Systemic treatment for hepatocellular carcinoma (HCC) is recommended for patients with advanced stage and for those who progressed on locoregional modalities. The first agent approved for advanced HCC was sorafenib, and it remains one of the cornerstones of systemic treatment. In the past years, immunotherapy has shown promising results and has been incorporated into the treatment armamentarium. The rates of recurrence and progression after locoregional therapies are significant, what highlights the need to explore systemic agents for preventing or delaying these negative outcomes. Recently, sorafenib was shown to benefit patients with unresectable HCC under transarterial chemoembolization (TACE) by delaying tumor progression and prolonging time to vascular invasion and extrahepatic spread. Although this result was reported in patients with intermediate stage, it provides background to test the strategy of combining systemic treatment plus TACE as a bridge therapy to HCC patients awaiting liver transplantation, for which the risk of dropout due to tumor progression impairs the possibility of cure.
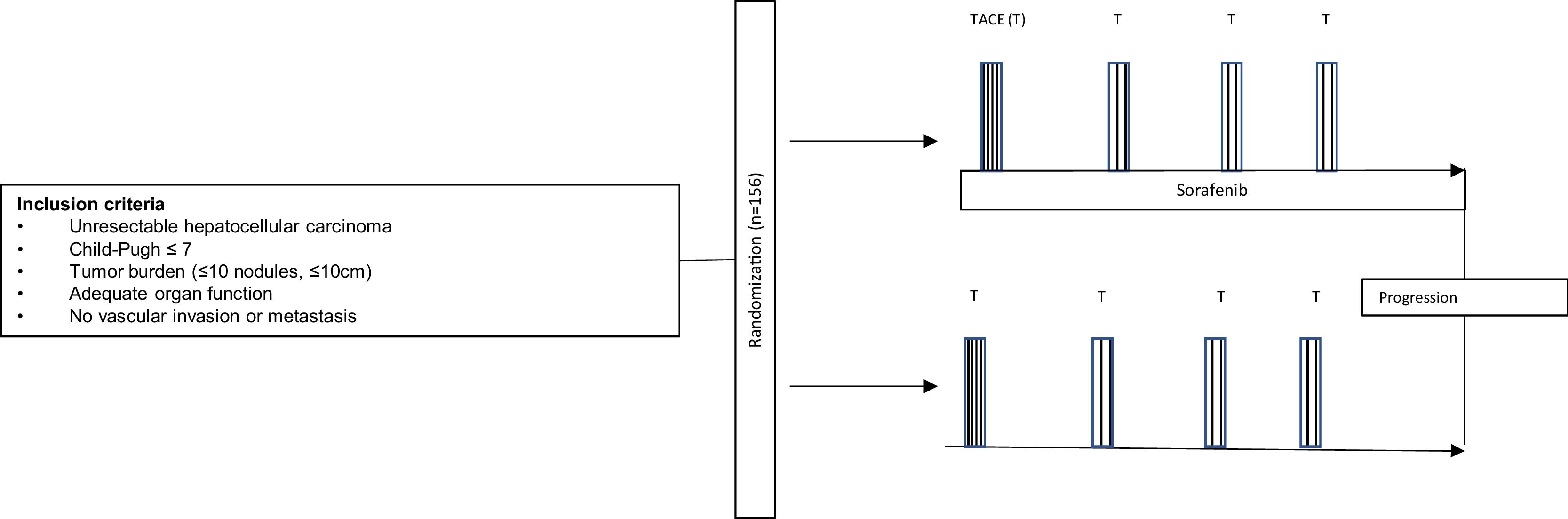
In a time when tyrosine kinase inhibitors seem to be drifted out of the landscape of hepatocellular carcinoma (HCC) by immune-oncology drugs, Kudo et al. [1] reported interesting data on the use of sorafenib plus transarterial chemoembolization (TACE) in TACTICS trial, published in Gut 2019. This was the first after a handful of negative studies [2–4] that finally showed benefit by the addition of sorafenib to TACE in HCC. The study was designed to randomize patients with unresectable HCC to TACE alone or TACE plus sorafenib (400mg daily for 2–3 weeks before TACE, followed by 800mg daily during on-demand conventional TACE) until disease progression (Fig. 1). Progression was defined as intrahepatic progression (25% increase vs baseline), deterioration of liver function, macrovascular invasion (MVI) or extrahepatic spread (EHS). After a median follow-up of 122.3 weeks and 156 patients randomized, the combination of TACE plus sorafenib was associated with a significant improvement in progression-free survival, although overall survival (OS) was not analyzed due to insufficient number of events.
If TACTICS does not reach survival significance at longer follow-up, it would be reasonable to conclude that sorafenib is able to provide a longer progression-free survival after TACE, but postponing sorafenib until progression is detected does not affect OS, as long as liver function and performance status are preserved. This could limit the adoption of sorafenib plus TACE according to TACTICS design as a routine. The recommendation to introduce sorafenib only at TACE-refractoriness or extrahepatic progression is well-established according to phase III trials (for i.e., SHARP and Asia-Pacific trial [5,6]) and is the current practice worldwide [7,8]. As a result, TACTICS has a low potential of being practice-changing.
Nevertheless, let us move and explore this data within another context: it is well known that liver transplantation offers the best chance of long-term survival in patients with unresectable HCC within Milan criteria. However, due to unpredictable waiting time for an organ and fear of tumor progression, the majority of patients receive some form of locoregional therapy while awaiting transplant. TACE is the most used modality in this scenario worldwide [9–11].
The goal of TACE as a bridge to transplant is to minimize the risk of dropout due to tumor progression, which includes EHS, MVI or intrahepatic progression beyond predefined criteria. The rate of dropout due to progression is reported to be around 4–23%[12,13], but it can be even higher in regions with shortage of organs, long waiting list and heterogeneous health structure.
The TACTICS trial reported that adding sorafenib to TACE provided an impressive progression-free survival improvement (25.2 vs 13.5 months; HR 0.59; CI95% 0.41–0.87). Additionally, there were improvements in MVI-free survival (31.3 vs 4.0 months; HR=0.26; 95%CI 0.09–0.75) and EHS-free survival (15.7 vs 6.9 months; HR=0.21; 95%CI 0.006–0.70) (1). EHS and MVI not only mean the dropout of the waiting list, but also that the opportunity of cure through liver translation (of both the tumor and the underlying hepatopathy) was missed. In patients with MVI or EHS, the prognosis is poor and systemic options are limited [14]. In this sense, MVI and EHS represent an unfavorable turnaround in the clinical course of HCC patients.
The TACTICS trial was not designed for evaluating patients who are candidates for liver transplantation, but it may open a different opportunity niche in HCC. Delaying the time to MVI and EHS sufficiently to ensure transplantation can theoretically reduce dropouts and improve cure rates. There are no prospective trials approaching the best bridging therapy, but TACTICS can ground a trial design to address this issue prospectively. In the real practice, adding sorafenib to TACE could be reasonably applied in sites with anticipated long waiting lists, in which patients are under prolonged risk of progression. Sorafenib is safe and most physicians involved in HCC care have experience in managing this agent. However, safety should be analyzed prospectively in order to determine if the antiangiogenic activity of sorafenib would impact on early post-transplant morbidity, arterial anastomosis complications or ischemic cholangitis[15].
Beyond the primary result, TACTICS trial can hand physicians with an alternative to improve outcomes in patients awaiting transplantation and support investigators with a background to keep exploring the use of sorafenib in HCC before it gets drowned out by the novel systemic agents for HCC.AbbreviationsHCC hepatocellular carcinoma transarterial chemoebolization macrovascular invasion extrahepatic spread overall survival
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.