Whether there is gender disparity in the recurrence of hepatocellular carcinoma (HCC) has been not fully addressed. This study aimed to investigate the impact of gender on HCC recurrence following curative hepatectomy.
Patients and methodsThis retrospective cohort study included 1087 patients with HCC (917 males, 170 females) who underwent curative hepatectomy. Cox regression models were constructed to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the risk parameters associated with HCC recurrence. In the sensitivity analysis, subgroup analysis, and propensity score matching (PSM) analysis were used. Logistic regression models were used to assess the odds ratio (OR) and 95% CI of the risk parameters related to early and late recurrence.
ResultsMale patients showed significantly higher risk for HCC recurrence than females, in both multivariate Cox regression analysis (HR [95% CI] = 1.480 [1.084–2.020], P = 0.014) and PSM analysis (HR [95% CI] = 1.589 [1.093–2.312], P = 0.015). Higher risk of HCC recurrence was again found in males in the subgroup analysis, but the effect of male versus female gender on HCC recurrence did not depend on any selected subgroups (all P for interaction > 0.05). Gender was an independent risk factor for early recurrence (OR [95% CI] = 1.864 [1.215–2.936], P = 0.006), but not for late recurrence.
ConclusionsThere is gender disparity in the recurrence of patients with HCC after curative hepatectomy: males had a higher risk for HCC recurrence than females.
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide and the third leading cause of tumor-related death [1]. It is believed to have various causes, such as hepatitis B virus and hepatitis C virus infection, and alcohol intake [2,3]. Hepatectomy provides the best opportunity for cure and offers great survival benefits for HCC. It is now considered the main treatment option in patients with HCC with a non-cirrhotic liver or selected patients with HCC with mild cirrhosis and preserved liver function. Unfortunately, tumor recurrence occurs in approximately 70% of cases within five years after curative hepatectomy [4]. Most studies advocate that recurrence within 2 years after hepatectomy is early recurrence (ER), while that occurring after more than 2 years is late recurrence (LR) [5]. According to reports, ER is often associated with the risk factors that are related to the tumor itself, while LR is more dependent on risk factors that are related to the background condition of the liver [6]. It is important to identify groups at high risk for recurrent HCC, which may help to develop a personalized follow-up strategy.
It is well documented that there is gender disparity in HCC morbidity and survival outcome: females have been reported to have a lower risk of suffering from HCC than males, with an approximate incidence rate ratio of 1:4 [7,8]. According to previous studies, the disparity in HCC morbidity and survival outcome by gender primarily relates to differences in the effects of hormonal, genetic, anatomical, and environmental factors and the differential levels of exposure to behavioral risk factors [9]. Higher HCC morbidity is found in postmenopausal females, and the use of oral contraceptive pills has been identified to be an independent favorable indicator for survival in female patients after curative treatment of HCC [10,11], revealing a protective effect of estrogen on HCC development. Experimental animal studies have also observed a protective effect of estrogen, which mediated the inhibition of interleukin-6 produced by Kupffer cells and subsequently reduced HCC risk [12]. Better overall survival and less risk for recurrence were found in female patients after curative treatment of HCC [11,13], and a lead-time bias in the diagnosis of HCC in favor of females has been suggested to account for this survival difference [8] because female patients less often had multiple tumors, vascular invasion, and larger tumors [13].
In addition to the differences in hormone levels and tumor-related parameters, males are more likely to develop behavioral risk habits such as cigarette smoking and alcohol intaking [14], which may influence the survival outcome of patients with HCC. Unfortunately, few studies have adequately controlled the balance of some parameters between males and females when exploring superiority of survival, such as cigarette smoking and alcohol intaking; in that condition, the male and female groups were not exactly comparable. In subgroup analysis, previous research has found that male patients experience worse survival in early-stage HCC but failed to demonstrate the same in late-stage HCC [15]. Although subgroup analyses have been reported in most previous studies, few have demonstrated an interaction effect between gender and their selected subgroups. Thus, the issue of whether there is gender disparity in HCC recurrence is still not fully addressed. We aimed to investigate the relationship between gender and HCC recurrence after curative liver hepatectomy.
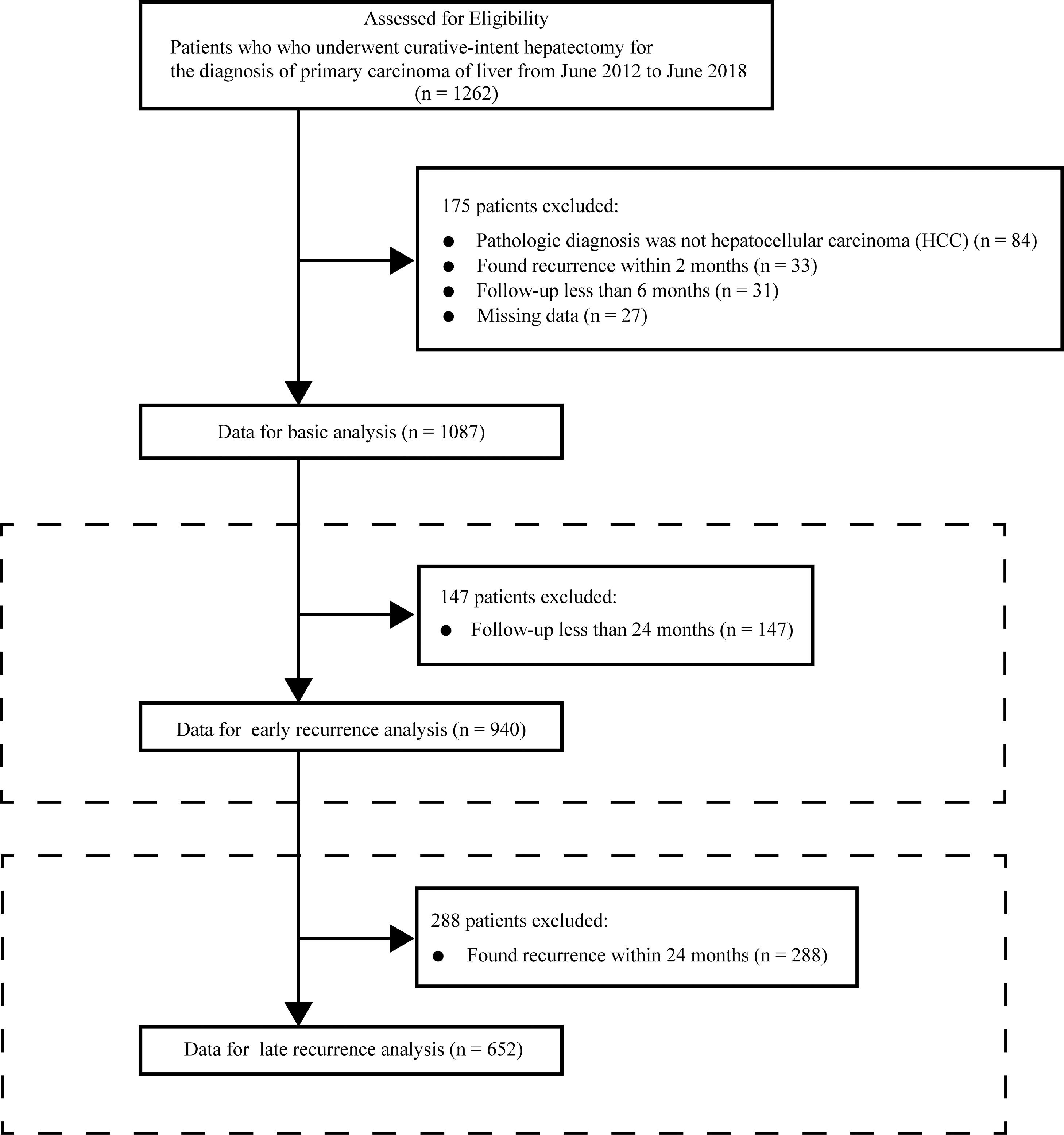
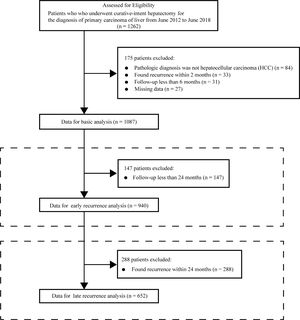
2Materials and methods2.1ParticipantsThis was a retrospective cohort study. The cohort comprised all consecutive patients in Guangxi Medical University who received their first curative-intention hepatectomy for the diagnosis of primary hepatic carcinom between June 2012 and June 2018. Patients who met the exclusion criteria were removed from the data analysis cohort. Patients whose pathological diagnosis on the resected tissue after hepatectomy was not HCC were excluded. Patients with apparent recurrence on imaging examination within 2 months were thought to have not received a curative hepatectomy (Chinese standardization for diagnosis and treatment of primary hepatic carcinom 2019 edition) and were also excluded. Patients who were finally selected for data analysis were followed up for at least 6 months and without missing data. The criteria for selecting the participants are shown in Fig. 1.
2.2HepatectomyAll patients who underwent hepatectomy were thoroughly evaluated before surgery, including <15% in indocyanine green retention rate at 15 min, residual liver volume of more than 40% of standard liver volume, and assessing surgical relative contraindications (age, heart function, lung function, and kidney function). The scope of hepatectomy was planned and determined by preoperative imaging and intraoperative ultrasound. Hepatectomy was accomplished by experienced hepatic surgeons. After the operation, the abdominal cavity was lavaged with sterile water, and an abdominal drainage tube was placed.
2.3ParametersWe took measurements for baseline clinicopathological data and prognostic parameters related to HCC recurrence, including parameters related to liver function (albumin (Alb), alanine aminotransferase (Alt), cirrhosis, Child-Pugh grade, etc.), parameters related to the tumor itself (alpha-fetoprotein level (AFP level), tumor diameter, tumor node, macrovascular invasion, Barcelona Clinic Liver Cancer staging (BCLC staging), etc.), and parameters related to hepatectomy (operation duration, blood loss, major resection, etc.).
2.4Follow-up and outcomeAll patients were followed up after hepatectomy every three months in the first year, and every six months thereafter, with physical examination, serum AFP concentration assay, liver biochemistry tests, and abdominal imaging examination during each visit. The primary outcome was HCC recurrence and the secondary outcome was all-cause death, which was compared between the male and female groups. HCC recurrence was diagnosed by radiologic evidence of a new tumor on contrast-enhanced computed tomography or magnetic resonance imaging showing arterial phase hyperenhancement and wash out appearance in the portal venous phase. Recurrence-free survival (RFS) was calculated from the date of hepatectomy to the date of the first diagnosis of recurrence after hepatectomy, or the date of the last follow-up. Overall survival (OS) was calculated from the date of hepatectomy to the date of all-cause death or the date of the last follow-up. We did not conduct competing risk analyses in the present study because there were no competing events (death without recurrence) in the RFS analysis and relatively few competing events (three patients died of a cause other than HCC) in the OS analysis.
2.5Statistical analysisContinuous parameters are displayed as the mean (SD) or median (IQR). Student's t-test and Wilcoxon rank sum test were then used. Categorical parameters are displayed as the number (centiles), and Chi-squared test and Fisher's exact test were used. Baseline parameters with an absolute standardized mean difference (ASMD) of 0.1 or greater were considered imbalanced [16], and we adjusted for those imbalanced parameters in the following analyses. The RFS and OS were calculated using the Kaplan-Meier method and compared using the log-rank method. The proportionality assumption was tested, and four Cox proportional hazard models were established to estimate the impact of gender on HCC recurrence. In model 1, we did not adjust for any other parameters. In model 2, we conducted a minimally adjusted model, which only adjusted for age. In model 3, we adjusted for parameters that were estimated to be imbalanced by the ASMD, comprising age, body mass index (BMI), cigarette smoking, alcohol intaking, hypertension, diabetes, Alb, Alt, hepatitis background, AFP level, and blood loss. In model 4, we adjusted for parameters that were expected to influence HCC recurrence after hepatectomy, comprising age, diabetes, cirrhosis, Child-Pugh grade, AFP level, tumor diameter, tumor node, macrovascular invasion, blood loss, and microvascular invasion (MVI). In the subgroup analysis (subgroups of age, BMI, hypertension, diabetes, cirrhosis, Child-Pugh grade, hepatitis background, AFP level, tumor diameter, tumor node, macrovascular invasion, BCLC stage, MVI), the interaction effects were explored, and the interaction P values were generated based on the relative differences in the HRs for males compared with females in the selected subgroups. In further analysis, the method of propensity score matching (PSM) was used. To calculate the propensity score, we enrolled all variables into the propensity score equation, and patients in the male and female groups were matched 1 to 1 on their raw propensity score without replacement using the greedy matching method with a fixed caliper width of 0.2 standard deviation. Logistic regression models were used in the early recurrence (ER) analysis and late recurrence (LR) analysis, and in multivariate logistic regression analysis, we adjusted for RFS using clinically meaningful parameters: AFP level, cirrhosis, Child-Pugh grade, tumor size, tumor node, macrovascular invasion, and MVI.
ASMD was calculated using R programing (https://www.r-project.org/) with the function of “CreateTableOne” in “tableone” package.
P-values less than 0.05 were considered statistically significant.
2.6Ethics statementThis study was performed with the approval of the institutional review boards of the first affiliated hospital of Guangxi Medical University, all the clinical routine data involved in this study were anonymous, including the management of data cleaning and statistical analyses.
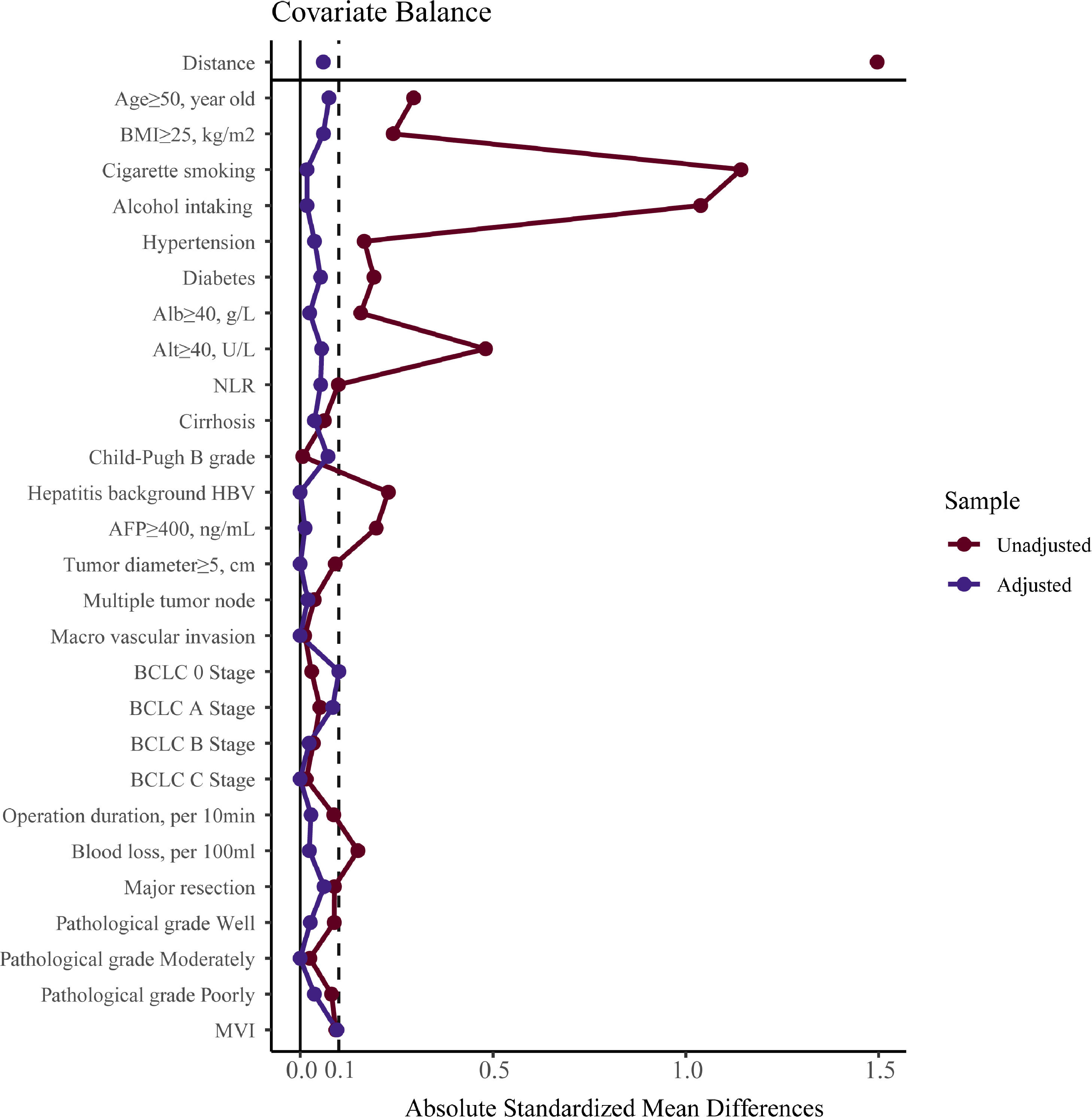
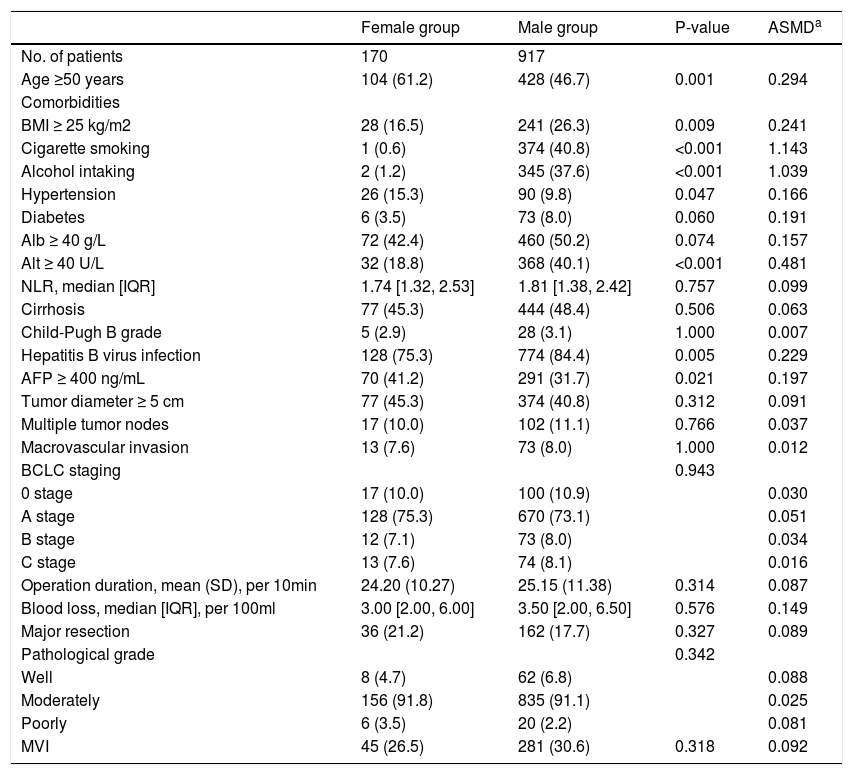
3Results3.1CharacteristicsAll patients’ demographic and clinicopathological characteristics were summarized in Table 1, and parameters whose ASMD test result was no less than 0.1 were regarded as imbalanced between the male and female groups. As shown in Table 1, the male group had higher rates of BMI ≥25 kg/m2 (ASMD = 0.241), cigarette smoking (ASMD = 1.143), alcohol intaking (ASMD = 1.039), diabetes (ASMD = 0.191), Alb ≥40 g/L (ASMD = 0.157), Alt ≥40 U/L (ASMD = 0.481), HBV infection (ASMD = 0.229), and had more blood loss (ASMD = 0.149) during surgery. However, females had a higher proportion with age ≥50 years (ASMD = 0.294), hypertension (ASMD = 0.166), and AFP ≥400 ng/mL (ASMD = 0.197). We adjusted for these imbalanced parameters in our subsequent Cox proportional hazard models.
Characteristics of the whole cohort.
| Female group | Male group | P-value | ASMDa | |
|---|---|---|---|---|
| No. of patients | 170 | 917 | ||
| Age ≥50 years | 104 (61.2) | 428 (46.7) | 0.001 | 0.294 |
| Comorbidities | ||||
| BMI ≥ 25 kg/m2 | 28 (16.5) | 241 (26.3) | 0.009 | 0.241 |
| Cigarette smoking | 1 (0.6) | 374 (40.8) | <0.001 | 1.143 |
| Alcohol intaking | 2 (1.2) | 345 (37.6) | <0.001 | 1.039 |
| Hypertension | 26 (15.3) | 90 (9.8) | 0.047 | 0.166 |
| Diabetes | 6 (3.5) | 73 (8.0) | 0.060 | 0.191 |
| Alb ≥ 40 g/L | 72 (42.4) | 460 (50.2) | 0.074 | 0.157 |
| Alt ≥ 40 U/L | 32 (18.8) | 368 (40.1) | <0.001 | 0.481 |
| NLR, median [IQR] | 1.74 [1.32, 2.53] | 1.81 [1.38, 2.42] | 0.757 | 0.099 |
| Cirrhosis | 77 (45.3) | 444 (48.4) | 0.506 | 0.063 |
| Child-Pugh B grade | 5 (2.9) | 28 (3.1) | 1.000 | 0.007 |
| Hepatitis B virus infection | 128 (75.3) | 774 (84.4) | 0.005 | 0.229 |
| AFP ≥ 400 ng/mL | 70 (41.2) | 291 (31.7) | 0.021 | 0.197 |
| Tumor diameter ≥ 5 cm | 77 (45.3) | 374 (40.8) | 0.312 | 0.091 |
| Multiple tumor nodes | 17 (10.0) | 102 (11.1) | 0.766 | 0.037 |
| Macrovascular invasion | 13 (7.6) | 73 (8.0) | 1.000 | 0.012 |
| BCLC staging | 0.943 | |||
| 0 stage | 17 (10.0) | 100 (10.9) | 0.030 | |
| A stage | 128 (75.3) | 670 (73.1) | 0.051 | |
| B stage | 12 (7.1) | 73 (8.0) | 0.034 | |
| C stage | 13 (7.6) | 74 (8.1) | 0.016 | |
| Operation duration, mean (SD), per 10min | 24.20 (10.27) | 25.15 (11.38) | 0.314 | 0.087 |
| Blood loss, median [IQR], per 100ml | 3.00 [2.00, 6.00] | 3.50 [2.00, 6.50] | 0.576 | 0.149 |
| Major resection | 36 (21.2) | 162 (17.7) | 0.327 | 0.089 |
| Pathological grade | 0.342 | |||
| Well | 8 (4.7) | 62 (6.8) | 0.088 | |
| Moderately | 156 (91.8) | 835 (91.1) | 0.025 | |
| Poorly | 6 (3.5) | 20 (2.2) | 0.081 | |
| MVI | 45 (26.5) | 281 (30.6) | 0.318 | 0.092 |
Values are n (%), mean (SD), or median [IQR].
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; Alb, albumin; Alt, alanine aminotransferase; NLR, neutrophil to lymphocyte ratio; AFP, alpha-fetoprotein; MVI, microvascular invasion; SD, standard deviation; IQR, interquartile range; ASDM, absolute standardized mean differences.
a, ASDM of 0.1 or more represent imbalanced.
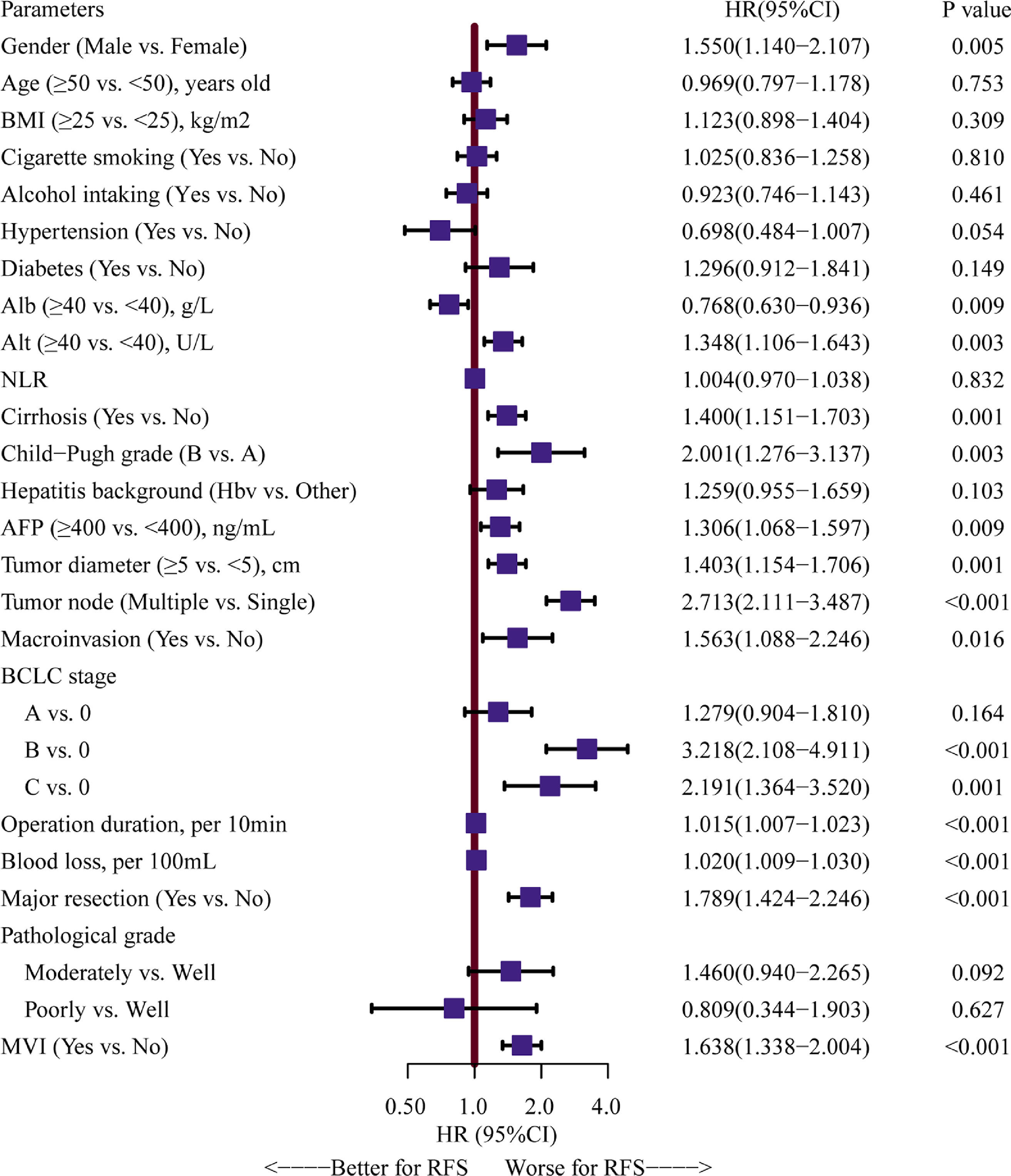
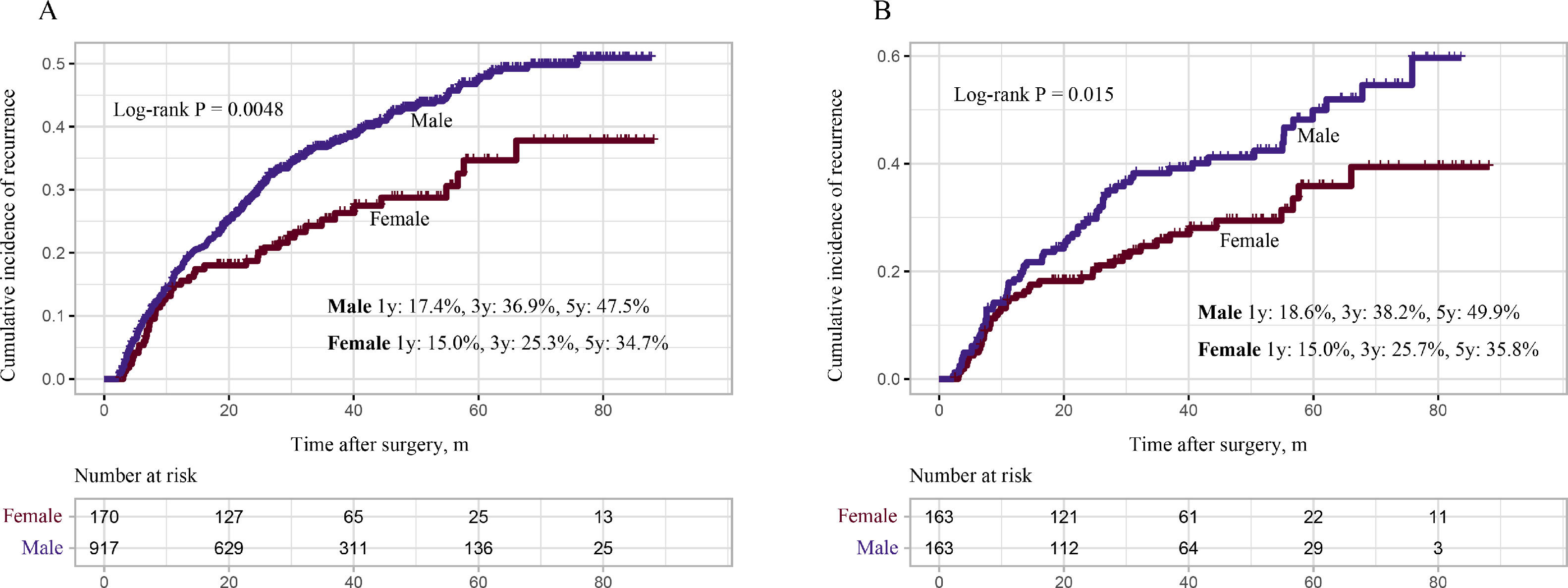
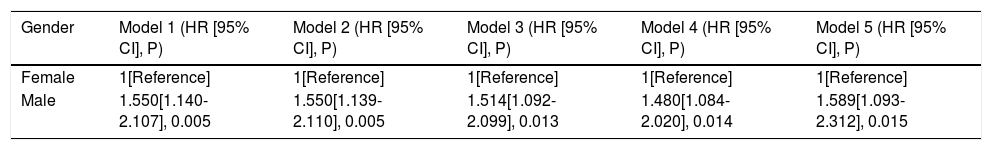
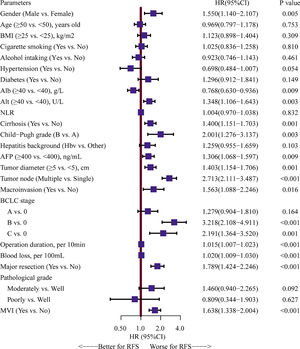
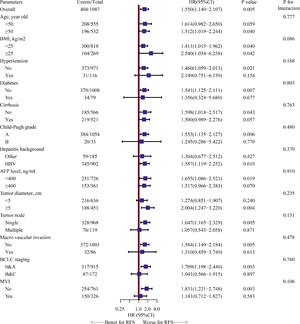
Univariate Cox analyses were performed on all the clinicopathologic parameters in the primary cohort (described in Fig. 2). Gender, Alb, Alt, cirrhosis, Child-Pugh grade, AFP level, tumor diameter, tumor node, macrovascular invasion, BCLC stage, operation duration, blood loss, major resection, and MVI were found to be significantly associated with RFS in univariate Cox analysis (P < 0.05). The RFS differed significantly by gender, and the male group showed a worse RFS (log-rank P = 0.0048) (Fig. 3A). The proportionality assumption in the primary cohort was tested (Fig. S1A, P = 0.556). Another three Cox proportional hazard models were conducted with different adjustment methods (shown in Table 2). In model 2, we built a minimally adjusted model, only adjusting for age, in which the male group showed a higher risk for recurrence (HR [95% CI] = 1.550 [1.139–2.110], P = 0.005). In model 3, we built a model adjusted for parameters that were imbalanced in Table 1, including age, BMI, cigarette smoking, alcohol intaking, hypertension, diabetes, Alb, Alt, hepatitis background, AFP level, and blood loss, which showed the same result: the male group again showed higher risk for recurrence (HR [95% CI] = 1.514 [1.092–2.099], P = 0.013). In model 4, we adjusted for parameters that were expected to have a clinically meaningful effect on RFS, consisting of age, diabetes, cirrhosis, Child-Pugh grade, AFP level, tumor diameter, tumor node, macrovascular invasion, blood loss, and MVI, and the results were replicated (HR [95% CI] = 1.480 [1.084–2.020], P = 0.014). Moreover, we repeated constructing model 2, model 3, model 4 with treating age, BMI, albumin, alanine aminotransferase, alpha-fetoprotein concentration and tumor diameter as their original continuous value formats, and also, we got the similar results (Table S1). In summary, male patients were more likely to suffer HCC recurrence than females.
Forest plot assessing risk factors on hepatocellular carcinoma recurrence.Note: HRs and 95% CIs are shown. HR and CI were derived from a univariate Cox proportional hazards model. BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; Alb, albumin; Alt, alanine aminotransferase; NLR, neutrophil to lymphocyte ratio; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MVI, microvascular invasion; HR: hazard ratio; CI: confidence interval.
Cox proportional hazard regression models examining the association between gender and hepatocellular carcinoma recurrence after curative hepatectomy.
| Gender | Model 1 (HR [95% CI], P) | Model 2 (HR [95% CI], P) | Model 3 (HR [95% CI], P) | Model 4 (HR [95% CI], P) | Model 5 (HR [95% CI], P) |
|---|---|---|---|---|---|
| Female | 1[Reference] | 1[Reference] | 1[Reference] | 1[Reference] | 1[Reference] |
| Male | 1.550[1.140-2.107], 0.005 | 1.550[1.139-2.110], 0.005 | 1.514[1.092-2.099], 0.013 | 1.480[1.084-2.020], 0.014 | 1.589[1.093-2.312], 0.015 |
Model 1: We did not adjust for any other parameter;
Model 2: Minimally adjusted model, we only adjusted for age;
Model 3: We adjusted for parameters that were estimated imbalanced by ASMDs, comprising age, body mass index, cigarette smoking, alcohol intaking, hypertension, diabetes, albumin, alanine aminotransferase, hepatitis background, alpha-fetoprotein level, blood loss;
Model 4: We adjusted for parameters that were expected to influence recurrence after hepatectomy, comprising age, diabetes, cirrhosis, Child-Pugh grade, alpha-fetoprotein level, tumor diameter, tumor node, macrovascular invasion, blood loss, microvascular invasion;
Model 5: Propensity score matching (PSM) model, we did a univariate Cox proportional hazard regression analysis in the PSM cohort;
HR: hazard ratio; CI: confidence interval.
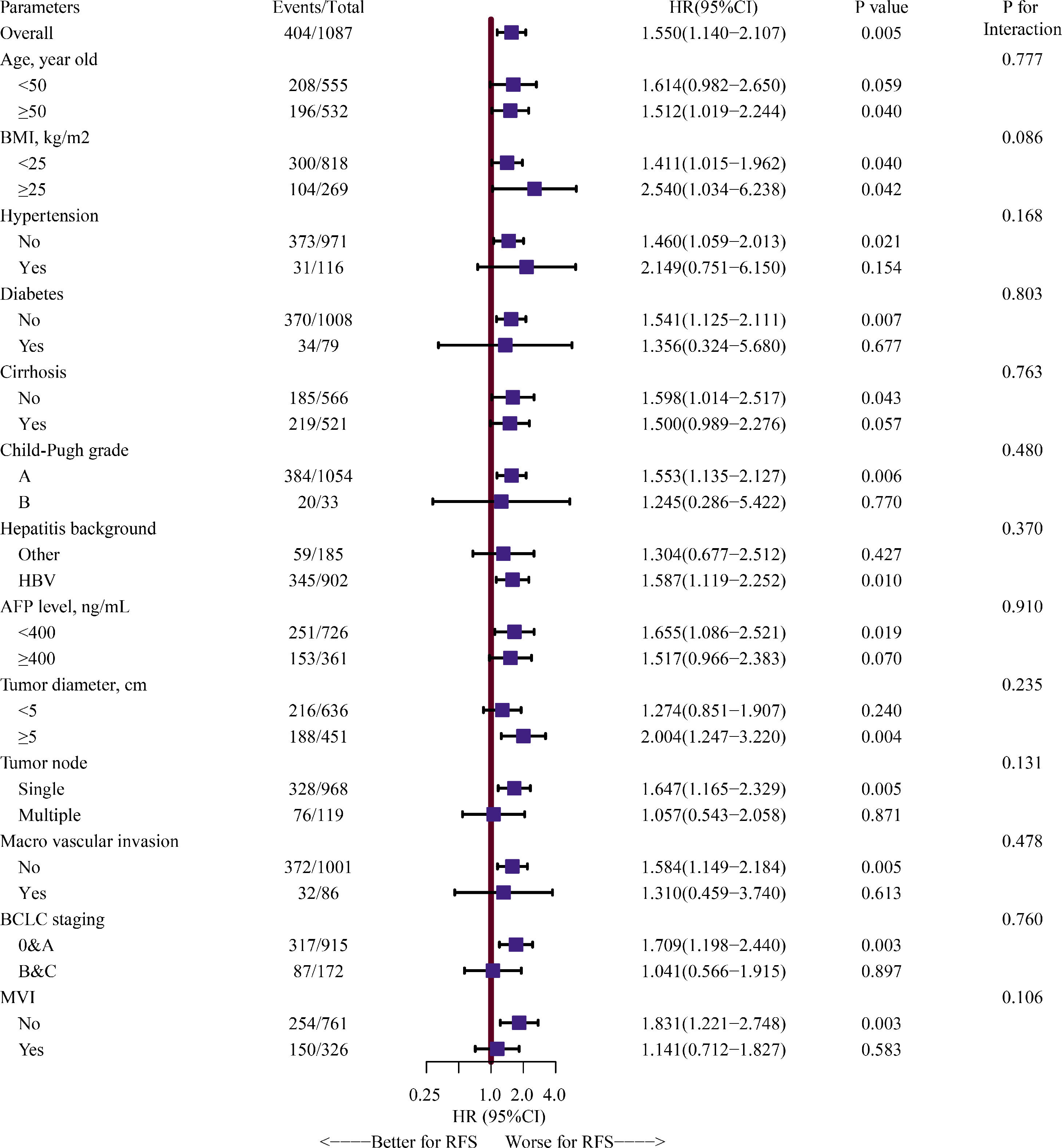
In another sensitivity analysis, we further explored the relationship between gender and RFS in different selected subgroups. We found that worse RFS was shown in the male group again in the subgroups of age ≥50 years, no diabetes, no cirrhosis, Child-Pugh A grade, HBV infection, AFP level <400 ng/mL, tumor diameter ≥5 cm, single tumor node, no macrovascular invasion, BCLC 0&A stage, no MVI. However, our findings also showed that the effect of male versus female on HCC recurrence did not depend on any of the subgroups described in Fig. 4 (all P for interaction >0.05).
Forest plot assessing interactions between the selected subgroups and the effect of male versus female on HCC recurrence.Note: HRs and 95% CIs are shown. The estimated overall HR and subgroups HRs were derived from a univariate Cox proportional hazards model, and we assessed the gender-by-interaction on the HCC recurrence, adjusting for the same parameters in multivariate Cox proportional hazards model, including age, diabetes, cirrhosis, Child-Pugh grade, alpha-fetoprotein level, tumor diameter, tumor node, macrovascular invasion, blood loss, and microvascular invasion. HR, hazard ratio; CI, confidence interval; HCC: hepatocellular carcinoma.
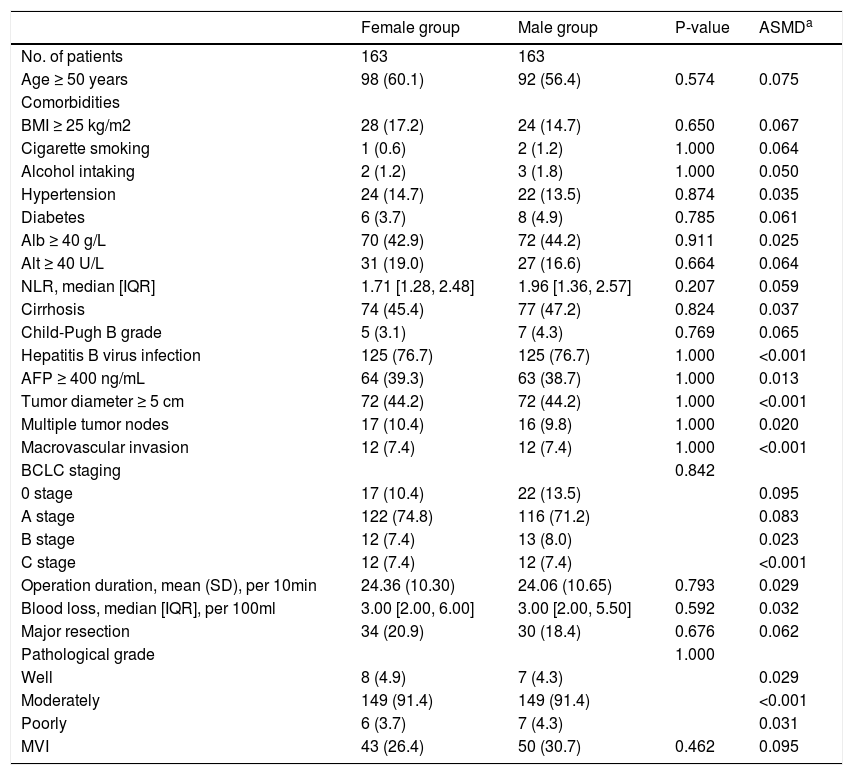
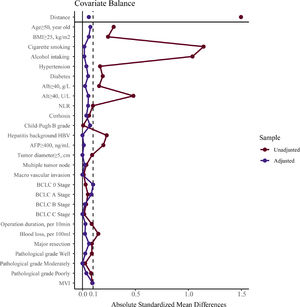
To verify our findings, we undertook a propensity score matching analysis. In the PSM cohort, all parameters tested were well balanced (all ASMD <0.1, Table 3 and Fig. 5). The results again demonstrated that the recurrence rates in the male group were significantly higher, (log-rank P = 0.015) (Fig. 3B), without violation of the proportional hazard assumption (Fig. S1B, P = 0.474). Univariate analysis in Cox proportional hazard model showed that the male group had a higher risk for recurrence (Table 2, Model 5 HR [95% CI] = 1.589 [1.093–2.312], P = 0.015).
Characteristics of the propensity score matching cohort.
| Female group | Male group | P-value | ASMDa | |
|---|---|---|---|---|
| No. of patients | 163 | 163 | ||
| Age ≥ 50 years | 98 (60.1) | 92 (56.4) | 0.574 | 0.075 |
| Comorbidities | ||||
| BMI ≥ 25 kg/m2 | 28 (17.2) | 24 (14.7) | 0.650 | 0.067 |
| Cigarette smoking | 1 (0.6) | 2 (1.2) | 1.000 | 0.064 |
| Alcohol intaking | 2 (1.2) | 3 (1.8) | 1.000 | 0.050 |
| Hypertension | 24 (14.7) | 22 (13.5) | 0.874 | 0.035 |
| Diabetes | 6 (3.7) | 8 (4.9) | 0.785 | 0.061 |
| Alb ≥ 40 g/L | 70 (42.9) | 72 (44.2) | 0.911 | 0.025 |
| Alt ≥ 40 U/L | 31 (19.0) | 27 (16.6) | 0.664 | 0.064 |
| NLR, median [IQR] | 1.71 [1.28, 2.48] | 1.96 [1.36, 2.57] | 0.207 | 0.059 |
| Cirrhosis | 74 (45.4) | 77 (47.2) | 0.824 | 0.037 |
| Child-Pugh B grade | 5 (3.1) | 7 (4.3) | 0.769 | 0.065 |
| Hepatitis B virus infection | 125 (76.7) | 125 (76.7) | 1.000 | <0.001 |
| AFP ≥ 400 ng/mL | 64 (39.3) | 63 (38.7) | 1.000 | 0.013 |
| Tumor diameter ≥ 5 cm | 72 (44.2) | 72 (44.2) | 1.000 | <0.001 |
| Multiple tumor nodes | 17 (10.4) | 16 (9.8) | 1.000 | 0.020 |
| Macrovascular invasion | 12 (7.4) | 12 (7.4) | 1.000 | <0.001 |
| BCLC staging | 0.842 | |||
| 0 stage | 17 (10.4) | 22 (13.5) | 0.095 | |
| A stage | 122 (74.8) | 116 (71.2) | 0.083 | |
| B stage | 12 (7.4) | 13 (8.0) | 0.023 | |
| C stage | 12 (7.4) | 12 (7.4) | <0.001 | |
| Operation duration, mean (SD), per 10min | 24.36 (10.30) | 24.06 (10.65) | 0.793 | 0.029 |
| Blood loss, median [IQR], per 100ml | 3.00 [2.00, 6.00] | 3.00 [2.00, 5.50] | 0.592 | 0.032 |
| Major resection | 34 (20.9) | 30 (18.4) | 0.676 | 0.062 |
| Pathological grade | 1.000 | |||
| Well | 8 (4.9) | 7 (4.3) | 0.029 | |
| Moderately | 149 (91.4) | 149 (91.4) | <0.001 | |
| Poorly | 6 (3.7) | 7 (4.3) | 0.031 | |
| MVI | 43 (26.4) | 50 (30.7) | 0.462 | 0.095 |
Values are n (%), mean (SD), or median [IQR].
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; Alb, albumin; Alt, alanine aminotransferase; NLR, neutrophil to lymphocyte ratio; AFP, alpha-fetoprotein; MVI, microvascular invasion; SD, standard deviation; IQR, interquartile range; ASDM, absolute standardized mean differences.
a, ASDM of 0.1 or more represent imbalanced.
Parameter balance measured by absolute standardized mean difference.Note: absolute standardized mean difference of 0.1 or greater were considered imbalanced. BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; Alb, albumin; Alt, alanine aminotransferase; NLR, neutrophil to lymphocyte ratio; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MVI, microvascular invasion.
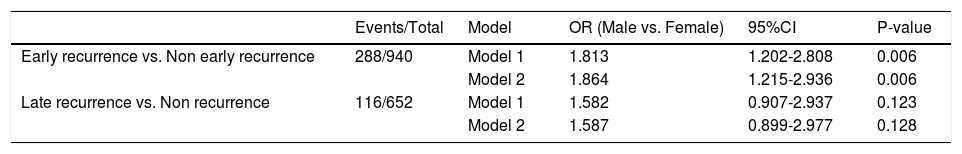
We subsequently explored the relationship between gender and different HCC recurrence patterns. Analysis of ER revealed that male patients had higher probability of suffering from ER (univariate analysis: OR [95% CI] = 1.813 [1.202–2.808], P = 0.006; multivariate analysis: OR [95% CI] = 1.864 [1.215–2.936], P = 0.006). However, in the analysis of LR, no difference was detected in the male group versus the female group (univariate analysis: OR [95% CI] = 1.582 [0.907–2.937], P = 0.123; multivariate analysis: OR [95% CI] = 1.587 [0.899–2.977], P = 0.128, Table 4). Furthermore, we repeated the ER analysis and LR analysis with treating alpha-fetoprotein concentration and tumor diameter as their original continuous value formats, and Table S2 showed the similar results.
Logistic regression analysis of early and late recurrence of patients with HCC.
| Events/Total | Model | OR (Male vs. Female) | 95%CI | P-value | |
|---|---|---|---|---|---|
| Early recurrence vs. Non early recurrence | 288/940 | Model 1 | 1.813 | 1.202-2.808 | 0.006 |
| Model 2 | 1.864 | 1.215-2.936 | 0.006 | ||
| Late recurrence vs. Non recurrence | 116/652 | Model 1 | 1.582 | 0.907-2.937 | 0.123 |
| Model 2 | 1.587 | 0.899-2.977 | 0.128 |
Model 1: We did not adjust for any other parameter;
Model 2: Multivariate logistic regression analysis, we adjusted for clinically meaningful parameters: alpha-fetoprotein level, cirrhosis, Child-Pugh grade, tumor diameter, tumor node, macrovascular invasion, and microvascular invasion;
OR: odds ratio, CI: confidence interval.
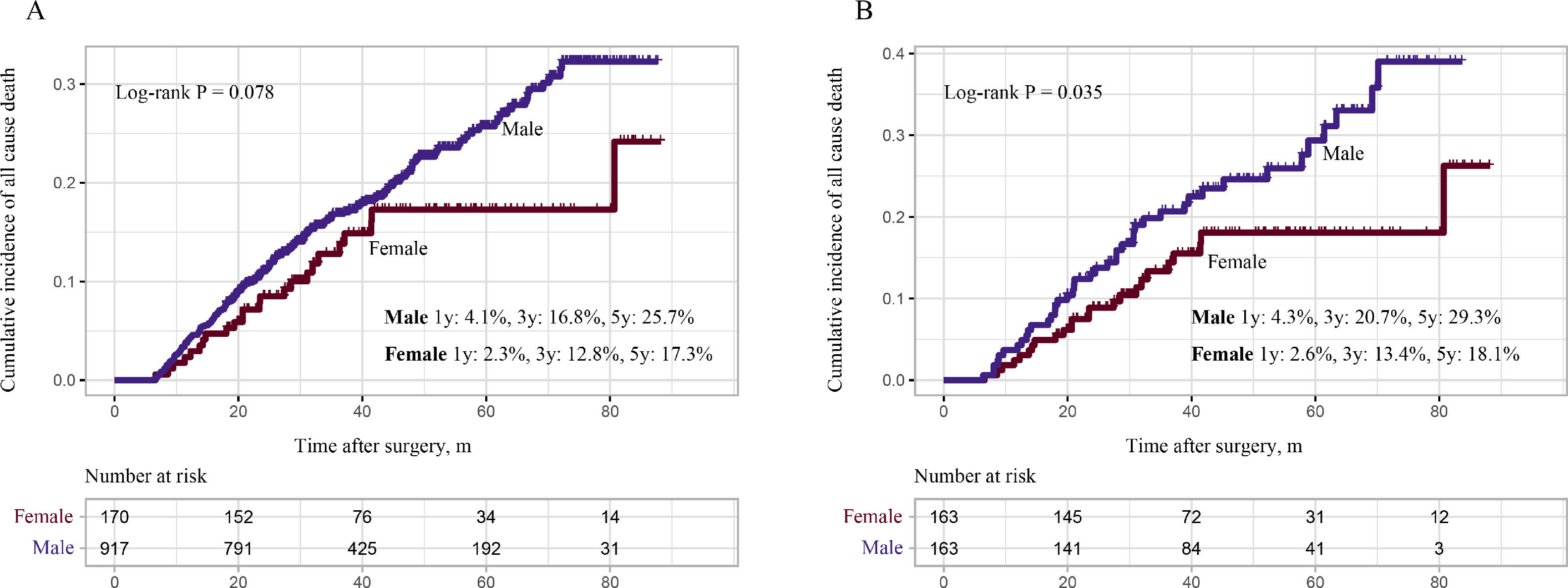
The above findings revealed that male patients were more likely to suffer from recurrence, and more likely to have an early recurrence. Therefore, we wanted to investigate whether worse OS was found in the male group. Consistent with the RFS analysis, the male group also showed a worse prognosis for OS. In the primary cohort, the male group showed higher all-cause mortality, though it did not reach statistical significance (male 1 year: 4.1%, 3 years: 16.8%, 5 years: 25.7%; female 1 year: 2.3%, 3 years: 12.8%, 5 years: 17.3%: log-rank P = 0.078, Fig. 6A). In the PSM cohort, the male group again showed higher all-cause mortality (male 1 year: 4.3%, 3 years: 20.7%, 5 years: 29.3%; female 1 year: 2.6%, 3 years: 13.4%, 5 years: 18.1%, log-rank P = 0.035, Fig. 6B).
4DiscussionIn this study, we demonstrated that male patients have a higher risk than females of HCC recurrence after curative hepatectomy; in particular, they are more likely to have an early recurrence. Our study also subsequently demonstrated that the male gender has an adverse impact on overall survival.
Many studies have reported that higher morbidity and worse survival outcomes are found in male patients with HCC [17–19]. Consistent with previous studies, our study also found an unfavorable RFS and OS in the male group. In contrast, several analyses did not succeed in demonstrating that gender is an independent variable affecting survival [20,21]. Christophe et al [22]. failed to reveal the survival difference by gender after hepatic resection, partially because only patients without cirrhosis and a relatively small number of patients were involved in their study.
Although many studies have studied the survival disparity associated with gender in HCC, few studies have taken the balance of parameters into consideration, which is the indicator of comparability between different groups of patients. Chi et al [11]. did not adjust for cigarette smoking and alcohol intaking in their multivariate Cox analysis for overall survival, though they showed a significant difference between male and female groups. In contrast, Yu et al [15]. adjusted for cigarette smoking in their multivariate Cox analysis, because differences in the prevalence of cigarette smoking in male and female patients may have influenced the survival difference [23]. In our study, we calculated the ASMD value of each parameter between these two groups, and parameters were thought to be imbalanced when their ASMDs were no less than 0.1. Four methods for adjusting the Cox proportional hazard model were used. The non-adjusted model (univariate analysis) and minimally adjusted model (adjusted only for age) demonstrated that male patients displayed worse RFS than female patients. In a further adjusted model, we adjusted for parameters that were shown to be imbalanced by ASMD analysis, including cigarette smoking and alcohol intaking, a method that few have reported in previous studies, and we gained the same result. In the final analysis, we constructed a traditional multivariate Cox model that adjusted for the most common clinically meaningful parameters, and the result was repeated.
In the sensitivity analysis, to explore further the relationship between RFS and gender, we used the method of propensity score matching, resampling the primary cohort to form a PSM cohort in which all parameters were well balanced. The univariate Cox analysis in the PSM cohort showed the same results as described above.
Early studies had found that in the subgroup with early-stage HCC, male patients showed worse survival outcomes, but not in the group with late-stage HCC, explaining that the methylation reversal of the estrogen receptor (ER) may only appear in early-stage HCC, which may restore the expression of the wild-type ER, contributing to the less aggressive growth of the tumor [11]. Our study also showed that in early-stage HCC (BCLC 0&A) males had a significantly worse RFS than females, but not in relatively late-stage HCC (BCLC B&C). However, in our further interaction analysis, the adverse effect of male gender relative to female was found not to be altered significantly by the subgroup of the BCLC stage.
Also, it has been reported that female patients have better HCC-specific survival than males only in the subgroup of age <50 years [13]. A possible explanation is that the risk of HCC is inversely related to the age of natural menopause and the use of hormone replacement therapy in females [24]. However, in our subgroup analysis, male patients had a worse RFS in both age subgroups (<50 and ≥50 years old), though it did not reach statistical significance in the age <50 subgroups (P = 0.059). One possible reason is that we did not categorize the age at menopause properly in our study. In the interaction analysis, our results showed that the effect of male versus female gender did not differ significantly in the different age subgroups.
As described in previous studies, ER is more often considered to be the consequence of occult metastasis from the original tumor, which is a characteristic of the tumor itself. In contrast, LR is often attributed to the development of a de novo tumor [25,26]. The median survival time for ER and LR patients was 15.8 months and 29.6 months, respectively, showing a more favorable prognosis in LR [27]. Yang et al [28]. revealed that the incidence of early recurrence was more than twice the incidence of late recurrence (68.03 % vs. 31.97 %) for patients after radiofrequency ablation for HCC meeting the up-to-seven criteria, and male gender had an adverse effect on HCC recurrence. Similar to the previous study, a disparity of recurrence pattern was also found in our study (ER: 30.6% vs. LR: 12.3%), and further, we found males had a higher risk for ER, but no significant difference was found in the LR analysis by gender. One possible reason may explain this that the intrahepatic micro metastasis before or during the hepatectomy, usually resulting to ER, can be impacted by the level of sex hormones then to different survival outcome. And former studies have demonstrated that cirrhosis is the main risk factor related to LR [27,29], but this shows less correlation with gender, which is similar to the results in our study.
There are some limitations of the present study. First, our study cohort mainly included HBV-related HCC and will differ from cohorts mainly with HCV infection and cohorts with neither infection. Second, unlike a randomized study, although we used a PSM method to achieve balance in baseline parameters between the two groups, the investigator was only able to balance the baseline parameters that were measured [30]. The unmeasured parameters may still be imbalanced, and thus may bias the results. Third, the study was a post hoc analysis. It was limited by the lack of data regarding the estrogen level and HBV-DNA replication level, which is important in the recurrence of HCC.
5ConclusionThere is gender disparity in the recurrence-free survival of patients with HCC after curative hepatectomy: male patients had a higher risk of recurrence, and the risk was mainly reflected in early recurrence.
FundingThis work was supported in part by the National Natural Science Foundation of China (No. 81802874, 81560535).
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributionsTianyi Liang and Tao Peng conceived and designed the manuscript; Yongfei He, Shutian Mo, Zijun Chen, Xiwen Liao, Xin Zhou, Chengkun Yang, Shuqi Zhao, Chuangye Han, Guangzhi Zhu, Hao Su, Xinping Ye made the acquisition of data and performed data analysis. Tianyi Liang wrote the manuscript, and Tao Peng guided and supervised the manuscript. All authors read and approved the final manuscript.
None.