The efficacy and safety of asunaprevir + daclatasvir combination therapy for treatment of hepatitis C virus (HCV) in compensated cirrhotic patients was not fully evaluated in real-world. Outcomes were assessed in cirrhotic patients with sustained viral response (SVR).
Material and methodsA total of 145 patients without resistance-associated substitutions (RASs) at L31 and Y93 in the nonstructural protein 5A of HCV genotype 1b, consisting of 49 hepatic cirrhotic and 96 non-cirrhotic patients, were enrolled to the therapy. The patients were treated with 100 mg asunaprevir twice daily plus 60 mg daclatasvir once daily for 24 weeks. The primary endpoint was SVR 24 weeks after completing treatment. In addition, we evaluated the improvement of liver function and development of HCC for 1 year from the end of treatment (EOT).
ResultsThe SVR24 rate was 96% (47/49) in the cirrhotic group and 96% (91/95) in the non-cirrhotic group (p = 0.69). During treatment, grade III/IV adverse events occurred more frequently in cir-rhotic patients (10/49; 20.4%) than in non-cirrhotic patients (10/96; 10.4%) (p = 0.099). After EOT, alanine aminotransferase and AFP levels were significantly decreased in cirrhotic patients with SVR. In addition, serum levels of albumin and platelet counts were significantly increased. On the other hand, the rates of HCC recurrence (43%) and development (7.4%) were higher in cirrhotic patients than in the non-cirrhotic patients (12.5% and 1.1%, respectively).
ConclusionRAS-oriented asunaprevir/daclatasvir therapy has a strong anti-HCV effect in patients with HCV genotype 1b. However, careful management is necessary in patients with cirrhosis.
Chronic hepatitis C (CHC) stemming from infection with the hepatitis C virus (HCV) affects ~170 million people worldwide and is the most common cause of chronic liver disease. Of all HCV-infected individuals, 20-30% eventually develop liver cirrhosis or hepatocellular carcinoma (HCC).1 The elimination of HCV is important for preventing HCC, particularly in aged patients with advanced fibrosis or CHC.2,3
Since 2010, direct-acting antiviral (DAA) regimens with or without interferon (IFN) have been evaluated and approved for anti-HCV therapy. In Japan, the protease inhibitors telaprevir, simeprevir, and vaniprevir have been approved for use as DAA combination therapy with pegylated interferon and ribavirin.4-7 Asunaprevir (ASV) and daclatasvir (DCV) combination therapy was developed as the first IFN-free anti-HCV therapeutic regimen in 2014. To date, this regimen has been approved by regulatory authorities in several countries across the Asia-Pacific region, including Korea, Taiwan, Australia, Singapore, and Mexico. In a phase III clinical trial in Japan, 168 (91.3%) of 214 patients without resistance-associated substitutions (RASs) at Y93 in the nonstructural protein (NS) 5A achieved sustained viral response (SVR).8 On the other hand, only 13 (43.3%) of 30 patients with RAS at Y93 achieved SVR. Therefore, the Japanese guidelines for HCV management recommend ASV + DCV combination therapy for patients without RAS at NS5A of HCV genotype 1b.9 In this phase III trial, 20 (90.9%) of 22 compensated cirrhotic patients achieved SVR. In a global phase III trial outside Japan, SVR12 was achieved by 171 (84%) of 206 cirrhotic patients and by 371 (85%) of 437 non-cirrhotic patients.10 There was no significant incidence of serious adverse effects (AEs). However, the mean age of enrolled patients was 5-6 years younger in the global study than in the Japanese trial. In addition, these phase III trials excluded cirrhotic or non-cirrhotic patients with a history of curative treatment for HCC. DAA therapy has now been approved for patients with a past history of HCC in Japan. It is necessary to evaluate the preventive effects of ASV + DCV therapy on the recurrence of HCC.
This study evaluated the anti-HCV effect and safety of ASV + DCV combination therapy for compensated cir-rhotic patients compared to non-cirrhotic patients. In addition, the development of HCC and liver function after therapy were evaluated in cirrhotic patients.
Material and MethodsPatientsBefore commencing treatment, we checked the RAS in NS5A of 350 patients with HCV genotype 1b (not shown). A total of 145 patients without RAS on L31 and Y93 within the NS5A domain were enrolled for treatment with ASV + DCV combination therapy, consisting of 49 hepatic cir-rhotic patients and 96 non-cirrhotic patients. Hepatic cirrhosis was defined as histological examination F4 or > 15.0 kPa according to transient elastography. The enrolled patients were aged 40-86 years old and were chronically infected with HCV genotype 1b with a serum HCV RNA viral load > 3 log copies/mL at baseline. Patients were excluded if they had medical contraindications for ASV and DCV, a history of DAA use, a history of drug use, decom-pensated cirrhosis, a Child-Pugh score > 6, or evidence of other significant liver diseases, including hepatitis B virus, autoimmune liver diseases, alcoholic liver disease, and viable HCC. None of the patients showed evidence of infection by human immunodeficiency virus (HIV). This study was performed in accordance with the 1975 Declaration of Helsinki (2004 version), and written informed consent was obtained from all patients prior to treatment. The study protocol was approved by the Ethics Committee of each hospital and by the Ethics Committee of Osaka City University Graduate School of Medicine (No. 2905). The trial was registered in UMIN (No. 000015806).
Study designThe patients were treated with 100 mg ASV (Sun-vepra®, Bristol-Myers Squibb, Tokyo, Japan) twice per day plus 60 mg DCV (Daklinza®, Bristol-Myers Squibb) once daily for 24 weeks. A total of 110 (76%) of the 145 patients were admitted to the hospital for 3-7 days at initiation and were educated regarding DAA therapy by a hospital pharmacist. A serum liver function test and an imaging test were performed regularly for 1 year from the end of treatment (EOT), in addition to the evaluation of serum HCV viral load.
Virological evaluationsHCV-RNA was determined by TaqMan HCV assay (COBAS® TaqMan® HCV assay, Roche Molecular Diagnostics, Tokyo, Japan) with a lower limit of quantification of 15 IU/mL and an upper limit of quantification of 6.9 x 107 IU/mL (1.2-7.8 log IU/mL). The HCV genotype was determined using an HCV genotype primer kit (Institute of Immunology Co., Ltd., Tokyo, Japan). RAS in the NS3/4A protease and the NS5A were evaluated using the direct sequencing method according to a previously reported protocol.11 Resistance analysis focused on amino acid polymorphisms Q80, R155, A156, D168, and V170 within NS3/4 A and of L28, R30, L31, Q54, P58, Q62, A92, and Y93 within the NS5A domain.
Virological response to the therapy was defined as follows: relapse showing undetectable HCV-RNA at EOT but detectable HCV RNA after EOT and re-elevation of HCV RNA at any time during treatment after a virological response (breakthrough). Patients in whom HCV-RNA remained stable during treatment were defined as non-responders.
EvaluationsThe rate of SVR24, defined as undetectable HCV-RNA at 24 weeks after completion of treatment, was used to evaluate primary efficacy. In addition, the improvement of liver function and HCC development were used as secondary evaluations after treatment. During the follow-up period, clinical, biochemical, and quantitative serum HCV-RNA assessments were evaluated at 1- to 3-month intervals. Safety evaluations included adverse event reporting, laboratory test values, and physical examinations. All methods used for assessing treatment efficacy were defined according to previously published guidelines.12
SNP genotypingWe examined genetic polymorphisms in the interleukin 28B (IL28B) gene in patients that consented to genetic anal-ysis.13 Whole blood was collected from patients and centri-fuged to separate the buffy coat. Genomic DNA was extracted from the buffy coat using a QIAamp® DNA Blood Midi Kit (Qiagen, Maryland, Germantown, MD). Genetic polymorphisms in IL28B rs8099917 and rs12979860 were genotyped by TaqMan SNP Genotyping Assay with the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). All samples were also subjected to direct sequencing to confirm the genotype. The primers and procedures used were as described previously.13,14
Statistical analysisData analyses were conducted using JMP software (ver. 9.0; SAS Institute, Cary, NC). Differences between groups were evaluated by Wilcoxon's two-sample test for numerical variables or Fisher's exact test for categorical variables. Variables with p < 0.1 in univariate analyses were subjected to stepwise multivariate logistic regression analyses. In the two-tailed test, p < 0.05 was taken to indicate statistical significance.
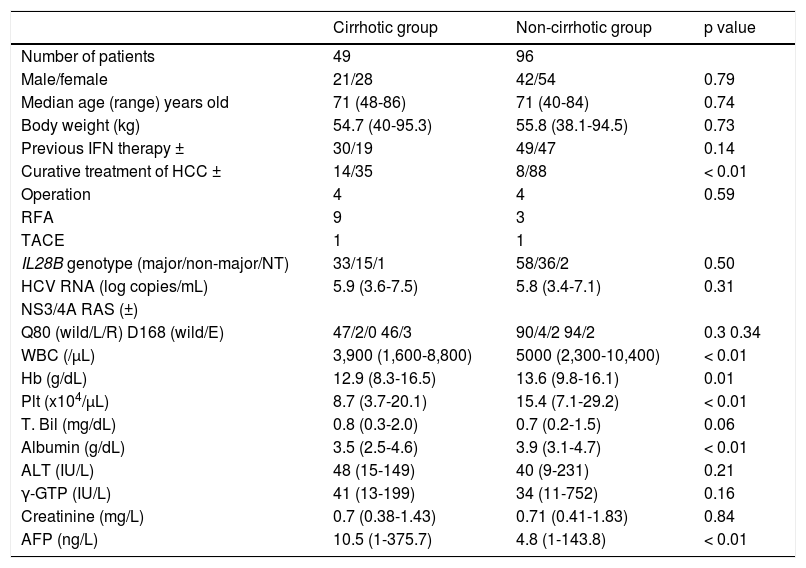
RESULTSPatients at baselineThe clinical background of 49 hepatic cirrhotic and 96 non-cirrhotic patients are shown in table 1. The cirrhotic group contained 21 females and 28 males, and the non-cir-rhotic group had 41 females and 54 males (p = 0.79). The median age was 71 years old (range: 48-86 years old) in the cirrhotic group, and 71 years old (range: 40-84 years old) in the non-cirrhotic group (p = 0.74). Previous IFN-based therapies were performed in 30 patients (61%) in the cirrhotic group and 49 (51%) in the non-cirrhotic group. The cirrhotic group had 14 (29%) patients with curative treatment for HCC, whereas the non-cirrhotic group had 8 (8%) patients with curative treatment for HCC (p < 0.02). There were significant differences in WBC, hemoglobin, platelet counts, and AFP levels at baseline between the cirrhotic and non-cirrhotic groups. The mean HCV-RNA level was 6.1 ± 0.5 log copies/mL in the cirrhotic group and 6.1 ± 0.8 log copies/mL in the non-cirrhotic group (p = 0.31). RASs in the HCV NS3/4A gene were detected in 15 (31%) of 49 patients in the cirrhotic group: Q80L, 2, D168E, 3, and V170I, 15. RASs were detected in 33 (34%) of 96 patients in the non-cirrhotic group: Q80L, 4, Q80R, 2, R155K, 1, D168E, 2, and V170I, 30. No differences in the distribution of the IL28B genotypes were detected between the two groups.
Baseline characteristics of patients with L31 and Y93 in NS5A of HCV genotype 1b infection in cirrhotic and non-cirrhotic groups.
| Cirrhotic group | Non-cirrhotic group | p value | |
|---|---|---|---|
| Number of patients | 49 | 96 | |
| Male/female | 21/28 | 42/54 | 0.79 |
| Median age (range) years old | 71 (48-86) | 71 (40-84) | 0.74 |
| Body weight (kg) | 54.7 (40-95.3) | 55.8 (38.1-94.5) | 0.73 |
| Previous IFN therapy ± | 30/19 | 49/47 | 0.14 |
| Curative treatment of HCC ± | 14/35 | 8/88 | < 0.01 |
| Operation | 4 | 4 | 0.59 |
| RFA | 9 | 3 | |
| TACE | 1 | 1 | |
| IL28B genotype (major/non-major/NT) | 33/15/1 | 58/36/2 | 0.50 |
| HCV RNA (log copies/mL) | 5.9 (3.6-7.5) | 5.8 (3.4-7.1) | 0.31 |
| NS3/4A RAS (±) | |||
| Q80 (wild/L/R) D168 (wild/E) | 47/2/0 46/3 | 90/4/2 94/2 | 0.3 0.34 |
| WBC (/μL) | 3,900 (1,600-8,800) | 5000 (2,300-10,400) | < 0.01 |
| Hb (g/dL) | 12.9 (8.3-16.5) | 13.6 (9.8-16.1) | 0.01 |
| Plt (x104/μL) | 8.7 (3.7-20.1) | 15.4 (7.1-29.2) | < 0.01 |
| T. Bil (mg/dL) | 0.8 (0.3-2.0) | 0.7 (0.2-1.5) | 0.06 |
| Albumin (g/dL) | 3.5 (2.5-4.6) | 3.9 (3.1-4.7) | < 0.01 |
| ALT (IU/L) | 48 (15-149) | 40 (9-231) | 0.21 |
| γ-GTP (IU/L) | 41 (13-199) | 34 (11-752) | 0.16 |
| Creatinine (mg/L) | 0.7 (0.38-1.43) | 0.71 (0.41-1.83) | 0.84 |
| AFP (ng/L) | 10.5 (1-375.7) | 4.8 (1-143.8) | < 0.01 |
IFN: interferon. HCC: hepatocellular carcinoma. RFA: radiofrequency ablation. TACE: trans-catheter arterial chemoembolization. RAVs: resistant associated variants. WBC: white blood cell. Hb: hemoglobin. Plt: platelet. ALT: alanine aminotransferase. γ-GTP: gamma-glutamyl transpeptidase. AFP: alpha protein.
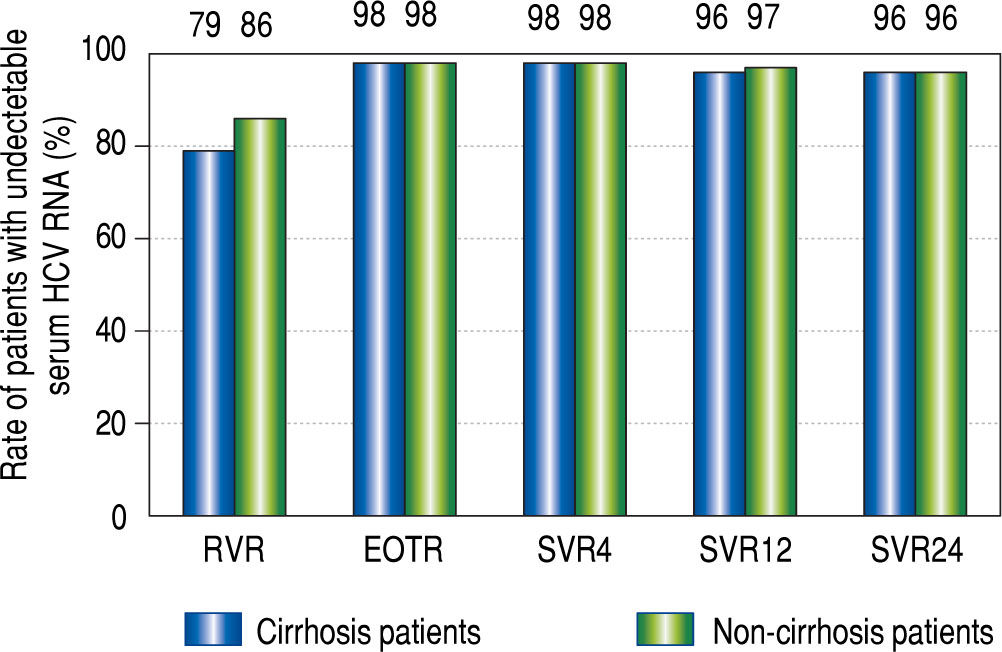
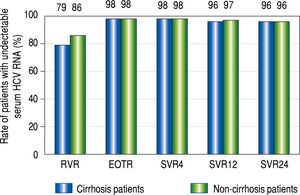
RVR rates were 79% (38/48) in the cirrhotic group and 86% (78/91) in the non-cirrhotic group (p = 0.45). The rates at the EOT were 98% (48/49) in the cirrhotic group and 98% (94/96) in the non-cirrhotic group (p = 0.55). The SVR24 rates were 96% (47/49) in the cirrhotic group and 96% (91/95) in the non-cirrhotic group (p = 0.69). There were no significant differences in the treatment response rate between the two groups (Figure 1). Viral breakthrough occurred in one patient in the cirrhotic group and two patients in the non-cirrhotic group (p = 0.55). At baseline RASs at D168E and V170I in the HCV NS3/4A were detected in two (31%) of three patients with viral breakthrough. Viral relapse occurred in one patient in the cirrhotic group and two patients in the non-cirrhot-ic group (p = 0.55). In three patients with relapse, ASV + DCV therapy was stopped due to adverse effects. At baseline RASs in the HCV NS3/4A were not detected in all of patients with viral relapse.
Rapid virological response (RVR), end-of-treatment response (EOTR), sustained virological response 12 weeks after completing treatment (SVR12), and SVR 24 weeks after completing treatment (SVR24) in cirrhot-ic and non-cirrhotic groups. RVR rates were 79% (38/48) in the cirrhotic group and 86% (78/91) in the non-cirrhotic group. EOT rates were 98% (48/ 49) in the cirrhotic group and 98% (94/96) in the non-cirrhotic group. SVR12 and SVR24 rates were 96% (47/49) and 96% (47/49) in the cirrhotic group, and 97% (92/95) and 96% (91/95) in the non-cirrhotic group, respectively. The rates at all-time points were not significantly different between the cir-rhotic and non-cirrhotic groups.
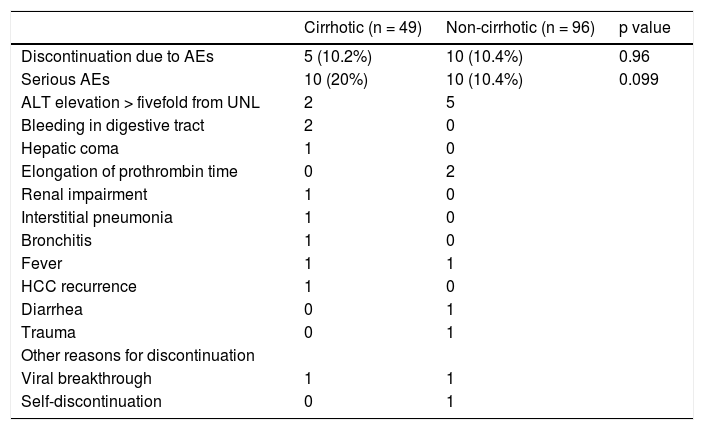
Treatment was discontinued for various reasons in the cirrhotic group, as follows (one patient each): ALT elevation, hepatic coma, institutional pneumonia, severe bronchitis, and HCC development. In the non-cirrhotic group, treatment was discontinued due to ALT elevation in five patients, prothrombin time elongation in two patients, trauma in one patient, and self-discontinuation in one patient.
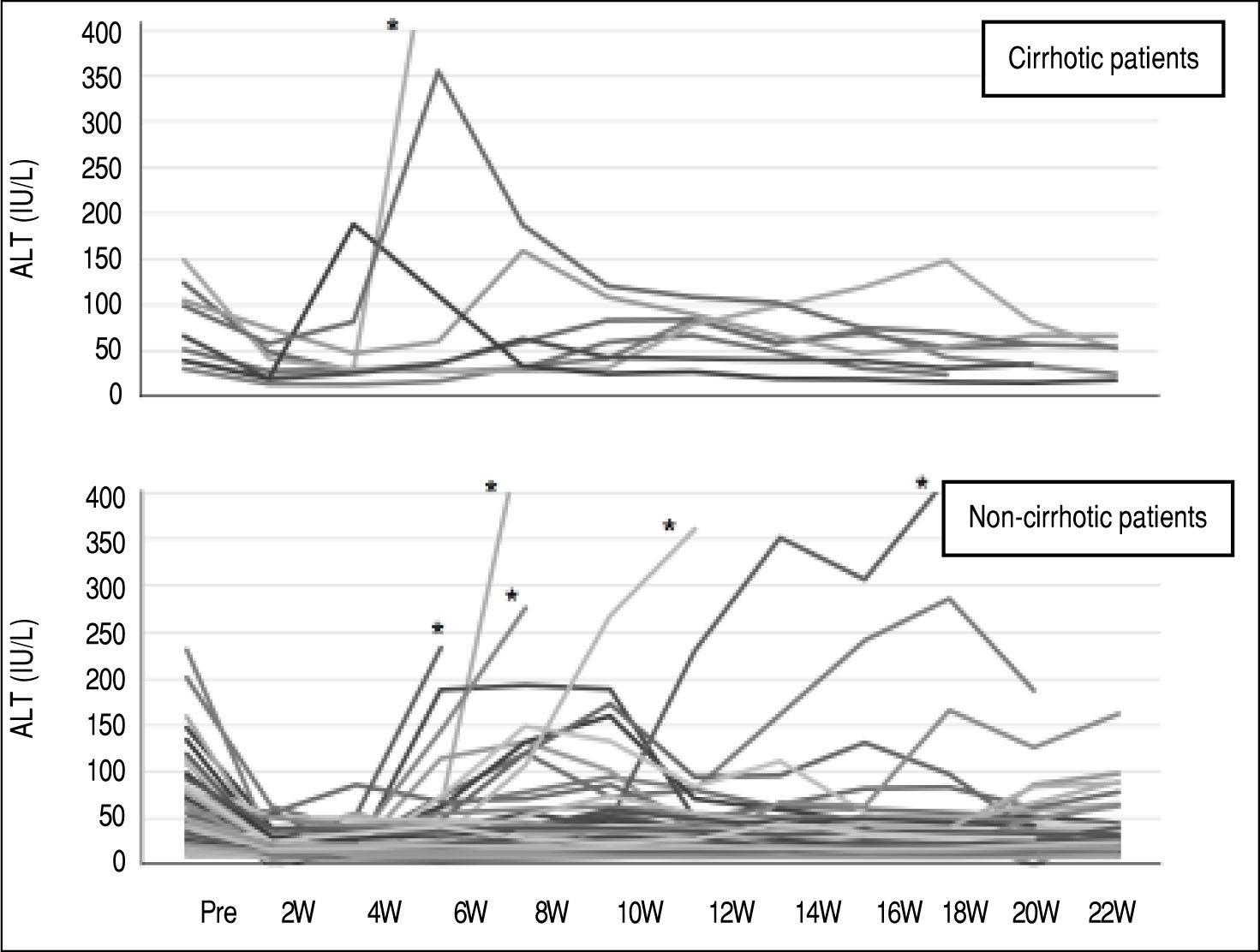
Grade III/IV adverse events occurred more frequently in the cirrhotic group (10/49; 20.4%) than in the non-cir-rhotic group (10/96; 10.4%) (p = 0.099). ALT elevation is an AE of DCV/ASV therapy, particularly in Japanese patients. The ALT level was elevated by more than twice the normal upper limit in 9 of 49 cirrhotic patients and 19 of 96 non-cirrhotic patients. There were no differences in the incidence of ALT elevation between the two groups (Table 2). DAA therapy was stopped in one cirrhotic patient and five non-cirrhotic patients due to ALT elevation (Figure 2). One non-cirrhotic patient died after ASV + DCV therapy due to events that were not related to the treatment.
Adverse events with and without treatment discontinuation in cirrhotic and non-cirrhotic groups.
| Cirrhotic (n = 49) | Non-cirrhotic (n = 96) | p value | |
|---|---|---|---|
| Discontinuation due to AEs | 5 (10.2%) | 10 (10.4%) | 0.96 |
| Serious AEs | 10 (20%) | 10 (10.4%) | 0.099 |
| ALT elevation > fivefold from UNL | 2 | 5 | |
| Bleeding in digestive tract | 2 | 0 | |
| Hepatic coma | 1 | 0 | |
| Elongation of prothrombin time | 0 | 2 | |
| Renal impairment | 1 | 0 | |
| Interstitial pneumonia | 1 | 0 | |
| Bronchitis | 1 | 0 | |
| Fever | 1 | 1 | |
| HCC recurrence | 1 | 0 | |
| Diarrhea | 0 | 1 | |
| Trauma | 0 | 1 | |
| Other reasons for discontinuation | |||
| Viral breakthrough | 1 | 1 | |
| Self-discontinuation | 0 | 1 |
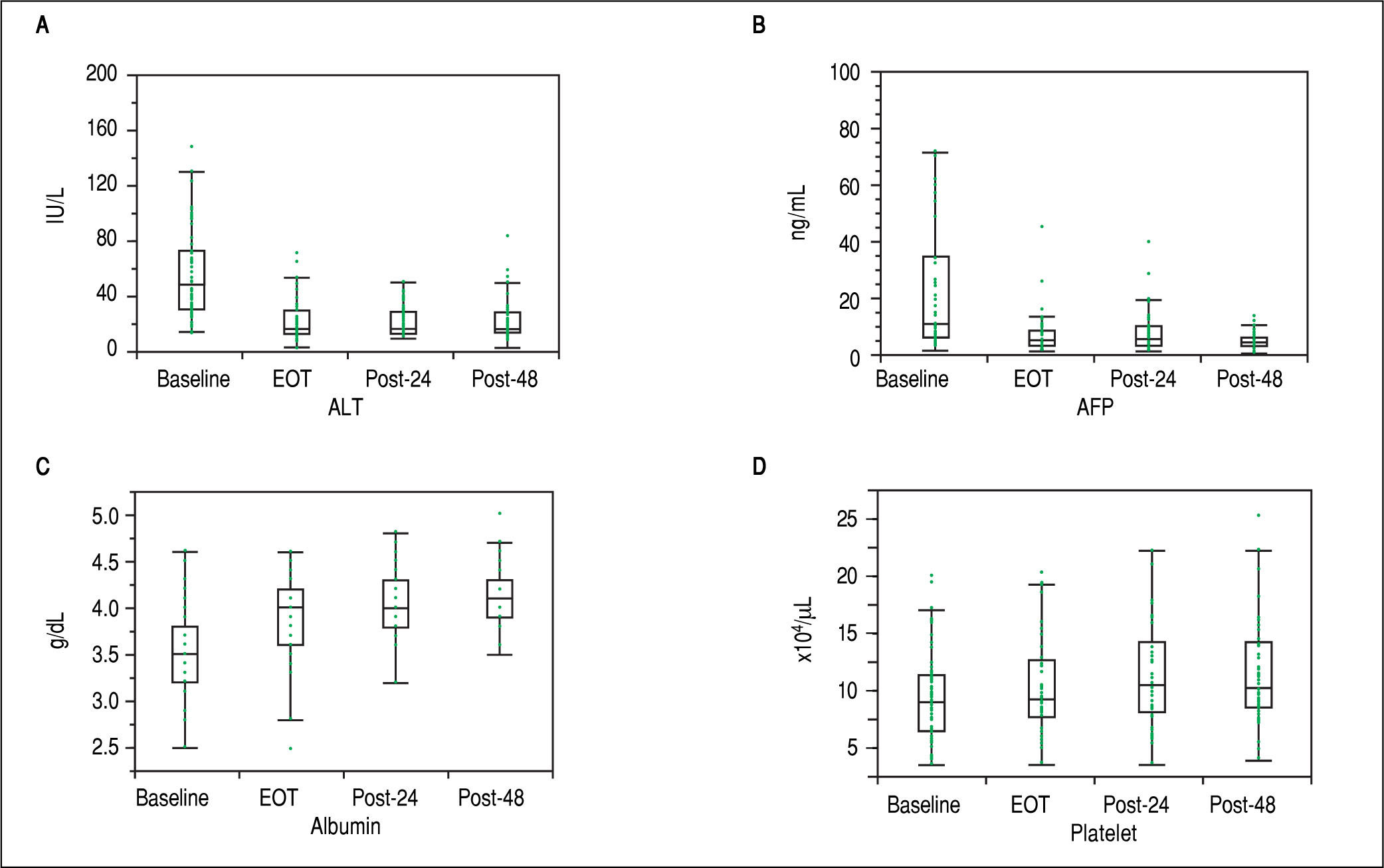
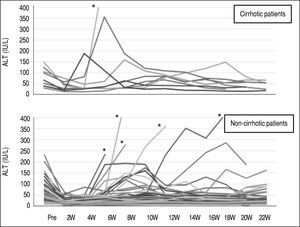
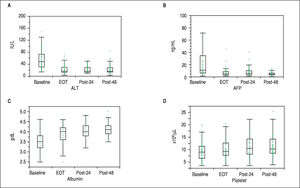
First, liver biochemical markers were evaluated in 47 cirrhotic patients that achieved SVR. Compared to the baseline, ALT and AFP levels were significantly decreased at EOT, post-24th week, and post-48th week (p < 0.01). In addition, albumin levels were gradually increased after ASV + DCV therapy (Figure 3).
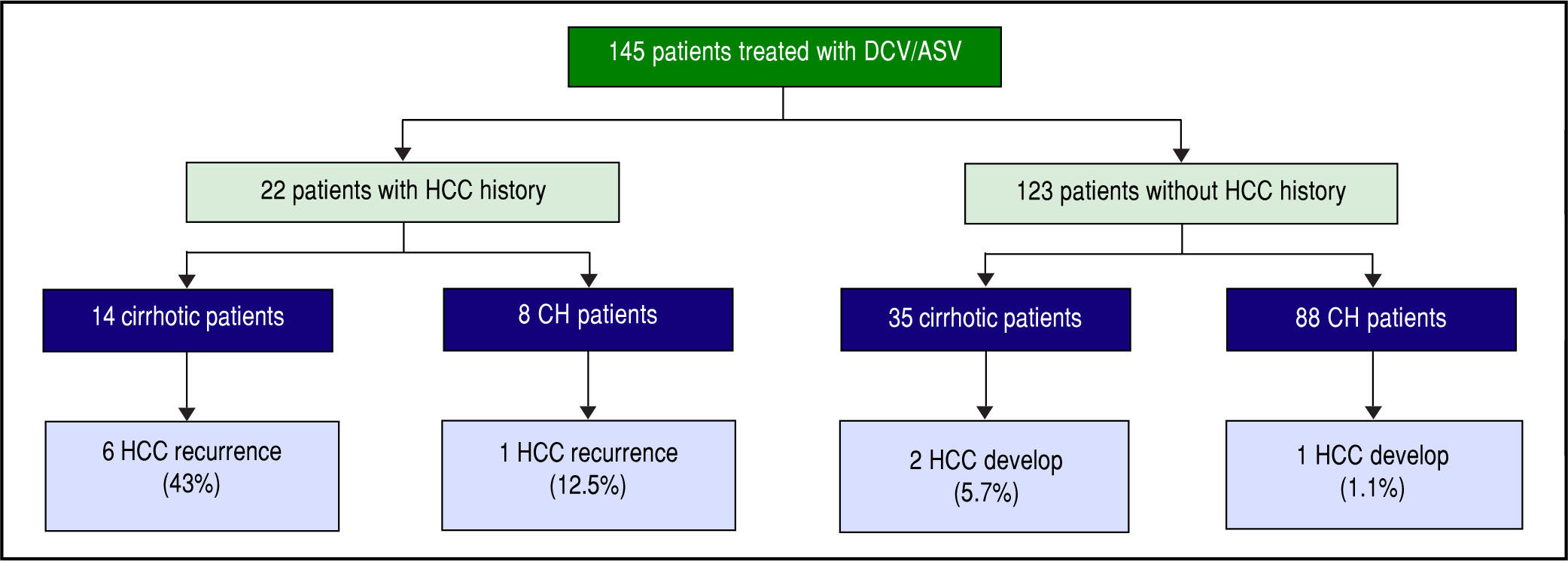
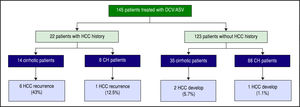
In all, 22 patients had received curative treatment for HCC before ASV + DCV therapy. After confirming the lack of HCC recurrence by contrast computed tomography or magnetic resonance imaging, ASV + DCV therapy was started. Over 48 weeks from the end of anti-HCV therapy, recurrence of HCC was observed in 6 (43%) of 14 cirrhotic, and 1 (12.5%) of 8 non-cirrhotic patients. On the other hand, HCC developed in 2 (5.7%) of 35 cirrhot-ic, and 1 (1.2%) of 88 non-cirrhotic patients (Figure 4). HCC recurrence and occurrence were observed more frequently in the cirrhotic group than in the non-cirrhotic group.
HCC recurrence and development during 1-year follow-up from the end of treatment. HCC recurred in 6 (43%) of 14 cirrhotic and 1 (12.5%) of 8 non-cirrhotic patients that had previous curative HCC therapy. HCC developed in 2 (7.4%) of 27 cirrhotic and 1 (1%) of 96 non-cirrhotic patients.
In the present study, 96% of cirrhotic and non-cirrhotic patients without RAS in NS5A achieved SVR after ASV + DCV therapy. RAS-oriented ASV + DCV therapy was also reasonable for HCV genotype 1b patients with cirrhosis. Previous reports have indicated SVR rates of 88.3-93.7% in patients without RAS in NS5A.15-17 The anti-viral effect observed in our study was slightly higher than those results. In our study, 76% patients started treatment at admission, and were instructed regarding DAA by a hospital pharmacist. Only 2 (1.7%) of 115 patients did not achieve SVR in the admitted group, and 4 (11%) of 35 patients without admission failed to achieve SVR (p = 0.039). These results suggest that admission with commencement of DAA be recommended, particularly in Japan.
On the other hand, AEs (adverse events) of grade III/IV occurred more frequently in cirrhotic patients than in non-cirrhotic patients during treatment. Elevation of ALT was a unique AE associated with ASV + DCV therapy. There were no differences in the incidence of serious ALT elevation between cirrhotic and non-cirrhotic groups. Bleeding in the digestive tract and hepatic coma were serious AEs seen only in the cirrhotic group. Compared to the Japanese phase III trial, there were some differences in AEs in the present study. These differences may have been due to the characteristics of the enrolled patients in terms of their age, gender, cirrhosis status, and history of HCC. Taken together, these results suggest that careful management is necessary for cirrhotic patients before and during ASV + DCV therapy.
A follow-up study showed that ALT levels and AFP levels decreased significantly after EOT in cirrhotic patients that achieved SVR. In addition, serum levels of albumin and platelet count significantly increased compared to baseline measurements. Our data are consistent with previous reports. Miyaki, et al. showed that serum fibrosis markers are improved in SVR patients.18 Mandorfer, et al. reported that DAA therapy decreases portal pressure in SVR patients with moderate hepatic fibrosis as determined by measuring hepatic venous pressure gradient.19 These data suggest that the elimination of HCV by DAA therapy improved liver function in cirrhotic patients.
On the other hand, the incidence rates of HCC development and recurrence were higher in cirrhotic patients. Conti, et al. reported that HCC was detected during 24-weeks of follow-up in 17 (29%) of 59 patients with previous HCC and 9 (3%) of 285 cirrhotic patients without HCC.20 Our observations are in line with those of other previous studies.21 Both the observation period and the incidence of HCC in our study were double those in these previous studies. Previous Japanese studies of IFN-based therapy showed that age and fibrosis at baseline were closely related to HCC development in SVR patients.3,22 Although the reasons for the high incidence of HCC development in patients treated with DAA are not clear, greater age and more-advanced fibrosis might be related to the development of HCC.
IFN-based therapy without DAA showed a preventive effect on the development of HCC in patients with HCV, even in those who did not achieve SVR. It was speculated that IFN had two effects, i.e., antivirus and antitumor effects. On the other hand, DAAs were designed as specific inhibitors of HCV and did not have direct antitumor sup-pressive effects. Minami, et al. compared the rate of HCC development between a DAA treatment group, an IFN-based therapy group, and a control group. The rate of early recurrence was not different between the three groups.23 It is important to monitor cirrhotic patients that achieve SVR with DAA therapy.
The present study had several limitations. First, the number of patients enrolled in the study was low, although the number was greater than in the phase III clinical trial. In September 2015, a 12-week IFN-free regimen was approved for HCV genotype 1 in Japan.24,25 It is difficult to implement a study in a larger cohort because patients prefer therapies with shorter durations. Second, there were no decompensated cirrhotic patients in the present study, as anti-HCV therapy is not approved for such patients in Japan. Finally, the follow-up period was insufficient to evaluate prognoses in cirrhotic patients with SVR. SVR is an important process but is not the goal of treatment in cases of chronic viral liver disease. It is necessary to evaluate long-term prognoses in cirrhotic patients with HCV, including the prevention of HCC development and the prevention of hepatic failure.
Taken together, our findings suggest that ASV + DCV therapy can achieve a high SVR rate in Japanese cirrhotic patients with NS5A wild-type HCV genotype 1b and also improve liver function, including albumin and AFP levels. However, AEs occur frequently in cirrhotic patients, particularly the recurrence and occurrence of HCC after therapy.
AcknowledgmentsThis study was supported in a part by Japan Society for the Promotion of Science grant 15k09019 (AT). We would like to thank Ms. Yoko Yasuhara, Ms. Tomoko Hirano and Ms. Sanae Deguchi for collecting the data.
Abbreviations- •
AEs: adverse events.
- •
ALT: alanine aminotransferase.
- •
EOTR: end of treatment response.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
IL28B: interleukin 28 B.
- •
RAS: resistance-associated substitution.
- •
RVR: rapid virological responders.
- •
SVR: sustained viral response.
- •
UNL: upper normal limit.
All authors declare that we have nothing to disclose regarding funding or conflict of interest with respect to the study reported in this manuscript.