Acute-on-chronic liver failure (ACLF) is associated with increased short and long-term mortality. Animal models of liver failure have demonstrated that granulocyte-colony stimulating factor (G-CSF) accelerates the liver regeneration process and improves survival. However, clinical evidence regarding the use of G-CSF in ACLF remains scarce. The aim of this study was to assess the benefits and harms of G-CSF in patients with acute-on-chronic liver failure. An electronic search was made in The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and LILACS up to November 2013. Randomized clinical trials comparing the use of any regimen of G-CSF against placebo or no intervention in patients with ACLF were included. Primary outcomes included overal mortality, mortality due multi-organ failure, and adverse events. Relative risk (RR) and mean difference (MD) were used. Two trials involving 102 patients were included. A significant reduction in short-term overall mortality was observed in patients receiving G-CSF compared to controls (RR 0.56; 95%CI 0.39,0.80). G-CSF failed to reduce mortality secondary to gastrointestinal bleeding (RR 1.45; 95%CI 0.50, 4.27). Adverse effects reported included: fever, rash, herpes zoster, headache and nausea. In conclusion, the use of G-CSF for the treatment of patients with ACLF significantly reduced short-term mortality. While the evidence is still limited, the apparent benefit observed on short-term mortality, mild adverse effects and lack of an alternative therapy make the use of G-CSF in ACLF patients a reasonable alternative when liver transplantation is contraindicated or unavailable.
Acute-on-chronic liver failure (ACLF) is an acute insult to the liver in a previously diagnosed or undiagnosed chronic liver patient.1 ACLF manifests with jaundice and coagulopathy,1 ascites and/or encephalopathy, increasing the mortality at 3 months mainly through multisystem organ failure.2 ACLF is a frequent complication of chronic liver disease, accounting for up to 30% of hospitalized patients with decompensated cirrhosis,3 and 28% of hospitalized patients with liver insufficiency and alcoholic cirrhosis.4 ACLF precipitating factors vary by geographic region. In Western countries, non-infectious etiologies, such as alcoholic hepatitis, variceal bleeding, or hepatotoxic drugs, represent the major causes. In Eastern countries, infectious etiologies such as reactivation of hepatitis B virus, hepatitis C virus and superinfection with hepatitis E virus account for the vast majority of cases.1,5
Regardless of the precipitating factor, the transition from stable cirrhosis to ACLF is strongly related to an exaggerated systemic inflammatory response.4 Initially, the release of proinflammatory cytokines (tumor necrosis factor-α, interleukin 2, 4, 6, 8, 10, Interferon-γ, interleukin 2 receptor) causes profound alterations in the macro and microcirculation ending in multi-organ failure. Subsequently, the immunologic response predisposes the patient to nosocomial infections and further deterioration.2,6 ACLF rapidly progresses into multi-organ dysfunction, septic shock and hypovolemic shock,3,7,8 producing a short and medium term mortality of 50-90%.9,10 Currently, orthotopic liver transplantation (OLT) remains the only definitive therapy for patients who fail to improve with life support measures.1 Yet, the availability of OLT for ACLF patients is very limited; a recent five-year follow-up Belgium cohort reported that only 7% of ACLF patients received liver transplantation.4
Granulocyte colony stimulating factor (G-CSF) is an immunomodulator glycoprotein produced by monocyte-macrophages, fibroblast and endothelial cells that could modify the course of ACLF. G-CSF acts primarily on neutrophils and neutrophilic precursors to promote cell growth, differentiation and function.11 G-CSF use has been approved in patients receiving myelosuppressive chemotherapy to accelerate immune reconstitution after hematopoietic stem cell transplant, mobilization and collection of peripheral blood stem cells for transplantation, and for severe chronic neutropenia. The most commonly reported adverse-effects of G-CSF include bone pain, headache, fatigue, nausea, fever, insomnia, anorexia and myalgia.12
In animal models the administration of G-CSF to rats with liver damage promoted liver repair by increasing the migration of bone marrow progenitors to the liver, and also by acting locally within the liver microenvironment facilitating the hepatic restoration program through oval cells.13 Another model of liver damage showed that the administration of G-CSF ameliorates the histological liver damage, accelerates the regeneration process and improves survival.14 Human trials have also shown beneficial effects of G-CSF on liver regeneration. In a randomized controlled trial including patients with alcoholic steatohepatitis, G-CSF therapy mobilized CD34+ cells, increased hepatocyte growth factor, and induced hepatic progenitor cells to proliferate.15 Mobilization of bone marrow-derived stem cells induced by G-CSF in patients with cirrhosis has also been reported.16
ACLF is a major chronic liver disease complication with no therapeutic alternatives beyond OLT. Recent evidence suggests G-CSF could prove beneficial in the treatment of patients with ACLF when transplantation is either contraindicated or unavailable. The present systematic review and meta-analysis aims to assess the benefits and harms of G-CSF in patients with acute-on-chronic liver failure.
Material and MethodsCriteria for considering the studies- •
Types of studies. Randomized clinical trials comparing the administration of G-CSF against placebo or no intervention for the treatment of patients with ACLF. Trials were included irrespective of publication status, language or blinding.
- •
Types of participants. Adult patients diagnosed with ACLF (according to the Asian-Pacific Association for the Study of the Liver or American Association for the Study of Liver Diseases-European Association for the Study of the Liver definition) regardless of the etiology of the preexisting liver disease, the precipitating event, or the severity of the disease.1,2
- •
Types of interventions. Use of G-CSF alone or in combination, regardless of dose, type of recombinant G-CSF, or route of administration. Control groups received either placebo or no intervention.
- •
Types of outcome measures. Primary outcomes: overall mortality, mortality due multi-organ failure, adverse events.17 Secondary outcomes: complications, hospitalization length, liver transplantation, changes in severity indices [Child-Turcotte-Pugh (CTP), Model of End-stage Liver Disease (MELD), Sequential Organ Failure Assessment (SOFA)], mortality secondary to gastrointestinal bleeding, changes in peripheral leukocyte and/or neutrophil counts, and changes in peripheral and hepatic CD34+ count.
- •
Search methods for identification of studies. Electronic searches. Relevant randomized trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2013) in The Cochrane Library, MEDLINE (1950 to November 2013), EMBASE (1980 to November 2013), and LILACS (1987 to November 2013). Search strategies and time span of the searches are given in table 1. Other sources. The reference list of all identified reports was inspected to identify further trials.
Table 1..Search strategy.
Number of search Search algorithm Results 1. Health problem “Acute liver failure” (“Liver Failure, Acute” [Mesh] OR “Failure, Acute Liver” OR “Fulminating Hepatic Failure” OR “Fulminating Hepatic Failures” OR “Hepatic Failure, Fulminating” OR “Hepatic Failures, Fulminating” OR “Liver Failure, Fulminant” OR “Fulminant Liver Failure” OR “Fulminant Liver Failures” OR “Liver Failures, Fulminant” OR “Hepatic Failure, Acute” OR “Acute Hepatic Failure” OR “Failure, Acute Hepatic” OR “Hepatic Failure, Fulminant” OR “Fulminant Hepatic Failure” OR “Fulminant Hepatic Failures” OR “Hepatic Failures, Fulminant” OR “Acute Liver Failure” OR “Fulminating Liver Failure” OR “Fulminating Liver Failures” OR “Liver Failure, Fulminating” OR “Liver Failures, Fulminating” 15,941 2. Intervention “Granulocyte colony stimulating factor” “Granulocyte Colony-Stimulating Factor” [Mesh]) OR “Recombinant proteins” OR “r-metHuG-CSF” OR “Recombinant-Methionyl Human Granulocyte Colony-Stimulating Factor” OR “G-CSF Recombinant, Human Methionyl” OR “Neupogen” OR “Amgen brand of Filgrastim” OR “Topneuter” OR “Filgrastim” OR “Lenograstim” OR “Pegfilgrastim” OR “Nivestim” OR “Ratiograstim” OR “Tevagrastim” OR “Zarzio” 195,754 3. Type of study “Randomized controlled trial” “randomized controlled trial”[Filter] OR “Controlled Clinical Trials, Randomized” OR “Clinical Trials, Randomized” OR “Trials, Randomized Clinical” OR Clinical Trials 43,908 4. Type of study “Systematic review” Review, systematic 1,802,535 5. Type of study “Meta-analysis” Meta-analysis 69,860 6. Types of study “Randomized controlled trial, systematic review, meta-analysis” ((((((randomized controlled trial) OR Controlled Clinical Trials, Randomized) OR Clinical Trials, Randomized) OR Trials, Randomized Clinical) OR Clinical Trials) OR Meta-Analysis) OR review, systematic 1,046,501 7. Health problem + intervention + types of study Search 1+ serch 2+ search 3 ((((“Liver Failure, Acute”[Mesh] OR “Failure, Acute Liver” OR “Fulminating Hepatic Failure” OR “Fulminating Hepatic Failures” OR “Hepatic Failure, Fulminating” OR “Hepatic Failures, Fulminating” OR “Liver Failure, Fulminant” OR “Fulminant Liver Failure” OR “Fulminant Liver Failures” OR “Liver Failures, Fulminant” OR “Hepatic Failure, Acute” OR “Acute Hepatic Failure” OR “Failure, Acute Hepatic” OR “Hepatic Failure, Fulminant” OR “Fulminant Hepatic Failure” OR “Fulminant Hepatic Failures” OR “Hepatic Failures, Fulminant” OR “Acute Liver Failure” OR “Fulminating Liver Failure” OR “Fulminating Liver Failures” OR “Liver Failure, Fulminating” OR “Liver Failures, Fulminating”)) AND (“Granulocyte Colony-Stimulating Factor”[Mesh]) OR “Recombinant proteins” OR “r-metHuG-CSF” OR “Recombinant-Methionyl Human Granulocyte Colony-Stimulating Factor” OR “G-CSF Recombinant, Human Methionyl” OR “Neupogen” OR “Amgen brand of Filgrastim” OR “Topneuter” OR “Filgrastim” OR “Lenograstim” OR “Pegfilgrastim” OR “Nivestim” OR “Ratiograstim” OR “Tevagrastim” OR “Zarzio”)) AND (“randomized controlled trial”[Filter] OR “Controlled Clinical Trials, Randomized” OR “Clinical Trials, Randomized” OR “Trials, Randomized Clinical” 6,187 8. Health problem + intervention + : type of study + filter: clinical trials Search 1+ search 2+ search 3 + Filter clinical trials ((((“Liver Failure, Acute” [Mesh] OR “Failure, Acute Liver” OR “Fulminating Hepatic Failure” OR “Fulminating Hepatic Failures” OR “Hepatic Failure, Fulminating” OR “Hepatic Failures, Fulminating” OR “Liver Failure, Fulminant” OR “Fulminant Liver Failure” OR “Fulminant Liver Failures” OR “Liver Failures, Fulminant” OR “Hepatic Failure, Acute” OR “Acute Hepatic Failure” OR “Failure, Acute Hepatic” OR “Hepatic Failure, Fulminant” OR “Fulminant Hepatic Failure” OR “Fulminant Hepatic Failures” OR “Hepatic Failures, Fulminant” OR “Acute Liver Failure” OR “Fulminating Liver Failure” OR “Fulminating Liver Failures” OR “Liver Failure, Fulminating” OR “Liver Failures, Fulminating”)) AND (“Granulocyte Colony-Stimulating Factor”[Mesh]) OR “Recombinant proteins” OR “r-metHuG-CSF” OR “Recombinant-Methionyl Human Granulocyte Colony-Stimulating Factor” OR “G-CSF Recombinant, Human Methionyl” OR “Neupogen” OR “Amgen brand of Filgrastim” OR “Topneuter” OR “Filgrastim” OR “Lenograstim” OR “Pegfilgrastim” OR “Nivestim” OR “Ratiograstim” OR “Tevagrastim” OR “Zarzio”)) AND (“randomized controlled trial”[Filter] OR “Controlled Clinical Trials, Randomized” OR “Clinical Trials, Randomized” OR “Trials, Randomized Clinical”) Filters: Clinical Trial 5,156 (Filter clinical trials) 9. Health problem + intervention + type of study Search 1+ search 2+ search 3+ Filter clinical trial +Filter meta-analysis + Filter systematic review ((((“Liver Failure, Acute”[Mesh] OR “Failure, Acute Liver” OR “Fulminating Hepatic Failure” OR “Fulminating Hepatic Failures” OR “Hepatic Failure, Fulminating” OR “Hepatic Failures, Fulminating” OR “Liver Failure, Fulminant” OR “Fulminant Liver Failure” OR “Fulminant Liver Failures” OR “Liver Failures, Fulminant” OR “Hepatic Failure, Acute” OR “Acute Hepatic Failure” OR “Failure, Acute Hepatic” OR “Hepatic Failure, Fulminant” OR “Fulminant Hepatic Failure” OR “Fulminant Hepatic Failures” OR “Hepatic Failures, Fulminant” OR “Acute Liver Failure” OR “Fulminating Liver Failure” OR “Fulminating Liver Failures” OR “Liver Failure, Fulminating” OR “Liver Failures, Fulminating”)) AND (“Granulocyte Colony-Stimulating Factor”[Mesh]) OR “Recombinant proteins” OR “r-metHuG-CSF” OR “Recombinant-Methionyl Human Granulocyte Colony-Stimulating Factor” OR “G-CSF Recombinant, Human Methionyl” OR “Neupogen” OR “Amgen brand of Filgrastim” OR “Topneuter” OR “Filgrastim” OR “Lenograstim” OR “Pegfilgrastim” OR “Nivestim” OR “Ratiograstim” OR “Tevagrastim” OR “Zarzio”)) AND (“randomized controlled trial”[Filter] OR “Controlled Clinical Trials, Randomized” OR “Clinical Trials, Randomized” OR “Trials, Randomized Clinical”) Filters: Clinical Trial; Meta-Analysis; Review 5784 (Filter clinical trial, systematic review and metaanalysis) 10. Health problem + intervention + type of study Search 1+search 2+search 3+search 4+ search 5 Search (((((((“Liver Failure, Acute”[Mesh] OR “Failure, Acute Liver” OR “Fulminating Hepatic Failure” OR “Fulminating Hepatic Failures” OR “Hepatic Failure, Fulminating” OR “Hepatic Failures, Fulminating” OR “Liver Failure, Fulminant” OR “Fulminant Liver Failure” OR “Fulminant Liver Failures” OR “Liver Failures, Fulminant” OR “Hepatic Failure, Acute” OR “Acute Hepatic Failure” OR “Failure, Acute Hepatic” OR “Hepatic Failure, Fulminant” OR “Fulminant Hepatic Failure” OR “Fulminant Hepatic Failures” OR “Hepatic Failures, Fulminant” OR “Acute Liver Failure” OR “Fulminating Liver Failure” OR “Fulminating Liver Failures” OR “Liver Failure, Fulminating” OR “Liver Failures, Fulminating”)) AND (“Granulocyte Colony-Stimulating Factor”[Mesh]) OR “Recombinant proteins” OR “r-metHuG-CSF” OR “Recombinant-Methionyl Human Granulocyte Colony-Stimulating Factor” OR “G-CSF Recombinant, Human Methionyl” OR “Neupogen” OR “Amgen brand of Filgrastim” OR “Topneuter” OR “Filgrastim” OR “Lenograstim” OR “Pegfilgrastim” OR “Nivestim” OR “Ratiograstim” OR “Tevagrastim” OR “Zarzio”)) AND (“randomized controlled trial”[Filter] OR “Controlled Clinical Trials, Randomized” OR “Clinical Trials, Randomized” OR “Trials, Randomized Clinical”)) OR Review, Systematic) OR Meta-Analysis) 1843931 11. Health problem + intervention + type of study Search 1+ search 2+ search 6 ((((((“Liver Failure, Acute”[Mesh] OR “Failure, Acute Liver” OR “Fulminating Hepatic Failure” OR “Fulminating Hepatic Failures” OR “Hepatic Failure, Fulminating” OR “Hepatic Failures, Fulminating” OR “Liver Failure, Fulminant” OR “Fulminant Liver Failure” OR “Fulminant Liver Failures” OR “Liver Failures, Fulminant” OR “Hepatic Failure, Acute” OR “Acute Hepatic Failure” OR “Failure, Acute Hepatic” OR “Hepatic Failure, Fulminant” OR “Fulminant Hepatic Failure” OR “Fulminant Hepatic Failures” OR “Hepatic Failures, Fulminant” OR “Acute Liver Failure” OR “Fulminating Liver Failure” OR “Fulminating Liver Failures” OR “Liver Failure, Fulminating” OR “Liver Failures, Fulminating”))) AND ((“Granulocyte Colony-Stimulating Factor”[Mesh]) OR “Recombinant proteins” OR “r-metHuG-CSF” OR “Recombinant-Methionyl Human Granulocyte Colony-Stimulating Factor” OR “G-CSF Recombinant, Human Methionyl” OR “Neupogen” OR “Amgen brand of Filgrastim” OR “Topneuter” OR “Filgrastim” OR “Lenograstim” OR “Pegfilgrastim” OR “Nivestim” OR “Ratiograstim” OR “Tevagrastim” OR “Zarzio”))) AND (((((((randomized controlled trial) OR Controlled Clinical Trials, Randomized) OR Clinical Trials, Randomized) OR Trials, Randomized Clinical) OR Clinical Trials) OR Meta-Analysis) OR review, systematic)) 49 - •
Data collection and analysis.Selection of studies. Two authors review each title and abstract to exclude reports that failed to comply with the inclusion criteria. For potentially relevant studies, the full-text report was obtained and reviewed independently by both authors. When needed, the authors of the original reports were contacted for clarification. In case of disagreement, a third author reviewed the article. Justification for study exclusion was documented. Data extraction and management. Two authors independently extracted all relevant data from included studies. In case of disagreement a third author extracted the data. Data extraction was discussed, decisions documented, and, when clarification was necessary, the authors of the original reports were contacted. Studies were identified with the last name of the first author and the year in which the report was published, and ordered chronologically. The following data were extracted, verified, and recorded: characteristics of the studies, characteristics of participants, characteristics of intervention, and characteristics of outcomes measured. Assessment of risk of bias in included studies. Following the Cochrane Handbook for Systematic Reviews of Interventions two authors independently assessed the risk of bias in the included trials. The following domains were assessed: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Any disagreement was resolved by discussion and, when needed, settled by a third author. The trial authors were contacted for clarification when needed. Measures of treatment effect. Dichotomous data of each trial was analyzed using the risk ratio (RR) and 95% confidence intervals; continuous data was analyzed with mean difference and 95% confidence intervals. Assessment of heterogeneity. To quantify the heterogeneity between trials we used the I2 test and Q statistic. Heterogeneity was considered significant when the Q statistic produced a P value ≤ 0.10 or when the I2 > 25%. Assessment of reporting biases. To assess publication bias, a funnel plot presenting the treatment effect against the trial precision was examined. Additional testing for funnel plot asymmetry was not performed due to the number of trials included. Data synthesis. Under no significant heterogeneity the Mantel-Haenszel method for fixed-effects was used to estimate the pooled RR and 95% CI.
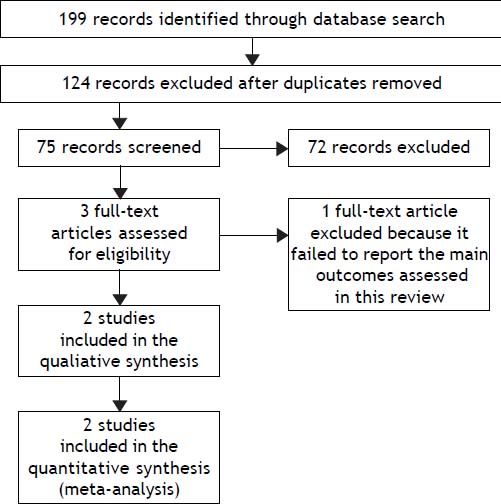
Seventy-five relevant references were initially identified and screened. Three articles were considered suitable for inclusion. One article was excluded because it did not consider the primary outcomes of this review.19 Finally, two trials were included for the analysis (Figure 1).
Study flowchart: search results. Seventy-five relevant references were initially identified and screened. Three articles were considered suitable for inclusion. One article was excluded because it did not consider the primary outcomes of this review. Finally, two trials were included for the analysis.
Two trials evaluating the effectiveness of G-CSF for the treatment of ACLF in 102 patients were included.20,21 One trial compared G-CSF against placebo.20 The second trial compared G-CSF against no intervention. Trials were conducted in India20 and China,21 and both were performed in hospitalized patients. One trial included only patients with HBV-associated ACLF,21 while alcoholic liver disease was the main etiology of the underlying chronic liver disease in the other trial.20 Both studies administered G-CSF subcutaneously at a dose of 5 µg/kg. In one trial, patients received a total of 6 doses in six consecutive days21 and in the other a total of 12 doses over one month (daily doses for the first five days and then every third day).20 Both studies aimed to evaluate the safety and efficacy of G-CSF and assessed short-term mortality, CD34+ cell mobilization, MELD and CTP. Risk of bias in the included studies. None of the trials were evaluated as having high risk of bias (Table 2).
Characteristics of included studies.
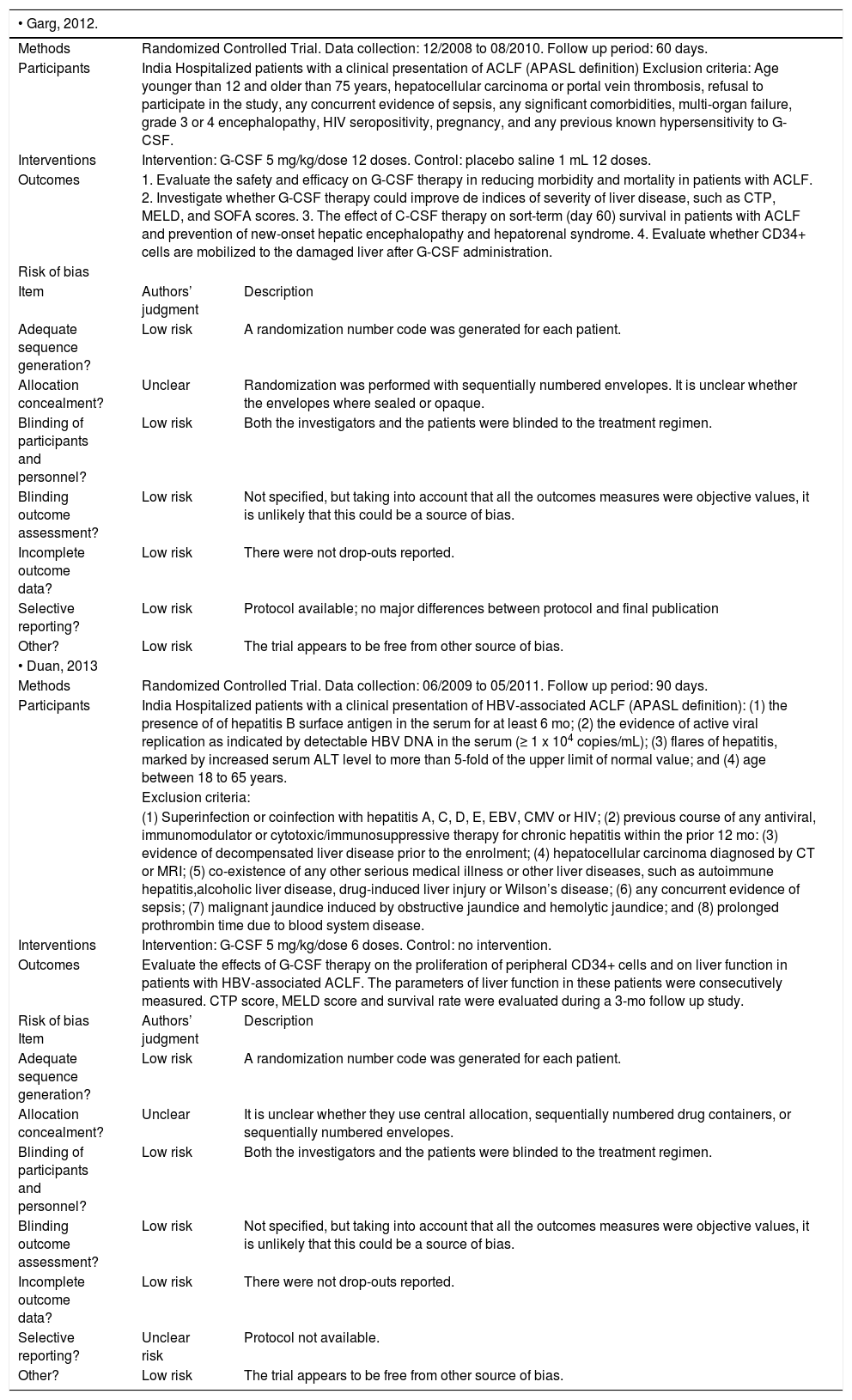
| • Garg, 2012. | ||
|---|---|---|
| Methods | Randomized Controlled Trial. Data collection: 12/2008 to 08/2010. Follow up period: 60 days. | |
| Participants | India Hospitalized patients with a clinical presentation of ACLF (APASL definition) Exclusion criteria: Age younger than 12 and older than 75 years, hepatocellular carcinoma or portal vein thrombosis, refusal to participate in the study, any concurrent evidence of sepsis, any significant comorbidities, multi-organ failure, grade 3 or 4 encephalopathy, HIV seropositivity, pregnancy, and any previous known hypersensitivity to G-CSF. | |
| Interventions | Intervention: G-CSF 5 mg/kg/dose 12 doses. Control: placebo saline 1 mL 12 doses. | |
| Outcomes | 1. Evaluate the safety and efficacy on G-CSF therapy in reducing morbidity and mortality in patients with ACLF. 2. Investigate whether G-CSF therapy could improve de indices of severity of liver disease, such as CTP, MELD, and SOFA scores. 3. The effect of C-CSF therapy on sort-term (day 60) survival in patients with ACLF and prevention of new-onset hepatic encephalopathy and hepatorenal syndrome. 4. Evaluate whether CD34+ cells are mobilized to the damaged liver after G-CSF administration. | |
| Risk of bias | ||
| Item | Authors’ judgment | Description |
| Adequate sequence generation? | Low risk | A randomization number code was generated for each patient. |
| Allocation concealment? | Unclear | Randomization was performed with sequentially numbered envelopes. It is unclear whether the envelopes where sealed or opaque. |
| Blinding of participants and personnel? | Low risk | Both the investigators and the patients were blinded to the treatment regimen. |
| Blinding outcome assessment? | Low risk | Not specified, but taking into account that all the outcomes measures were objective values, it is unlikely that this could be a source of bias. |
| Incomplete outcome data? | Low risk | There were not drop-outs reported. |
| Selective reporting? | Low risk | Protocol available; no major differences between protocol and final publication |
| Other? | Low risk | The trial appears to be free from other source of bias. |
| • Duan, 2013 | ||
| Methods | Randomized Controlled Trial. Data collection: 06/2009 to 05/2011. Follow up period: 90 days. | |
| Participants | India Hospitalized patients with a clinical presentation of HBV-associated ACLF (APASL definition): (1) the presence of of hepatitis B surface antigen in the serum for at least 6 mo; (2) the evidence of active viral replication as indicated by detectable HBV DNA in the serum (≥ 1 x 104 copies/mL); (3) flares of hepatitis, marked by increased serum ALT level to more than 5-fold of the upper limit of normal value; and (4) age between 18 to 65 years. | |
| Exclusion criteria: | ||
| (1) Superinfection or coinfection with hepatitis A, C, D, E, EBV, CMV or HIV; (2) previous course of any antiviral, immunomodulator or cytotoxic/immunosuppressive therapy for chronic hepatitis within the prior 12 mo: (3) evidence of decompensated liver disease prior to the enrolment; (4) hepatocellular carcinoma diagnosed by CT or MRI; (5) co-existence of any other serious medical illness or other liver diseases, such as autoimmune hepatitis,alcoholic liver disease, drug-induced liver injury or Wilson’s disease; (6) any concurrent evidence of sepsis; (7) malignant jaundice induced by obstructive jaundice and hemolytic jaundice; and (8) prolonged prothrombin time due to blood system disease. | ||
| Interventions | Intervention: G-CSF 5 mg/kg/dose 6 doses. Control: no intervention. | |
| Outcomes | Evaluate the effects of G-CSF therapy on the proliferation of peripheral CD34+ cells and on liver function in patients with HBV-associated ACLF. The parameters of liver function in these patients were consecutively measured. CTP score, MELD score and survival rate were evaluated during a 3-mo follow up study. | |
| Risk of bias Item | Authors’ judgment | Description |
| Adequate sequence generation? | Low risk | A randomization number code was generated for each patient. |
| Allocation concealment? | Unclear | It is unclear whether they use central allocation, sequentially numbered drug containers, or sequentially numbered envelopes. |
| Blinding of participants and personnel? | Low risk | Both the investigators and the patients were blinded to the treatment regimen. |
| Blinding outcome assessment? | Low risk | Not specified, but taking into account that all the outcomes measures were objective values, it is unlikely that this could be a source of bias. |
| Incomplete outcome data? | Low risk | There were not drop-outs reported. |
| Selective reporting? | Unclear risk | Protocol not available. |
| Other? | Low risk | The trial appears to be free from other source of bias. |
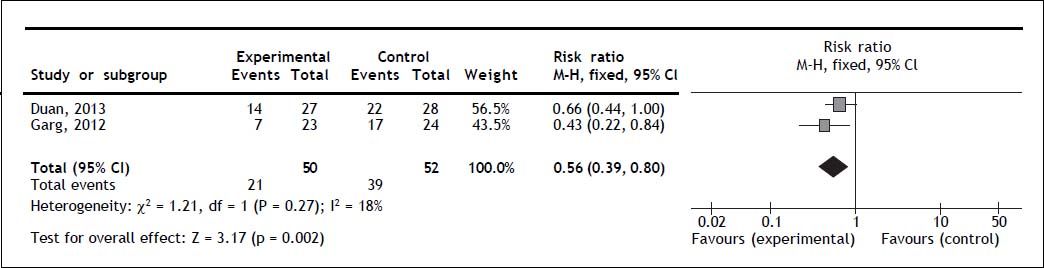
Two trials reported overall mortality. G-CSF significantly decreased overall mortality (RR 0.56; 95% CI 0.39, 0.80; P = 0.002); no heterogeneity (Q = 0.27; I2 = 18%) nor significant asymmetry in the funnel plot were detected (Figure 2). Mortality secondary to multi-organ failure. One trial reported multi-organ failure as cause of death.20 The mortality rate secondary to multi-organ failure was 13% in the experimental and 50% in the control group (P = 0.011).20
Adverse eventsOnly minor adverse events were reported in the treatment group. One study reported fever, headache and nausea,21 while the other reported rash, herpes zoster, and fever.20
Development of complicationsOnly one trial reported complications.20 The treatment arm showed a lower probability of hepatorenal syndrome than controls (19 vs. 71%, respectively; p ≤ 0.001), new-onset encephalopathy (19 vs. 66%, respectively; p = 0.001) and sepsis (14 vs. 41%, respectively; p = 0.04).20
Changes in severity indicesBoth trials reported the changes in CTP from baseline. One trial reported the outcome as the median change from baseline. At day 60, the median change in the treatment group was -33% compared to +7% in the control group.20 The second trial reported the CTP score at baseline and at day 30 the percentage of change was -8.9% and +4.9% in the experimental and control group respectively.21 Both trials reported changes in the MELD score. One trial reported the outcome as the median change from baseline.20 At day 60, the median change was -15.3% in the treatment group and +11.7% in the control group. The second trial reported the MELD score at baseline and at day 30; the percentage of change was -7.2% and +13.3% in the experimental and control group respectively.21 Only one trial reported changes in SOFA score from baseline to day 60. Median change was -50% in the treatment group and +50% in the control group.20
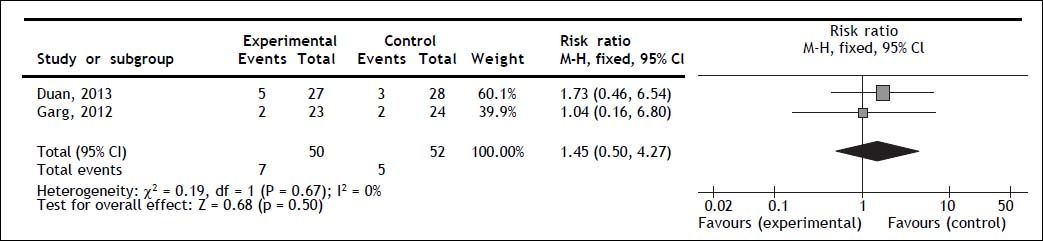
Mortality secondary to gastrointestinal bleedingBoth trials reported gastrointestinal bleeding mortality; no statistically significant difference for G-CSF therapy was observed (RR 1.45; 95% CI 0.50, 4.27; P = 0.50); no heterogeneity (Q = 0.67; I2 = 0%) nor significant asymmetry in the funnel plot were observed (Figure 3).
Changes in peripheral leukocyte and/or neutrophil countsOne trial reported a greater increase from baseline in total leukocyte count at days 2, 4, 7, 14, 21, and 30 in the treatment group compared to the control group.20 At days 42 and 60, no difference was detected. The absolute value of the leukocyte count and the units used to measure this outcome were not specified.20 The second trial reported the peripheral neutrophil count at days 3, 7, and 15. At day 15 was 5.8 ± 3.6 x 109/L vs. 4 ± 1.3 x 109/L, P = 0.032 in treatment and the control group, respectively.21
Changes in peripheral CD34+ countOne trial reported the peripheral vein CD34+ cells at day 30 as percentage.20 They reported no significant difference in peripheral CD34+ cells between groups (0.43% treatment vs. 0.41% control; P = 0.803).20 The second trial reported the peripheral CD34+ cells at days 3, 7, and 15 as mean and SD.21 At day 15, were 4.92 ± 1.63 x 109/L vs. 2.11 ± 1.39 x 109/L, P ≤ 0.001, in the treatment and control groups respectively.21
Changes in hepatic CD34+ countOnly one trial assessed this outcome.20 The percentage of hepatic CD34+ cells at baseline and day 30 was reported. The group that received G-CSF experienced a significant increase from baseline (27.5 to 40%, P = 0.001); meanwhile the control group experienced a significant decrease (30 to 20% P = 0.03).
No information regarding days of hospitalization or number of patients requiring transplantation was reported.
DiscussionThe present systematic review and meta-analysis aimed to analyze the benefits and harms of G-CSF in patients with ACLF. Patients treated with G-CSF experienced a 44% reduction in short-term mortality (60-90 days) compared to controls, as well as an improvement in liver function, and an increase in peripheral neutrophil/leukocyte counts, and peripheral and intrahepatic CD34+ cell count. Patients under G-CSF were also less likely to experience important complications such as hepatorenal syndrome, new onset encephalopathy and sepsis. Furthermore, no significant adverse events were reported in any of the trials.
Recombinant G-CSF was developed in 1985 and approved in 1991 to reduce the incidence of neutrophenic fever after myelosuppressive chemotherapy in patients with non-myeloid malignancies.23 Since its approval, several trials in animal models and humans have demonstrated G-CSF efficacy in stimulating the proliferation and differentiation of neutrophil progenitor cells as well as activation of mature neutrophils.22,24,25 These findings are consistent with the increase in the peripheral neutrophil/leukocytes and CD34+ cells observed after the administration of G-CSF in both trials included.
Bone marrow cell mobilization towards the liver is the most likely mechanism for G-CSF liver regeneration. Animal models of acute and chronic liver failure have shown that under severe liver damage bone marrow-derived stem cells can migrate and engraft into the liver.13–16,26–31 The evidence regarding the latest is not universally conclusive, since some studies have been unable to reproduce these findings.32–34 In our review, only one study evaluated the presence of CD34+ cells (a marker of hematopoietic stem cells) in liver biopsies after G-CSF treatment, confirming their presence.20 However, the effects of G-CSF in liver regeneration are not limited to the mobilization of bone marrow-derived stem cells. G-CSF exerts autocrine and paracrine effects in the liver, promoting and enhancing the oval cell reaction.13 The synergistic contribution of the bone marrow-derived stem cells and the oval cells might be responsible for the liver function improvement observed in this review.
The observed effect of G-CSF on mortality is likely the product of both liver regeneration and improved immune response. Both studies included in this review provided evidence of improved liver function demonstrated by the reduction in CTP, MELD and SOFA scores. Yet, another potential beneficial effect of G-CSF is the increase, activation and correction of neutrophil defects.35,36 The improvement in neutrophil activity can result in the reversal of the severe immunologic dysfunction that characterizes the physiopathology of ACLF, as well as the prevention of sepsis and finally in reducing the mortality.
To our knowledge, this is the first systematic review and meta-analysis to assess the role of G-CSF in the treatment of patients with ACLF. Some important limitations must be mentioned. The number of trials included in this review is small and restricted to Asian populations, limiting the external validity of the results. Eligible reports failed to report important information such as number of patients requiring transplantation, and total length of hospitalization, precluding the analysis of these important outcomes. Inconsistent data reporting made overall mortality and mortality secondary to gastrointestinal bleeding the only outcomes susceptible of meta-analysis. Both trials were considered as having low risk of bias. Taking into account that the amount of information remains scarce, the quality of the evidence should be considered limited and results interpreted carefully. Still, it is promissory that both studies included converged to the same positive result, even when different treatment regimens were used.
ACLF is associated with a very high short-term mortality and to date OLT is the only effective treatment available. However, in most cases, transplantation is not an option due to elevated costs, shortage of organs, or lack of experienced personnel. G-CSF therapy demonstrated a significant reduction in short-term mortality and improvement in liver function in ACFL patients with no significant adverse events. G-CSF could represent an alternative to improve the life quality and expectancy of ACLF patients when OLT is contraindicated or unavailable. Further randomized controlled trials evaluating the effectiveness of G-CSF in patients with ACLF are required.
AcknowledgmentNCT conceived of the study, and participated in its design and coordination, data analysis and interpretation, and helped to draft the manuscript. IMP conceived of the study, performed the literature research and selection of the included studies, and performed the data extraction. VOA collaborated with the data management, data interpretation and data analysis, and to draft the manuscript. DVP conceived of the study, performed the literature research and selection of the included studies. GDS conceived of the study, performed the literature research and selection of the included studies. CHN conceived of the study, performed the literature research and selection of the included studies, and performed the data extraction. MU provided general advice and reviewed the final manuscript. TBG conceived of the study, and participated in its design and coordination, data analysis and interpretation, and helped to draft the manuscript.
Abbreviations- •
ACLF: acute-on-chronic liver failure.
- •
CTP: Child-Turcotte-Pugh.
- •
G-CSF: granulocyte colony-stimulating factor.
- •
MELD: model of end-stage liver disease.
- •
OLT: orthotopic liver transplantation.
- •
RR: risk ratio.
- •
SOFA: sequential organ failure assessment.
No grants or financial support to declare.