer staging system (BCLC).15BCLC stage A (Early stage) is defined by a single lesion between 2-5 cm or ≤ 3 lesions each < 3 cm, without portal vein thrombosis or extrahepatic metastasis. BCLC stage 0 (very early HCC) was defined as a single lesion < 2 cm without vascular involvement or metastasis. Late stage was defined as the combination of intermediate (BCLC stage B)/advanced (BCLC stage C) HCC.15
Laboratory evaluationPeripheral blood was collected from each participant at the time of the office visit. Sera were stored at -80 °C until measurements of OPN, AFP. The following laboratory tests were performed: hepatitis B surface antigen (HBsAg), hepatitis B core antibody (HBc-Ab), antibody to hepatitis C virus (anti-HCV), quantitative PCR (VERSANT HCV RNA 3.0 Assay) (Siemens Medical Solutions, Puteaux, France), HCV genotyping using INNO-LiPA III provided by (Inno-Lipa, Innogenetics, Genetics, Belgium),27serum aspartate aminotransferase (AST) level, serum alanine aminotransferase (ALT) level. HBsAg and anti-HCV were detected in serum by radioimmunoassay (Lumipulse II HBsAg; Fujirebio Co. Inc, Tokyo, Japan) and a second-generation enzyme-linked immunosorbent assay (Lumipulse II Ortho HCV; Ortho-Clinical Diagnostics Co Inc, Tokyo, Japan).
OPN ELISA assayPlasma concentrations of OPN were measured by capture enzyme linked immunosorbent assay (ELISA) according to the protocol provided by the manufacturer (R&D, San Diego, CA). The optical density was measured at 450 nm using a microplate reader (Thermo-Lab Systems). The I-smart program was used to create a regression curve. All the experiments were performed in duplicates.
Measurement of Plasma AFP LevelPlasma AFP levels were measured with the same plasma sample within 3 days after the measurement of OPN level by the chemiluminescence method using Elecsys AFP kits (Roche Diagnostic GmbH, Mannheim, Germany) according to the manufacturer’s instructions.
EthicsThe study protocol was approved by the Institutional Ethics Committee of School of Medicine, Minia University, Egypt, and all patients gave informed consent to participate in this study. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice.
Statistical AnalysisMedian, range, mean, and standard deviation were used for descriptive statistics, as appropriate. Categorical variables were tested with Fisher’s exact test or χ2test. Continuous variables were tested with Student t-test or analysis of variance (ANOVA). Comparison of plasma OPN levels and clinical characteristics among the three groups of subjects were analyzed using ANOVA test, post hoc tests, and Mann-Whitney U test. Correlation between plasma levels of OPN and AFP were analyzed using Spearman’s correlation coefficient.
Receiver operating characteristics (ROC) analysis was used to evaluate the diagnostic value of OPN and AFP and to identify the optimal threshold values. Sensitivity and specificity, positive and negative predictive values of OPN and AFP were profiled by curves. Calculations were done with the Statistical Package for the Social Sciences version 11 (SPSS, Inc., Chicago, IL, USA).
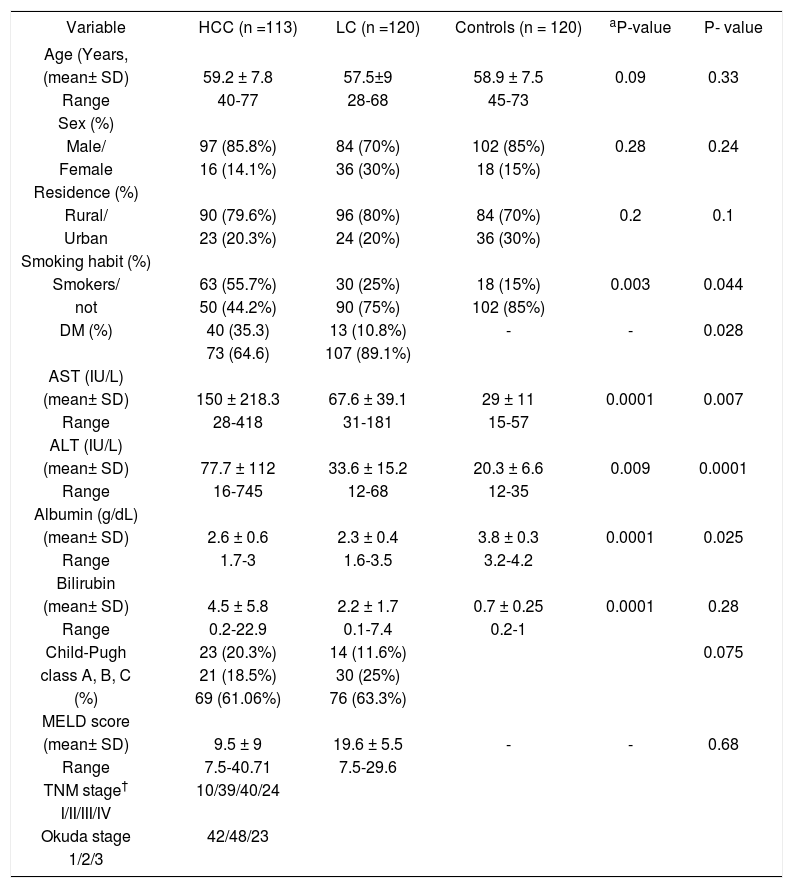
ResultsCharacteristics of the patientsThe baseline socio-demographic data, functional status of liver in terms of Child-Pugh class, and tumor stage of the subject patients are summarized in table 1. All recruited patients were positive for HCV antibodies, PCR for HCV RNA and all had genotype-4. In HCC group (Group 1); mean age was 59.2 ± 7.8; 97 (85.8%) patients were males and 16 (14.1%) patients were females. In liver cirrhosis (Group 2), mean age was 54.5 ± 9 and 84 (70%) patients were males, 36 (30%) patients were females. In control group (Group 3) mean age was 58.9 ± 7.5 and 102 (85%) patients were males, 18 (15%) patients were females. The frequency of smoking shows a significant trend to be increased by passing between the three groups of the study (55.7%, 25%, and 15%; P = 0.003).
Baseline demographics of the hepatocellular carcinoma, liver cirrhosis and healthy controls groups
| Variable | HCC (n =113) | LC (n =120) | Controls (n = 120) | aP-value | P- value |
|---|---|---|---|---|---|
| Age (Years, | |||||
| (mean± SD) | 59.2 ± 7.8 | 57.5±9 | 58.9 ± 7.5 | 0.09 | 0.33 |
| Range | 40-77 | 28-68 | 45-73 | ||
| Sex (%) | |||||
| Male/ | 97 (85.8%) | 84 (70%) | 102 (85%) | 0.28 | 0.24 |
| Female | 16 (14.1%) | 36 (30%) | 18 (15%) | ||
| Residence (%) | |||||
| Rural/ | 90 (79.6%) | 96 (80%) | 84 (70%) | 0.2 | 0.1 |
| Urban | 23 (20.3%) | 24 (20%) | 36 (30%) | ||
| Smoking habit (%) | |||||
| Smokers/ | 63 (55.7%) | 30 (25%) | 18 (15%) | 0.003 | 0.044 |
| not | 50 (44.2%) | 90 (75%) | 102 (85%) | ||
| DM (%) | 40 (35.3) | 13 (10.8%) | - | - | 0.028 |
| 73 (64.6) | 107 (89.1%) | ||||
| AST (IU/L) | |||||
| (mean± SD) | 150 ± 218.3 | 67.6 ± 39.1 | 29 ± 11 | 0.0001 | 0.007 |
| Range | 28-418 | 31-181 | 15-57 | ||
| ALT (IU/L) | |||||
| (mean± SD) | 77.7 ± 112 | 33.6 ± 15.2 | 20.3 ± 6.6 | 0.009 | 0.0001 |
| Range | 16-745 | 12-68 | 12-35 | ||
| Albumin (g/dL) | |||||
| (mean± SD) | 2.6 ± 0.6 | 2.3 ± 0.4 | 3.8 ± 0.3 | 0.0001 | 0.025 |
| Range | 1.7-3 | 1.6-3.5 | 3.2-4.2 | ||
| Bilirubin | |||||
| (mean± SD) | 4.5 ± 5.8 | 2.2 ± 1.7 | 0.7 ± 0.25 | 0.0001 | 0.28 |
| Range | 0.2-22.9 | 0.1-7.4 | 0.2-1 | ||
| Child-Pugh | 23 (20.3%) | 14 (11.6%) | 0.075 | ||
| class A, B, C | 21 (18.5%) | 30 (25%) | |||
| (%) | 69 (61.06%) | 76 (63.3%) | |||
| MELD score | |||||
| (mean± SD) | 9.5 ± 9 | 19.6 ± 5.5 | - | - | 0.68 |
| Range | 7.5-40.71 | 7.5-29.6 | |||
| TNM stage† | 10/39/40/24 | ||||
| I/II/III/IV | |||||
| Okuda stage | 42/48/23 | ||||
| 1/2/3 |
P-value of the χ2-test between three groups. P-value of the χ2-test for the HCC vs. the LC group. AFP: a-fetoprotein. ALT: alanine aminotransferase. BMI: body mass index. CI: confidence interval. DM2: type 2 diabetes mellitus. HCC: hepatocellular carcinoma. HCV. LC: liver cirrhosis. SD: standard deviation.
The plasma OPN levels in the HCC group, the CLD group, and the normal healthy control group are displayed in figure 1. The median plasma OPN level was significantly higher in the HCC group than in the CLD group or in the normal control group as determined by Mann-Whitney U test (p-value < 0.001). OPN levels were not differed significantly in correlation with the degree of liver function deterioration in terms of advanced Child-Pugh class (p-value < 0.9) (Figure 2).
Plasma osteopontin (OPN) levels in patients with hepatocellular carcinoma (HCC), chronic liver diseases (CLD), and healthy controls. Bars are median values of each disease group. The median plasma OPN level in HCC was significantly higher than in CLD or healthy controls (p-value < 0.001 by Mann-Whitney U test).
Plasma OPN levels according to Child-Pugh class in patients with hepatocellular carcinoma (HCC) and liver cirrhosis (LC). Bars are median values of each disease group. The plasma OPN level wasn’t progressively increased according to the Child-Pugh class, a classical marker for underlying liver function (P = 0.9).
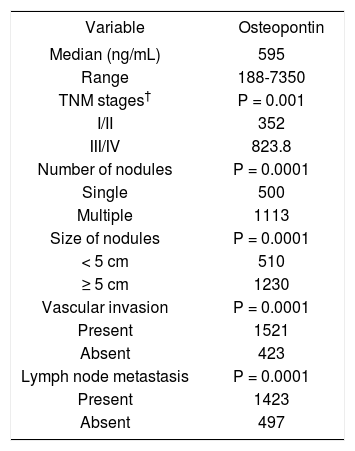
Plasma OPN levels were not significantly affected by age or sex in all three groups of the subjects. OPN levels in HCC group according to various pathological parameters are summarized in table 2. HCC patients in TNM stage I and II showed significantly lower OPN levels (352 ng/mL) than in stage III and IV HCC patients (825.8 ng/mL) (p-value = 0.001). A parallel increase of plasma OPN levels depending on the tumor stage by Okuda was also observed (data not showed). Plasma OPN were associated with number of nodules (p value = 0.0001), size of tumor nodules (p value = 0.0001), vascular invasion (p value = 0.0001), and lymph node metastasis (p value = 0.0001).
Relationship between osteopontin (OPN) and pathologic features in 113 patients with hepatocellular carcinoma (HCC).
| Variable | Osteopontin |
|---|---|
| Median (ng/mL) | 595 |
| Range | 188-7350 |
| TNM stages† | P = 0.001 |
| I/II | 352 |
| III/IV | 823.8 |
| Number of nodules | P = 0.0001 |
| Single | 500 |
| Multiple | 1113 |
| Size of nodules | P = 0.0001 |
| < 5 cm | 510 |
| ≥ 5 cm | 1230 |
| Vascular invasion | P = 0.0001 |
| Present | 1521 |
| Absent | 423 |
| Lymph node metastasis | P = 0.0001 |
| Present | 1423 |
| Absent | 497 |
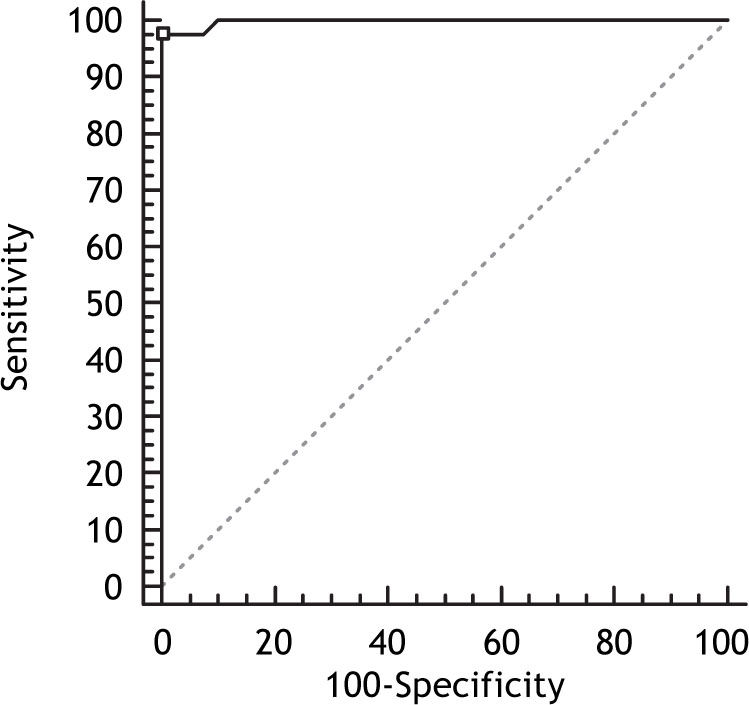
When all patients with HCC were evaluated, the AUC for OPN was (0.998; 95% Confidence Interval (CI) (0.952-1), p-value = 0.0001) which was significantly higher than that yielded by AFP (0.91; with 95% CI (0.826-0.961; p-value = 0.0001) (Figures 3 and 4), which suggested that the diagnostic accuracy of plasma OPN was superior to AFP for HCC at least under the current settings. The correlation between concurrently measured AFP levels and OPN levels was non significant (Spearman rho value of 0.312; p-value = 0.308), by Spearman rank correlation test.
When only Early stage HCC (BCLC stage 0 and BCLC stage A) was compared to cirrhosis controls, OPN yield better AUC than AFP (0.88 vs. 0.56; P = 0.0001, respectively) as shown in figure 5. When intermediate-advanced stage HCC was compared to cirrhotic controls, OPN had also highest AUC compared to total AFP (0.991 vs. 0.899; p = 0.01), but with improvement in the performance of AFP (Figure 6), indicating that OPN was more predictive of early and late stages HCC.
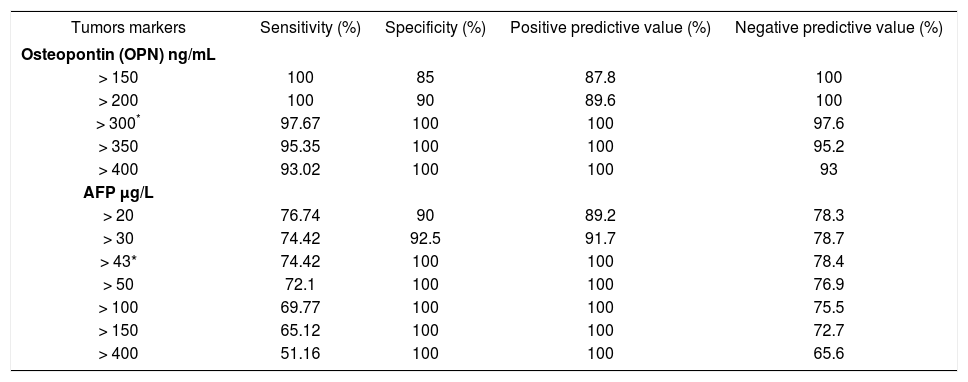
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of plasma OPN levels in HCC patients relative to the CLD group were 97.67% and 100%, 100%, 97.6% respectively, at a cut-off value of 300 ng/mL. The sensitivity ranged from 93 to 100% at different OPN levels with the specificity increasing sequentially from 85 to 100%. For AFP at a cut-off value > 43 ng/mL (the optimal cutoff); the values of sensitivity, specificity, PPV and NPV were 74.4%, 100%, 100%, 78.4%. The sensitivity ranged from 51.1 to 76.6% at different AFP levels with the specificity increasing sequentially from 90 to 100% (Table 3).
The sensitivity, specificity, positive predictive value and negative predictive value of Osteopontin and Alpha fetoprotein in diagnosis of HCC from chronic liver disease.
| Tumors markers | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| Osteopontin (OPN) ng/mL | ||||
| > 150 | 100 | 85 | 87.8 | 100 |
| > 200 | 100 | 90 | 89.6 | 100 |
| > 300* | 97.67 | 100 | 100 | 97.6 |
| > 350 | 95.35 | 100 | 100 | 95.2 |
| > 400 | 93.02 | 100 | 100 | 93 |
| AFP μg/L | ||||
| > 20 | 76.74 | 90 | 89.2 | 78.3 |
| > 30 | 74.42 | 92.5 | 91.7 | 78.7 |
| > 43* | 74.42 | 100 | 100 | 78.4 |
| > 50 | 72.1 | 100 | 100 | 76.9 |
| > 100 | 69.77 | 100 | 100 | 75.5 |
| > 150 | 65.12 | 100 | 100 | 72.7 |
| > 400 | 51.16 | 100 | 100 | 65.6 |
In this study, the plasma OPN levels were significantly higher in HCV-related HCC patients than in healthy control individuals and even higher than in patients with CLDs. Within the HCC group, OPN levels in contrast to á-fetoprotein correlated with advancing degree of tumor stage as number of nodules, size of tumor nodules, vascular invasion, lymph node metastasis and TNM staging. Additionally, the diagnostic efficacy of OPN was superior to AFP in terms of AUC, sensitivity, specificity, PPV and NPV whereas the correlation between OPN and AFP levels was not significant.
Ideal tumor biomarkers should possess high specificity and sensitivity not to be detected in prema-lignant liver disease. It should be easily accessed, easily measurable, minimally invasive, inexpensive, accurate acceptable to patients and physicians.28
OPN expression is common in many types of cancer and is correlated with poor prognosis of pa-tients.29-32OPN expression in tumors from diverse origins implies that either OPN plays a fundamental role in tumor formation and progression or in the host antitumor response. However, the role of OPN in tumor development is complex and may be affected by many factors. OPN is an attractive potential tumor marker, because it exists not only as an immobilized extracellular matrix molecule but also in a secreted form in body fluids including plasma.23,24,33
In this study; the AUC of plasma OPN and AFP were 0.998 and 0.91 respectively. The sensitivity, specificity, PPV and NPV of plasma OPN levels in HCC patients relative to the CLD group were 97.67% and 100%, 100%, 97.6% respectively, at a cut-off value of 300 ng/mL, which were higher than that for AFP at a cut-off value > 43 ng/mL (the optimal cutoff); the values of sensitivity, specificity, PPV and NPV were 74.4%, 100%, 100%, 78.4%. This suggested OPN levels might serve as a potential candidate biomarker for HCC, with superior diagnostic accuracy than AFP at least under the current settings. The correlation between concurrently measured AFP levels and OPN levels was non significant (Spearman rho value of 0.312; p-value = 0.308), by Spearman rank correlation test.
The wide application of more effective surveillance strategies which lead to earlier HCC detection and earlier qualification for effectively potential curative therapies requires more novel tumor markers for diagnosis. In the view of that most HCC develop in patients with underlying cirrhosis, early detection of HCC in setting of liver cirrhosis represent a great challenge. Given that the values of OPN are significantly higher in HCC patients than in patients with CLD and the AUC of OPN in differentiating early HCC from CLD in comparison with AFP was (0.88 vs. 0.56; P = 0.0001, respectively); OPN has clearly potential value as a diagnostic tumor marker for HCC during surveillance period, especially in cancers without detectable AFP.
Although the available traditional biomarkers as serum AFP may be useful as tools for the diagnosis of HCC;34,35there is no ideal marker associated properly with the prognosis of HCC patients. Plasma OPN might be a new biomarker for predicting the prognosis in those patients. In this study; OPN levels in contrast to a-fetoprotein correlated with advancing degree of tumor stage as number of nodules, size of tumor nodules, vascular invasion, lymph node metastasis and TNM staging. This in consistent with abundant data in the literature.23,24,33,36,37In contrast AFP; found to be not correlated well with tumor size. AFP simultaneous sensitivity decreases much more from about 50% for lesions of > 3 cm in diameter to about 25% for potentially resectable tumors of less than 3 cm in diameter. 20-30% of AFP sensitivity coincides with cut-off values > 100 μg/L, which means that 70-80% of the results of AFP are falsely negative. Based on these data 70-80% of liver tumors, normally resectable are non-detectable. For this reason only one patient out of 5 can receive potentially curative treatment.38,39In addition to that; the emerging of novel molecular targeted therapy for HCC requires novel tools for the evaluation of its efficacy beyond the traditional markers have been used for years.
The underlying etiology of HCC may affect the performance of different tumor markers. Worldwide, and especially in Egypt there is significant change in etiologies and risk factors of HCC. Now HCV are the most common etiology in HCC, in Egypt 80% of all HCC patients are anti-HCV positive.14Ideal tumor biomarker should perform by the same accuracy in all HCC etiologies. Unfortunate, PPV for AFP are paradoxically significantly lower among patients with HCC viral etiology than non-viral (PPV: 70% vs. 94%, P < 0.05).34We report in this study the accurate performance of OPN in HCV-related HCC. Our findings in HCV-infected subjects support the abundant available data in the literature indicate that OPN has a prognostic and diagnostic significance in HBV-related HCC.37
Collectively, these data suggest that the OPN concentration perform well in patients of HCC with viral etiology. This highlights that OPN may be incorporated in hepatocarcinogensis independent of the etiology of HCC. Also it worth mention that OPN mediate carcinogenesis through mediating hepatic inflammatory environment;40-42which are nearly similar in both viral hepatitis B and C. Further studies are keenly needed in this issue to explore the role of OPN in setting of other different etiologies.
This study has undoubtedly some limitations, since it is case-controlled one, the possibility of over estimation of the accuracy of biomarkers can’t be completely excluded and the diagnostic performance of the biomarker cannot be completely assed. Although we don’t detect the expression of OPN in HCC tissues, detecting plasma level of OPN is well validated, less invasive, reproducible, easier and applicable.43
In conclusion; in this study plasma OPN level is elevated in HCV-related HCC patients than patients with CLD. These results suggest that plasma OPN level is a potential diagnostic marker for HCC, especially among high-risk group of patients. This value extends beyond the traditional tumor biomarkers as AFP, as it possess good prognostic value. Although, AFP has to be considered ‘the golden standard, for HCC serum markers for years, in the view of our data and that of others; the usefulness of AFP testing for the population at risk should be seriously questioned. The ultimate diagnostic utility and implication of plasma OPN in HCC needed to be validated in other large multicenter cohort studies.