Vitamin D has been associated with chronic liver diseases and low vitamin levels may contribute to progression of chronic hepatitis C. The aim of this study was to evaluate the influence of vitamin D serum levels and GC gene polymorphisms in the severity of liver fibrosis in patients with chronic hepatitis C genotype 1.
Material and methodsCross-sectional study that enrolled 132 adult patients with chronic hepatitis C genotype 1 attended at the outpatient Clinic of Gastroenterology Division at Hospital de Clínicas de Porto Alegre. At the time of enrollment patients had a blood withdraw for serum 25(OH)D determination and genotypic analysis of rs7041 and rs4588 polymorphisms in GC gene. None/mild fibrosis was considered as METAVIR F0, F1 and F2 and severe fibrosis as METAVIR F3 and F4.
ResultsMedian 25(OH)D levels in the sample were 19.9 ng/mL (P25-P75: 14.0-29.4). Fifty percent of patients presented vitamin D deficiency (< 20 ng/mL). In stepwise multiple linear regression the variables associated with 25(OH)D levels were blood withdrawn in Winter/spring season, the haplotypes AT/AT + AG/AT of rs7041 and rs4588 and female sex. For evaluation of severe fibrosis, variables associated in logistic regression were age, vitamin D severe deficiency (< 10 ng/mL), glucose levels, BMI and platelets count.
ConclusionsVitamin D levels are associated with severity of liver fibrosis in chronic hepatitis C genotype 1 patients. Although the rs7041 and rs4588 GC polymorphisms are strong predictors of vitamin D levels, they do not play a direct role in liver fibrosis.
Liver fibrosis is defined by extracellular matrix (ECM) accumulation with consequent loss of liver function, due to acute or chronic damage.1 Chronic hepatitis C (CHC) leads to liver fibrosis and 20-30% of patients progress to cirrhosis after 25-30 years of infection.2 Several factors influence the evolution of CHC and formation of liver fi-brosis, such as age, obesity, virus C genotype, alcohol consumption, duration of infection, presence of viral co-infections and metabolic disorders.2
Vitamin D has been associated with chronic liver diseases and low vitamin levels may contribute to progression of CHC.3 Vitamin D is mainly synthesized in the skin by 7-dehydrocholesterol redutase (DHCR7) in pre-vitamin D, which is converted into vitamin D by isomeri-zation. Vitamin D and its metabolites circulate bounded to vitamin D-binding protein (GC-globulin). Vitamin D goes under hydroxylation in the liver by cytochrome P450 enzymes forming 25-hydroxyvitamin D (25(OH)D) and is used to evaluate vitamin D status. The metabolite 25(OH)D is hydroxylated by CYP27B1 in the kidney, producing 1,25-dihydroxyvitamin D (1,25(OH)D), the active form that binds to vitamin D receptor (VDR).4 Vitamin D deficiency is a risk factor for all-cause mortality in the general population5 as well as for cirrhotic patients.6
GC-globulin is coded by GC gene. The three protein isoforms are coded by different alleles, which are formed by single nucleotide polymorphisms (SNPs). Polymorphism rs7041 has two alternative alleles, containing either A or C, and polymorphism rs4588 has either G or T. Each person carries two copies of the GC gene; therefore possible genotypes for rs7041 are AA, AC or CC, for example. Different SNPs at the same gene are inherited together forming a haplotype. For rs7041 and rs4588 possible haplo-types are CT, CG or AT. These two SNPs are responsible for the three major circulating isoforms of GC-globulin Gc1F (CT), Gc1S (CG) and Gc2rs (AT).7 The Gc-2 iso-form is described to have the lowest affinity for its ligand.8 The three common haplotype variants combined form CT-CT, CT-CG, CG-CG, CG-AT, CT-AT and AT-AT.
It is known that several polymorphisms involved in the vitamin D metabolism are associated with its serum con-centrations.9,10 The aim of this study was to evaluate the influence of vitamin D serum levels and GC polymorphisms in the severity of liver fibrosis in patients with chronic hepatitis C genotype 1.
Material and MethodsThis was a cross-sectional study that enrolled 132 adult patients with chronic hepatitis C genotype 1 attended at the outpatient Clinic of Gastroenterology Division at Hospital de Clínicas de Porto Alegre, Brazil, from June 2013 to February 2015. Hepatitis C virus infection was confirmed by anti-HCV ELISA 3 and by detection of viral RNA by polymerase chain reaction (HCV RNA PCR). Patients infected by the human immunodeficiency virus (HIV) or hepatitis B virus (HBV), alcohol abusers (> 40g/ day), chronic renal injured, liver transplanted, decompen-sated cirrhotic and those with hepatocellular carcinoma (HCC) or in immunosuppressive or antiviral treatment were excluded. Both naïve and previously PEG-INF treated patients were included.
At the time of the enrollment patients had a blood withdraw for vitamin D serum levels determination and geno-typic analysis. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl-transferase (GGT), platelets, glucose, cholesterol and body mass index (BMI) were extracted from patient's chart from the previous 6 months. Severity of fibrosis was evaluated by the last liver biopsy done by the outpatients Clinic routine. Liver biopsies were evaluated by the same pathologist and scored with METAVIR system.11 Serum 25(OH)D levels were determined using a chemoluminescent immunoassay on a Liaison automatic analyzer (Dia-Sorin). Vitamin D levels below 10 ng/mL were considered severe deficiency; 10 to 20 ng/mL deficiency; 20 to 30 ng/ mL insufficiency; and above 30 ng/mL sufficiency.12 Date of sample collection was categorized into two seasons, Summer/autumn (December-May) and Winter/spring (June-November). The study was conducted according to the principles of the Declaration of Helsinki and was approved by the hospital Ethical Committee. All patients gave written informed consent to participate in the study.
Genotypic analysisThe genomic DNA was extracted from whole blood EDTA using Wizard® Genomic DNA Purification Kit (Promega) according to manufacturer's instructions. The polymorphisms rs7041 and rs4588 from the GC gene were identified using hydrolysis probes (TaqMan Assays; ABI), IDs: C_3133594_30 and C_8278879_10, respectively. A total of 10 ng of DNA was used in a final reaction volume of 12.5 μL for real-time PCR on StepOne™ System (Thermo Fisher).
Statistical analysisCategorical variables are presented as frequencies. Continuous variables with normal distribution are presented as mean and standard deviation and variables with non-normal distribution as median and interquartile range (P25-P75). To check the normal distribution of the variables, a Sha-piro-Wilk test was applied. Associations between continuous variables were carried out with T test, Mann-Whitney or Kruskall-Wallis as appropriated and associations between categorical variables were evaluated with Fisher's exact test. Stepwise multiple linear regression analysis with a forward approach using a cutoff value of 0.05 was performed to identify independent predictors of 25(OH)D levels. Step-wise logistic regression analysis with a forward approach using a cutoff value of 0.05 was performed to identify independent predictors of liver fibrosis. Variables included in the model were sex, age, fibrosis (none/mild or severe), diabetes mellitus, BMI, ALT, AST, GGT, cholesterol, glucose, platelets, rs4588 and rs7041 haplotypes (AT/AT + AG/ AT vs. others), season of blood withdrawn (Summer/Autumn vs. Winter/Spring). For analysis purposes none/mild fibrosis was considered as METAVIR F0, F1 and F2 and severe fibrosis as METAVIR F3 and F4 (cirrhosis). A χ2 test was employed to verify whether the proportions of the two polymorphisms were distributed in accordance with the Hardy-Weinberg Equilibrium. Haplotypes for rs7041 and rs4588 were statistically reconstructed using the PHASE program, version 2.1, which implements a Bayesian statistical method for haplotyping reconstructing from genotype data.13,14 Statistical analysis was performed using SPSS 20.0 (SPSS Inc, Chicago, Il, USA). Significance level was set at α< 0.05.
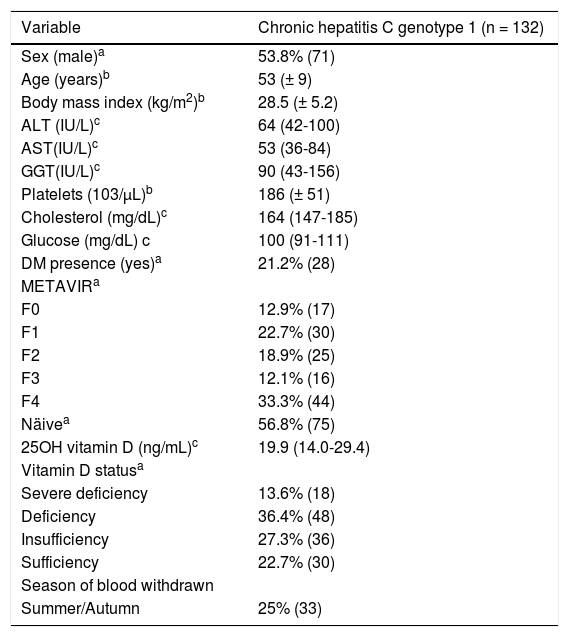
ResultsPatient featuresDemographical, clinical, biochemical and genotypic features of enrolled patients are described in table 1. Patients’ age varied from 26 to 74 years. The majority of patients were cirrhotic (33.3%). Median 25(OH)D levels were 19.9 ng/mL (14.0 - 29.4). Fifty percent of patients presented deficiency or severe deficiency of vitamin D.
Demographic, clinical and biochemical features of chronic hepatitis C genotype 1 patients.
| Variable | Chronic hepatitis C genotype 1 (n = 132) |
|---|---|
| Sex (male)a | 53.8% (71) |
| Age (years)b | 53 (± 9) |
| Body mass index (kg/m2)b | 28.5 (± 5.2) |
| ALT (IU/L)c | 64 (42-100) |
| AST(IU/L)c | 53 (36-84) |
| GGT(IU/L)c | 90 (43-156) |
| Platelets (103/μL)b | 186 (± 51) |
| Cholesterol (mg/dL)c | 164 (147-185) |
| Glucose (mg/dL) c | 100 (91-111) |
| DM presence (yes)a | 21.2% (28) |
| METAVIRa | |
| F0 | 12.9% (17) |
| F1 | 22.7% (30) |
| F2 | 18.9% (25) |
| F3 | 12.1% (16) |
| F4 | 33.3% (44) |
| Näivea | 56.8% (75) |
| 25OH vitamin D (ng/mL)c | 19.9 (14.0-29.4) |
| Vitamin D statusa | |
| Severe deficiency | 13.6% (18) |
| Deficiency | 36.4% (48) |
| Insufficiency | 27.3% (36) |
| Sufficiency | 22.7% (30) |
| Season of blood withdrawn | |
| Summer/Autumn | 25% (33) |
DM: diabetes mellitus. a Percentage (n). b Mean (standard deviation). c Median (IQR P25-P75). Total sample for: BMI = 125; platelets = 131.
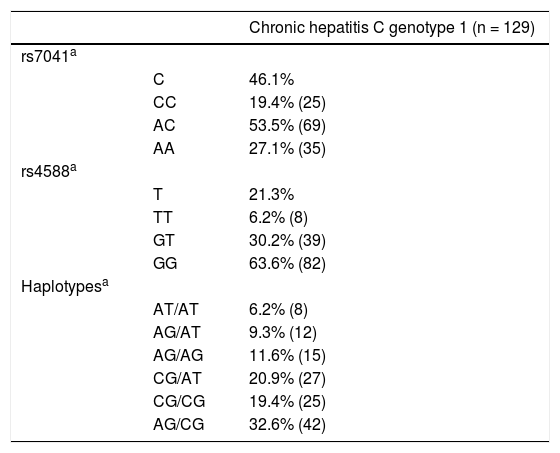
Genotypes and alleles frequencies of rs7041 and rs4588 were in Hardy Weinberg Equilibrium. The most frequent genotypes were AC for rs7041 and GG for rs4588 (Table 2). Finally, for haplotypes, GC/TA, GC/GC and GC/GC were the most common ones.
Allelic, genotypic and haplotypic data of rs7041 and rs4588 polymorphisms in chronic hepatitis C genotype 1 patients.
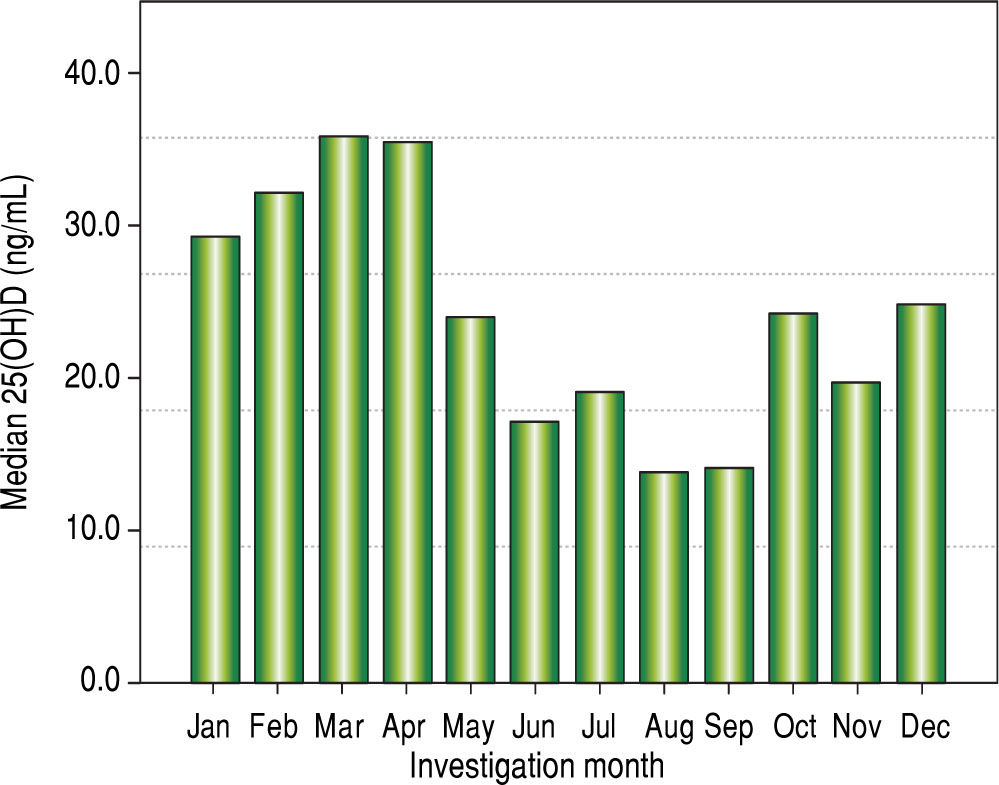
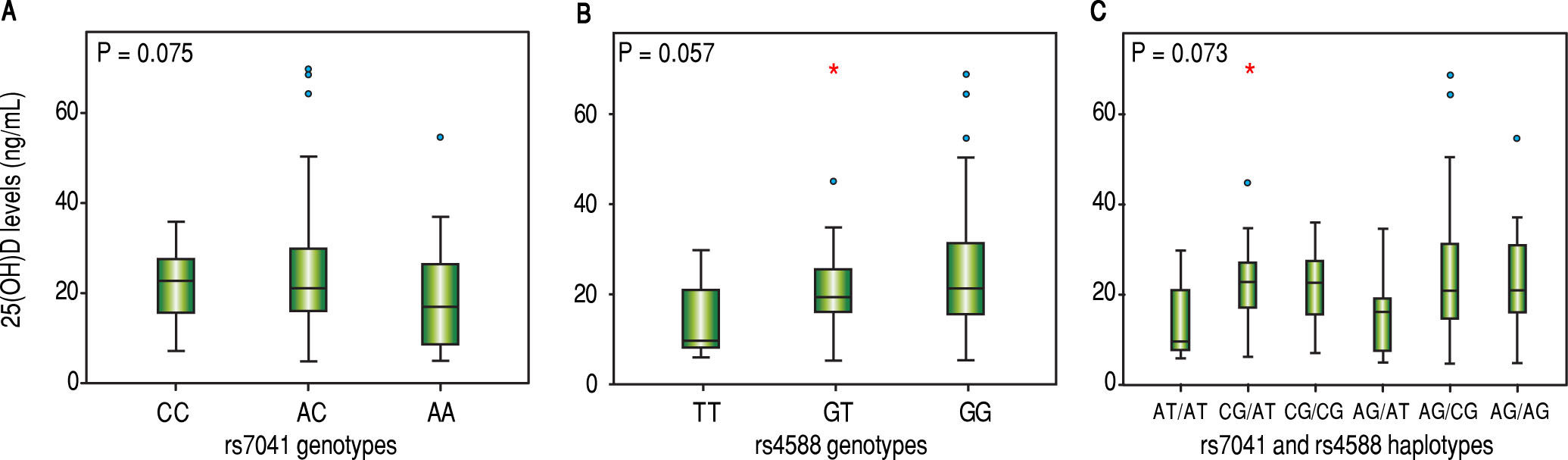
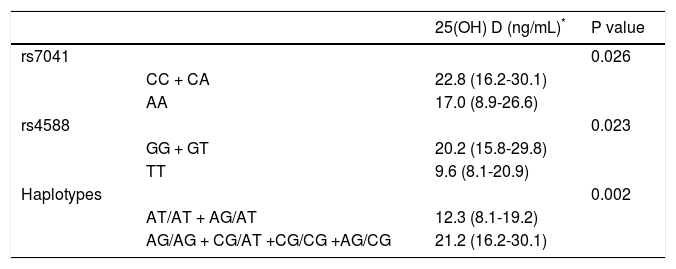
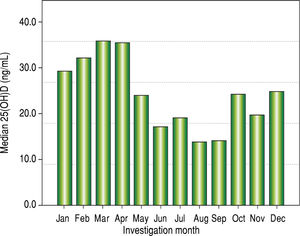
Vitamin D levels were higher in patients enrolled in Summer/Autumn than in Winter/Fall [31.2ng/mL (IQR 19.8-35.0) vs. 18.4ng/mL (IQR 12.8-24.1); P < 0.001] (Figure 1). Median 25(OH)D levels was 18.0 ng/mL (12.7 - 23.2) for females and 24.0 ng/mL (17.2 - 31.8) for males (P = 0.002). Vitamin D did not differ between näive and PEG-INF treated patients (P = 0.921). When 25(OH)D serum levels were stratified among rs7041 and rs4588 genotypes and haplotypes, there was no statistical significance (P > 0.05), however a trend is graphically observed for AA genotype of rs7041 and TT genotype of rs4588, as well as for AT/AT and AG/AT haplotypes (Figure 2). Transforming the mentioned genotypes and haplo-types into dichotomous variables, it is observed that 25(OH)D levels are lower for AA genotype of rs7041, TT genotype of rs4588 and AT/AT + AG/AT haplotypes when compared to their counterparts (Table 3). In multiple linear regression the variables associated with diminished 25(OH)D circulating levels were blood withdrawn in Winter/Spring, the risk haplotypes AT/AT + AG/AT and female sex (Table 4).
Box plots showing the distribution of 25(OH) D serum levels stratified according to (A) rs7041 genotypes, (B) rs4588 genotypes and (C) rs7041 and rs4588 haplotypes. Upper horizontal line of box, 75th percentile; lower horizontal line of box, 25th percentile; horizontal bar within box, median; upper horizontal bar outside box, 90th percentile; lower horizontal bar outside box, 10th percentile. * Circles represent outliers. P values for Kruskal-Wallis test.
Median 25 (OH)D stratified according to risky genotypes and haplotypes of rs7041 and rs4588.
| 25(OH) D (ng/mL)* | P value | ||
|---|---|---|---|
| rs7041 | 0.026 | ||
| CC + CA | 22.8 (16.2-30.1) | ||
| AA | 17.0 (8.9-26.6) | ||
| rs4588 | 0.023 | ||
| GG + GT | 20.2 (15.8-29.8) | ||
| TT | 9.6 (8.1-20.9) | ||
| Haplotypes | 0.002 | ||
| AT/AT + AG/AT | 12.3 (8.1-19.2) | ||
| AG/AG + CG/AT +CG/CG +AG/CG | 21.2 (16.2-30.1) |
Stepwise forward multiple linear regression analysis for variables associated with 25(OH)D serum levels.
| Variables | β | SE | P value |
|---|---|---|---|
| Winter/Spring vs. Summer/Autumn | -0.375 | 2.003 | < 0.001 |
| Female sex | -0.261 | 1.740 | 0.001 |
| Haplotypes AT/AT + AG/AT vs. others | -0.259 | 1.254 | 0.001 |
Variables included in the model: sex, age, fibrosis (none/mild or severe), diabetes mellitus, BMI, ALT, AST, GGT, cholesterol, glucose, platelets, rs4588 and rs7041 haplotypes (AT/AT + AG/AT vs. others), season of blood withdrawn (Summer/Autumn vs. Winter/Spring). β: standardized coefficient beta. SE: standard error.
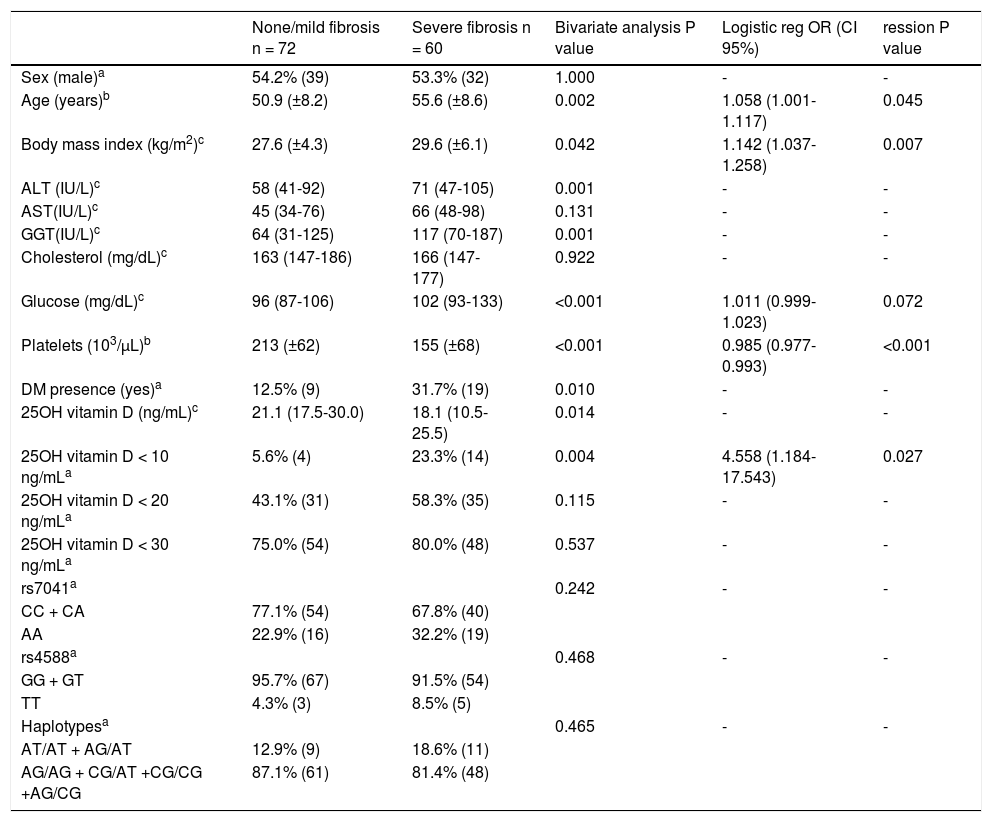
In table 5 are presented the association of studied variables with severity of liver fibrosis. Older age; higher BMI, ALT, GGT and glucose; lower platelets and 25(OH)D; diabetes and severe vitamin D deficiency were related to severe liver fibrosis in bivariate analysis. None of the evaluated genotypes and haplotypes of rs7041 and rs4588 were associated with fibrosis severity. In the logistic regression analysis were included sex, age, 25(OH)D > 10 ng/mL, season of blood withdrawn (Summer/Autumn vs. Winter/Spring), diabetes mellitus, BMI, ALT, AST, GGT, cholesterol, glucose, platelets, rs4588 and rs7041 haplo-types (AT/AT + AG/AT vs. others). The variables that remained in the model were age, 25(OH)D < 10 ng/mL, glucose levels, BMI and platelets count. When 25(OH)D is included as a continuous variable it loses statistical significance in the model.
Bivariate and logistic regression analysis of risk factors associated with severe fibrosis in chronic hepatitis C genotypes 1 patients.
| None/mild fibrosis n = 72 | Severe fibrosis n = 60 | Bivariate analysis P value | Logistic reg OR (CI 95%) | ression P value | |
|---|---|---|---|---|---|
| Sex (male)a | 54.2% (39) | 53.3% (32) | 1.000 | - | - |
| Age (years)b | 50.9 (±8.2) | 55.6 (±8.6) | 0.002 | 1.058 (1.001-1.117) | 0.045 |
| Body mass index (kg/m2)c | 27.6 (±4.3) | 29.6 (±6.1) | 0.042 | 1.142 (1.037-1.258) | 0.007 |
| ALT (IU/L)c | 58 (41-92) | 71 (47-105) | 0.001 | - | - |
| AST(IU/L)c | 45 (34-76) | 66 (48-98) | 0.131 | - | - |
| GGT(IU/L)c | 64 (31-125) | 117 (70-187) | 0.001 | - | - |
| Cholesterol (mg/dL)c | 163 (147-186) | 166 (147-177) | 0.922 | - | - |
| Glucose (mg/dL)c | 96 (87-106) | 102 (93-133) | <0.001 | 1.011 (0.999-1.023) | 0.072 |
| Platelets (103/μL)b | 213 (±62) | 155 (±68) | <0.001 | 0.985 (0.977-0.993) | <0.001 |
| DM presence (yes)a | 12.5% (9) | 31.7% (19) | 0.010 | - | - |
| 25OH vitamin D (ng/mL)c | 21.1 (17.5-30.0) | 18.1 (10.5-25.5) | 0.014 | - | - |
| 25OH vitamin D < 10 ng/mLa | 5.6% (4) | 23.3% (14) | 0.004 | 4.558 (1.184-17.543) | 0.027 |
| 25OH vitamin D < 20 ng/mLa | 43.1% (31) | 58.3% (35) | 0.115 | - | - |
| 25OH vitamin D < 30 ng/mLa | 75.0% (54) | 80.0% (48) | 0.537 | - | - |
| rs7041a | 0.242 | - | - | ||
| CC + CA | 77.1% (54) | 67.8% (40) | |||
| AA | 22.9% (16) | 32.2% (19) | |||
| rs4588a | 0.468 | - | - | ||
| GG + GT | 95.7% (67) | 91.5% (54) | |||
| TT | 4.3% (3) | 8.5% (5) | |||
| Haplotypesa | 0.465 | - | - | ||
| AT/AT + AG/AT | 12.9% (9) | 18.6% (11) | |||
| AG/AG + CG/AT +CG/CG +AG/CG | 87.1% (61) | 81.4% (48) |
Bivariate analysis: a Percentage (n) Fisher's Exact Test. b Mean (standard deviation) T test. c Median (IQR P25-P75) Mann-Whitney. Variables included in the stepwise logistic regression model with forward approach: sex, age, 25(OH)D > 10 ng/mL, season of blood withdrawn (summer/autumn vs. winter/spring), diabetes mellitus, BMI, ALT, AST, GGT, cholesterol, glucose, platelets, rs4588 and rs7041 haplotypes (AT/AT + AG/AT vs. others). OR: odds ratio. CI: confidence interval.
In recent years, the influence of vitamin D in liver diseases has been broadly discussed since it undergoes hepatic metabolism. Herein we evaluated the association of vitamin D serum levels and of rs7041 and rs4588 polymorphisms from GC gene in severe liver fibrosis in a sample of 132 chronic hepatitis C genotype 1 patients.
In this sample, vitamin D median levels were 19.9 ng/ mL and deficiency prevalence was 50%. In a study with 496 CHC patients from Swiss hospitals, median 25(OH)D was 13.4 ng/mL, and 74% presented levels below 20.0 ng/ mL.15 It has been described that the prevalence of vitamin D insufficiency and deficiency in patients with chronic liver disease ranges between 64 and 92%.16
Serum levels of 25 (OH)D fluctuate strongly as a result of variations in sunlight exposure during the season, type of food, presence of comorbidities, vitamin D supplementation and others. It is important to also evaluate the different genetic variants of vitamin D metabolism, which could provide an assessment of long term vitamin D status. In multiple regression analysis, 25(OH)D levels were influenced by season, sex and genetic factors. Polymorphisms in genes involved in synthesis, transport and vitamin D metabolism are known to affect its circulating levels, especially in GC, DHCR7 and CYP2R1 loci.7,8,17 The GC gene codifies the protein responsible for carrying vitamin D and its metabolites in the circulation and in our study the haplotypes AT/AT and AG/AT were responsible for the lower levels of vitamin D, showing that the T allele of rs4588 has the biggest influence in vitamin D levels. Our findings are similar to Santos, et al., which found the same results in healthy Brazilian girls.18 The Gc-2 isoform is described to have the lowest affinity for its ligand, which is in accordance confirming the lowest levels of vitamin D described in both studies.10
Vitamin D deficiency seems to be associated with a poorer prognosis of the liver disease. In this sample, a difference in 25(OH)D serum levels was observed in none/ mild and severe fibrosis (21.1 vs. 18.1 ng/mL; P = 0.014). Baur, et al. reported that individuals with CHC METAVIR 2 presented 15.5 ng/mL 25(OH)D levels compared to 22.2 ng/mL of individuals METAVIR 0/1.19 Meanwhile, Petta described that CHC G1 with Scheuer scoring 0-2 presented 25.5ng/mL vs. 21.9 ng/mL from patients Scheuer scoring of 3-4.20 In our sample the overall prevalence of severe deficiency was 13.6% (18/132), in patients with none/mild fibrosis was 5.6% (4/72) and in patients with severe fibro-sis was 23.3% (14/60). In a cohort of German CHC patients, severe deficiency prevalence was 25%.21 In logistic regression, severe vitamin D deficiency was associated with severe fibrosis along with age, BMI and platelets count. Although the studied polymorphisms have presented association with vitamin D circulating levels, they were not related to liver fibrosis. There are studies that have shown that polymorphisms of vitamin D metabolism relate to the stages of liver disease. Studies from Petta and Grünhage identified the polymorphism rs12785878 in the gene of cholesterol reductase (DHCR7) associated with a greater degree of liver fibrosis.22,23 This polymorphism was not evaluated in the present sample. Another gene that is important in liver fibrosis is VDR, since it can down-regulate the activation of hepatic stellate cells by competing for several genomic sites related to fibrosis, which are activated by SMAD3, an effector of the transforming growth factor beta 1 (TGFβ1).24 The rs1544410, rs7975232 and rs731236 polymorphisms have been associated with the rate of progression of fibrosis and cirrhosis.19
As limitation of this study we could cite that some co-founding factors were not analyzed, such as smoking, intravenous drug use, duration of HCV infection. Also, it was a cross-sectional study that analyzed only two polymorphisms. Although the sample size is relatively small, we considered as a main strength the use of a well-defined cohort of HCV genotype 1 patients that represent the real-world experience. Our results corroborate the literature findings, whereas lower vitamin D levels are found among patients with higher liver fibrosis. Identifying those patients is important since oral supplementation of vitamin D is a very simple therapeutic measure. Several studied have demonstrated that vitamin D deficiency I is likely to be related to lower odds of sustained virological response (SVR) to treatment with pegylated interferon plus ribavi-rin.25 and recently, in a Japanese trial, vitamin D3 supplementation increased SVR rates on refractory CHC patients on treatment with simeprevir, pegylated interferon plus ribavirin.26
In summary, vitamin D levels were associated with severity of liver fibrosis in chronic hepatitis C genotype 1 patients. Although the rs7041 and rs4588 GC gene polymorphisms are strong predictors of vitamin D levels, they do not play a direct role in liver fibrosis.
Abbreviations- •
1,25(OH)D: 1,25-dihydroxyvitamin D.
- •
25(OH)D: 25-hydroxyvitamin D.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BMI: body mass index.
- •
CHC: chronic hepatitis C.
- •
DHCR7: 7-dehydrocholesterol redutase.
- •
ECM: extracellular matrix.
- •
GGT: gamma-glutamyl-transferase.
- •
SVR: sustained virological response.
- •
VDR: vitamin D receptor.
The authors would like to thank Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), Programa de Apoio à Pós-Graduação da Coorde-nação de Aperfeiçoamento de Pessoal de Nível Superior (PROAP-CAPES) and Conselho Nacional de Desen-volvimento Científico e Tecnológico (CNPq).
Authors ContributionStudy conception and design: LAA; UM; TRS; MRAS. Performed the experiments: LAA; JWB; JPB. Analyzed the data: LAA. Wrote the paper: LAA; UM; TRS; JPB; JWB; MRAS. Critical revision: UM; TRS; MRAS.