The most practical screening test for hepatitis C virus antibodies are second and third-generation enzyme immunoassays. We evaluated the usefulness of the third generation microparticle enzyme immunoassay (MEIA) in predicting HCV viraemia in anti-HCV positive patients. Serum samples from 106 patients with positive anti-HCV were obtained. To evaluate the diagnostic value of the MEIA test in predicting HCV viraemia, anti-HCV positive patients were categorized in two groups according to the presence or absence of serum HCV-RNA. Among the 106 patients, 26 had non detectable serum HCV-RNA and 80 had detectable HCV-RNA by PCR. The assay automatically calculates a result based on the ratio of sample rate to the cut-of rate for each sample and control (S/CO). When the means of S/CO values for patients with detectable and non detectable HCV-RNA were analyzed, a statistically significant difference was found, (79.3 SD 22.2 vs. 8.2 SD 6.4, respectively) (p 0.0001). We further analyzed the best cut-off value of the S/CO in differentiating viremic from non viremic patients. The S/CO value of 26 showed a sensitivity of 99% and a specificity off 96% in discriminating both categories of HCV infected patients. In conclusion, our data demonstrate that viremic HCV patients had higher S/CO values in the MEIA test in comparison with non viremic patients. Hence, this assay may be used to predict HCV viraemia in anti-HCV positive individuals.
Hepatitis C is a major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma world-wide.1 Only a minority of infected people (less than 30%) resolves acute infection, with most developing chronic infection.2
Diagnosis of HCV infection can be established readily by sensitive and specific serological assays which incorporates a mixture of viral polypeptides on the solid phase.3 These antigens include both structural proteins, such as the putative nucleocapside protein, and non-structural region polypeptides such as NS3, NS4, and NS5.
Although successful specific antibody response, the presence of antibodies directed against most viral antigens hardly differentiates between patients who developed spontaneous clearance of the virus from those with persistent infection. The gold standard for the accurate evaluation of these two possibilities is by the use of molecular detection of RNA of the virus.
However, training of quality-control measures technologies as well as proficient testing are necessary to ensure accuracy.4,5 Furthermore, polymerase chain reaction is not easily available in clinical laboratories.
Recent report have suggested that it is possible to evaluate antiviral response in HCV treated patients, using a third generation anti-HCV assay.6
In the present study, we aimed to determine the usefulness of the above mentioned test in predicting HCV viraemia in anti-HCV positive patients, since we have previously observed that the ratio of the sample rate to the cut-off rate (S/CO) of third generation anti-HCV assay was substantially different when we compare viremic with non viremic patients.
Patients and methodsBetween January 2000 to January 2001, 106 anti-HCV positive patients confirmed by a third-generation supplemental test, were included in this study.
Patients were evaluated at the Liver Unit of the Argerich County Hospital, and were 57 male and 49 female, mean age 43 years, range 21 to 68.
No patient had a previous history of autoimmune disease, alcohol intake, current intravenous drug use or other chronic liver disease. All patients were negative for hepatitis B surface antigen and anti-HIV and patients with immunodeficiency conditions were excluded from the study.
Serum was assayed for anti-HCV using HCV version 3.0, AxSYM® (Abbott Diagnostics, Chicago, IL, USA). The third generation microparticle enzyme immunoassay (MEIA), contains four recombinant proteins: HCr43, a fusion protein consisting of parts of the structural core region and the NS3 region; c200, containing parts of the NS3 and NS4 regions; c1003, containing a shorter sequence of the NS3 region and the same part of the NS4 region; and parts of the NS5 region.
The assay 3.0 automatically calculates a result based on the ratio of the sample rate to the cut-off rate for each sample and control (S/CO). In the anti-HCV test, an S/CO equal to or grader than 1.00 is considered reactive.
Anti-HCV reactivity was confirmed by an independent assay in all samples by a line immunoassay, LIA TEK III®, Organon Teknika, according to the manufacturer’s instructions.
Detection of serum HCV-RNA was performed by a home made reverse-transcription polymerase chain reaction (RT-PCR) in all the samples in at least two different samples using primers from the 5’-non coding region of the HCV genome.
Total RNA was extracted by the acid guanidium-phenol-chloroform method as previously described.7 Briefly, 150 μl serum were mixed with 500 μL of denaturing solution (4 M guanidium thiocyanate, 25 mM sodium citrate pH 7, 0.5% sarcosyl, 0.1M 2-mercaptoethanol, 50 μl of 2 M NaAc (pH 4.0), 500 μl of phenol and 100 μl of chloroform. After centrifugation, aqueous phase was recovered and precipitated over night at -20°C with 650 μL of isopropanol and 20 μg Dextran T500. The resulting pellet was washed with 70% ethanol and re-suspended in 9 μL of water. RNA obtained was denatured at 78 °C for 5 min and primed with 0.4 μg of random hexamers. Reverse transcription conditions were: 50 mM TrisHCl (pH 8.3), 25 mM KCl, 3 mM MgCl2), 0.1 mM DTT, 1 mM dNTPs, 18 U of ribonuclease inhibitor (Promega) and 100 U of M-MLV reverse transcriptase (Gibco), reaction was performed for 90 min at 37 °C. After heat inactivation at 95°C for 5 min and chilling on ice, the cDNA was amplified. The 50 ul PCR reaction contained: 20 mM TrisHCl, 50 mM KCl, 50 pmoles of each primer for the 5UT region of HCV genome, 5UT1 (5’ CCTGTGAGGAACTACT-GTCTTCACGC 3’) and 5UT2 (5’ AGGTCTCGTAGA CCGTGCACC 3’) and 1.25 U of Taq. The PCR reaction consisted of 40 cycles each with denaturing at 94°C for 30 sec, annealing at 55°C 30 sec, and polymerization at 72°C for 45 sec. Nested PCR was done with 2 μL of PCR product as template, using internal primers 5UT3 (5’ TCT AGC CAT GGC GTT AGT GCG AGT GT 3) and 5UT4 (5’ CAC TCG CAA GCA CCC TAT CAG GCA GT 3), in the same conditions of the first round. PCR products were analyzed by ultraviolet fluorescence after ethidium bromide staining.
The lower limit of HCV-RNA detection was 200 genome copies/mL.
To evaluate the diagnostic value of the MEIA test in predicting HCV viraemia, anti-HCV positive patients were categorized in two groups according to presence or absence of serum HCV-RNA.
S/CO values were analyzed in each group; sensitivity, specificity, positive and negative predictive values of different S/CO values in detecting viremic patients were calculated.
Receiver-operating characteristic curves, in which the sensitivity is plotted against the false-positive rate (1-the value of specificity) was generated to evaluate the best cut-off point of the S/CO value of the assay.8
All patients provided informed consent, and the study was approved by the Institutional review board of our Hospital.
Statistic analysisResults were expressed as mean + standard deviation. A p value < 0.05 was considered statistically significant. Sensitivity, specificity, positive and negative predictive values were calculated for different values of S/CO of the MEIA test. Receiver operating characteristic (ROC) curve was performed for S/CO values. The area under the ROC curve and its standard error was calculated using the non parametric method.8 Student’s T test was used for comparisons.
ResultsAmong the 106 patients, 26 had non detectable serum HCV-RNA and 80 had detectable HCV-RNA by PCR.
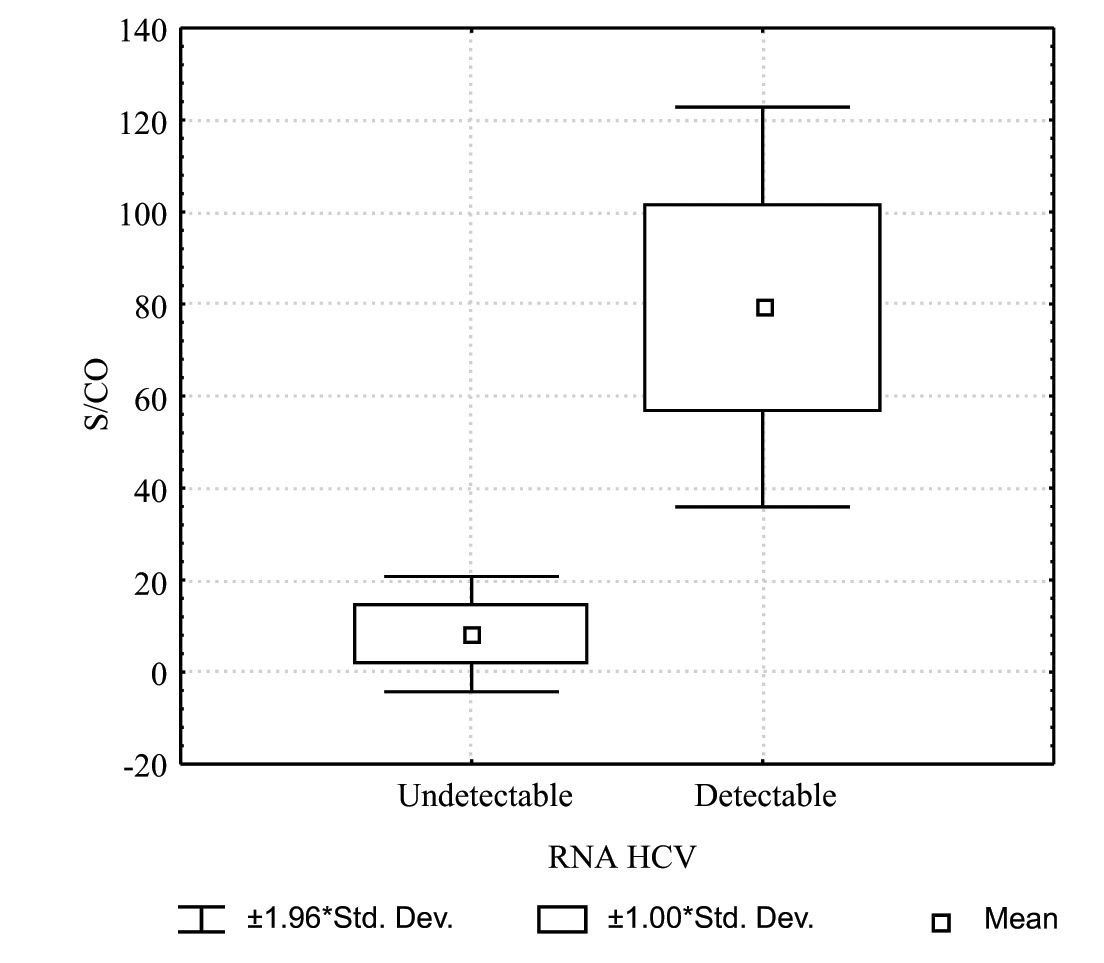
When the means of S/CO values for patients with detectable and non detectable HCV-RNA were analyzed, a statistically significant difference was found, (79.3 SD 22.2 vs 8.2 SD 6.4, respectively) (p 0.0001). Figure 1 shows S/CO values according to the HCV-RNA status.
We further analyzed the best cut-off value of the S/CO in differentiating viremic from non viremic patients. The S/CO value of 26 showed a sensitivity of 99% and a specificity of 96% in discriminating both categories of HCV infected patients (Table I).
Ratio of sample rate to the cut-off (S/CO) value in predicting HCV viraemia in anti-HCV positive patients.
| Detectable HCV-RNA | Non detectable HCV-RNA | |
|---|---|---|
| S/CO value > or equal 26 | 79 | 1 |
| S/CO value < or equal 25 | 1 | 25 |
| Sensitivity | 99 % | |
| Specificity | 96 % | |
| Positive predictive value | 99 % | |
| Negative predictive value | 96 % |
Receiver operating characteristic (ROC) curves demonstrates the relationship between true positive ratio (sensitivity) and false positive ratio (1-specificity) as one modifies the definition of a positive test.
While there are several reasons for examining ROC curves, in this study we have used ROC curves to discriminate sensitivity and specificity for different S/CO values (Figure 2) and to compare the area under the two curves: one plotting different S/CO values and the other plotting the presence of HCV-RNA. As the presence of HCV-RNA represents the gold standard, the area under its curve was 1 and the standard error was 0. The area under the curve of S/CO values was 0.9991 and the standard error was 0.0013. No significant difference was found between the areas under the two curves.
DiscussionThe most practical screening test for hepatitis C virus antibodies are second and third-generation enzyme immunoassays.3 The specificity of currently available EIAs for anti-HCV antibodies is higher than 99 percent. However, a positive result does not differentiate between viremic and non viremic patients, thus patients should be tested for HCV RNA by PCR to confirm viraemia.
In this study, we evaluated the performance of different MEIA S/CO values in the identification of viremic from non viremic anti-HCV positive patients. Our data demonstrate that viremic HCV patients had higher S/CO values in the MEIA test in comparison with non viremic patients. Hence, this assay may be used to predict HCV viraemia in anti-HCV positive individuals.
Immunoassay tests analyze diseases and other medical conditions by measuring the body’s antigen/antibody reaction.
Antibodies against HCV has been reported to be present in the serum of patients with chronic hepatitis as well as in the serum of patients who have cleared the virus.9 However, evidence that titre of anti-HCV antibodies decrease in subjects with spontaneous resolution of the infection comes from several studies,10,11 suggesting that the presence of HCV antigens plays an important role in the maintenance of a continuos antigenic stimulation of the humoral response.
On the other hand, Baumert et al., recently described that chronic HCV patients who successfully cleared the virus after interferon therapy, had a gradual decline of anti-HCV titres.12 Additionally, Tung et al., reported that they can differentiate sustained virological responders from non responders to antiviral therapy using the S/CO of the MEIA test, since it decreases significantly in responders patients.6
In conclusion, by establishing 26 as cut-off value of the S/CO in the third generation anti-HCV assay, it is possible to differentiate viremic from non viremic patients.
This assay has the advantages of the enzyme immunoassay - it is simple to use, allows to process a variety of immune diagnostic tests simultaneously at conventionally settings, has low variability and relatively low expense (3)-, and subsequently, may predict HCV viraemia. In this regard, clinicians may be informed not only about the antibody existence against HCV, but also they may infer that patients having a high S/CO value in the MEIA test may be viremic.
However, the gold standard for detecting HCV viraemia is by qualitative polymerase chain reaction, which is indeed recommended by all the guidelines.