Background. Carvedilol appears to be more effective than propranolol in the treatment of portal hypertension in cirrhotic patients.
Aim. To compare the effects of carvedilol vs. propranolol on systemic and splanchnic haemodynamics and to evaluate the adverse events associated with these treatments.
Material and methods. We performed a systematic review following the Cochrane and PRISMA recommendations. Randomised controlled trials comparing carvedilol versus propranolol, in the treatment of portal hypertension in cirrhotic patients with oesophageal varices, with or without bleeding history were included. The primary outcome measure was the haemodynamic response to treatment.
Results. Four randomised trials and 153 patients were included; 79 patients received carvedilol (6.25-50 mg/d) and 74 patients received propranolol (10-320 mg/d). The hepatic vein pressure gradient (HVPG) decreased more with carvedilol than with propranolol (MD -2.21; 95% CI: −2.83 to −1.60, I2 = 0%, P < 0.00001). Carvedilol was superior to propranolol for reducing HVPG by ≥ 20% from the baseline value or to ≤ 12 mmHg (OR: 2.93; 95% CI: 1.50 to 5.74, I2 = 22%, P = 0.002). Overall adverse events did not differ between. In conclusion, there is limited evidence suggesting that carvedilol is more effective than propranolol for improving the haemodynamic response in cirrhotic patients with portal hypertension. Long-term randomized controlled trials are needed to confirm this information.
The exact worldwide prevalence of cirrhosis is undefined, but it is estimated at about 0.15% or 400,000 people in the USA.1 Natural history of cirrhosis leads to portal hypertension and the development of varices and gastrointestinal bleeding.2–5 At diagnosis, varices are present in 30-40% of compensated patients and in 60% of those with ascites.6 In cirrhotic patients without varices, the annual incidence of new varices is 5-10 %.7–9 The first haemorrhagic event is a signal of decompensate disease; the 1-year rate of this event is about 5% for small varices and 15% for large varices.10 Variceal bleeding is associated with a 6-week mortality rate of 10-20 %,11 and the one-year mortality rate is 57%. 2
The risk of bleeding can be reduced significantly by decreasing the hepatic vein pressure gradient (HVPG) to < 12 mmHg or by 20% from the baseline value.12,13 The HVPG can be reduced by administration of a non-selective beta-blocker (NSBB), such as propranolol. NSBB alone is recommended for prevention of the first bleeding episode and in combination with band ligation for prevention of re-bleeding.11 Propranolol is recommended widely in the treatment of portal hypertension; unfortunately, more than a half of patients fail to achieve the haemodynamic objective because of lack of drug efficacy, intolerance to the drug, or adverse effects.
Carvedilol, an NSBB with weak anti-α1 adrenergic activity, appears to be more effective than propranolol in the reduction of portal hypertension,14–17 but there is inconsistency between studies involving head-to-head comparisons of Carvedilol vs. propranolol treatment. The primary aim of this systematic review and meta-analysis was to compare carvedilol versus propranolol for haemodynamic control of portal hypertension in cirrhotic patients. The secondary aim was to evaluate the adverse events associated with both interventions.
Material and MethodsTypes of studiesThe present systematic review and meta-analysis is based on Cochrane and PRISMA recommendations.18,19
We included randomised clinical trials that compared the efficacy of carvedilol versus propranolol therapy for control of portal hypertension in cirrhotic patients. There was no restriction regarding time, language, or publication status.
Types of participantsAll adult patients diagnosed with liver cirrhosis, portal hypertension and/or oesophageal varices with or without a history of variceal bleeding were included.
Types of interventionsStudies that compared the acute or chronic effect of carvedilol versus propranolol on haemodynamic control of portal hypertension were included. We excluded trials that evaluated interventions other than carvedilol vs. propranolol monotherapy.
Types of outcome measuresThe primary outcome measure was the haemodynamic control of portal hypertension defined as the proportion of patients with HVPG reduced ≥ 20% from the baseline or to ≤ 12mmHg. Secondary outcome measures were other haemodynamic parameters (as wedged hepatic venous pressure, free hepatic venous pressure, azygos blood flow, hepatic blood flow, heart rate, cardiac output, mean pulmonary artery pressure, wedge pulmonary artery pressure, right atrial pressure, systemic vascular resistance), adverse events including hypotension, reduction in mean arterial pressure, renal function deterioration, variceal bleeding, and bleeding-related mortality.
Search methods to identify studies1Electronic searches were performed in the Co-chrane Library and EMBASE, LILACS and MEDLINE. The literature search was performed using the medical subject headings terms “Propranolol” AND “Carvedilol” AND “Hypertension, Portal”. No limits were applied. The search results were examined for abstracts and full versions, and suitable trials were identified. The search update was performed in March 2013.
Searching other resourcesReferences of original and review articles were also reviewed to identify other relevant trials.
Selection of studiesScreening of abstracts and a selection of full-text articles were performed by two principal reviewers (NAO, NCT). They independently inspected each trial and applied the inclusion criteria. In case of disagreement, a third author reviewed the article. Justification for study exclusion was documented.
Data extraction and managementData were extracted from reports by the same two authors in an independent manner. The extracted data included the year of trial, location, participants’ characteristics, number of subjects treated in each group, dose and duration of propranolol and carvedilol treatments, outcome measures, and risk of bias. Discrepancies were resolved through discussion with the other authors.
Assessment of risk of bias in included studiesThe risk of bias was assessed following the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.О.19 The methodological quality of the trials focused on randomisation methods assessed by allocation sequence generation and allocation concealment. We included evaluations of blinding, selective reporting, and incomplete outcome data.
Measures of treatment effect and data analysisData analyses were performed using Review Manager (RevMan), version 5.2. (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). For summary measures, the results of continuous data are expressed as the mean difference (MD) with 95% confidence interval (CI), and dichotomous measures are presented as odds ratio (OR) with 95% CI. For synthesis of the results, we analysed the data using both fixed- and random-effects models; when both models produced similar estimates, the fixed-effect result is reported. Number needed to treat was calculated.
Assessment of heterogeneityHeterogeneity of effects across trials was evaluated by visual inspection of the forest plots and x2 and I2 statistic for heterogeneity. Statistical heterogeneity was defined as a P value ≤ 0.10 for χ2 or a I2 value > 25%.
Sensitivity analysisWe analysed the data using both fixed- and random-effect models. When both models produced similar estimates, the fixed-effect result is reported. Primary outcome is reported in an intention-to-treat manner.
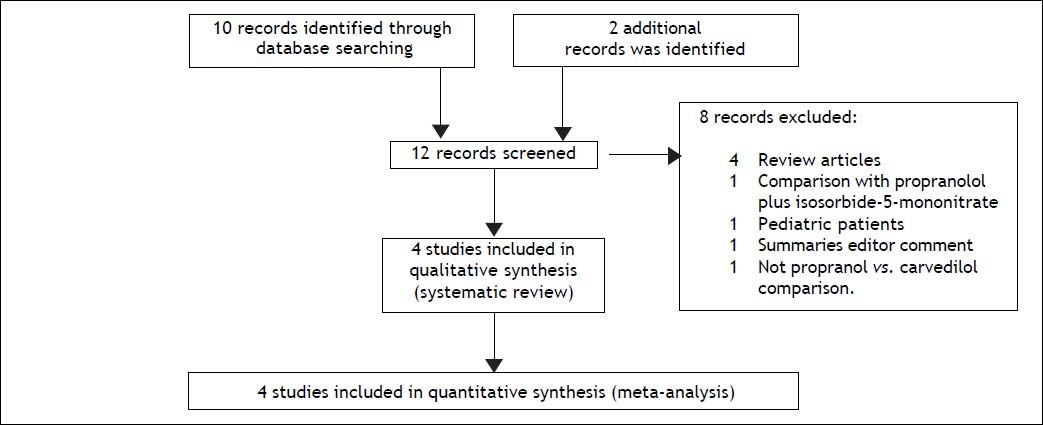
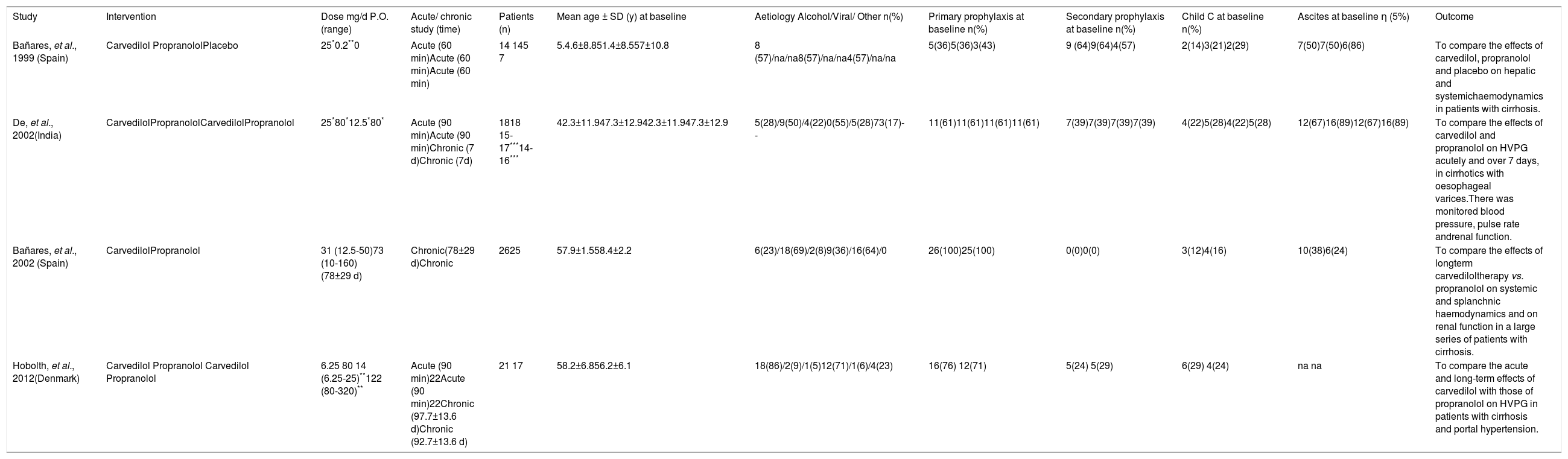
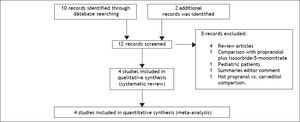
Results and DiscussionStudy selection and study characteristicsThe literature search identified 12 trials and no additional records after thorough examination of the references of review articles. A total of four head-to-head randomised trials14–17 were included in the systematic review and meta-analysis (Figure 1). The trials were conducted in Spain,14,16 India,15 and Denmark,17 and included a total of 153 patients, 79 of whom were given carvedilol (dose 6.25-50 mg/d) and 74 were given propranolol (dose 10-320 mg/d). The characteristics of the included trials are shown in Table 1. Alcoholic cirrhosis was the principal aetiology reported. All patients had severe portal hypertension (HVGP > 12 mmHg) and the presence of oesophageal varices with or without a history of variceal bleeding. The percentage of patients with primary prophylaxis was 36-100% in the studies; three trials included patients with ascites.14–16
Characteristics of head-to-head comparing included trials.
| Study | Intervention | Dose mg/d P.O. (range) | Acute/ chronic study (time) | Patients (n) | Mean age ± SD (y) at baseline | Aetiology Alcohol/Viral/ Other n(%) | Primary prophylaxis at baseline n(%) | Secondary prophylaxis at baseline n(%) | Child С at baseline n(%) | Ascites at baseline η (5%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bañares, et al., 1999 (Spain) | Carvedilol PropranololPlacebo | 25*0.2**0 | Acute (60 min)Acute (60 min)Acute (60 min) | 14 145 7 | 5.4.6±8.851.4±8.557±10.8 | 8 (57)/na/na8(57)/na/na4(57)/na/na | 5(36)5(36)3(43) | 9 (64)9(64)4(57) | 2(14)3(21)2(29) | 7(50)7(50)6(86) | To compare the effects of carvedilol, propranolol and placebo on hepatic and systemichaemodynamics in patients with cirrhosis. |
| De, et al., 2002(India) | CarvedilolPropranololCarvedilolPropranolol | 25*80*12.5*80* | Acute (90 min)Acute (90 min)Chronic (7 d)Chronic (7d) | 1818 15-17***14-16*** | 42.3±11.947.3±12.942.3±11.947.3±12.9 | 5(28)/9(50)/4(22)0(55)/5(28)73(17)-- | 11(61)11(61)11(61)11(61) | 7(39)7(39)7(39)7(39) | 4(22)5(28)4(22)5(28) | 12(67)16(89)12(67)16(89) | To compare the effects of carvedilol and propranolol on HVPG acutely and over 7 days, in cirrhotics with oesophageal varices.There was monitored blood pressure, pulse rate andrenal function. |
| Bañares, et al., 2002 (Spain) | CarvedilolPropranolol | 31 (12.5-50)73 (10-160)(78±29 d) | Chronic(78±29 d)Chronic | 2625 | 57.9±1.558.4±2.2 | 6(23)/18(69)/2(8)9(36)/16(64)/0 | 26(100)25(100) | 0(0)0(0) | 3(12)4(16) | 10(38)6(24) | To compare the effects of longterm carvediloltherapy vs. propranolol on systemic and splanchnic haemodynamics and on renal function in a large series of patients with cirrhosis. |
| Hobolth, et al., 2012(Denmark) | Carvedilol Propranolol Carvedilol Propranolol | 6.25 80 14 (6.25-25)**122 (80-320)** | Acute (90 min)22Acute (90 min)22Chronic (97.7±13.6 d)Chronic (92.7±13.6 d) | 21 17 | 58.2±6.856.2±6.1 | 18(86)/2(9)/1(5)12(71)/1(6)/4(23) | 16(76) 12(71) | 5(24) 5(29) | 6(29) 4(24) | na na | To compare the acute and long-term effects of carvedilol with those of propranolol on HVPG in patients with cirrhosis and portal hypertension. |
All studies evaluated the intervention effect on HVGP. The first study evaluated only the acute effect (60 minutes),14 the second trial reported the acute and chronic effect (90 minutes and 7 days, the last measurement was used for analysis),15 the third investigated the longer-term response (77.7 days),16 and the fourth evaluated both acute and chronic effects (90 minutes and 92.7 days).17
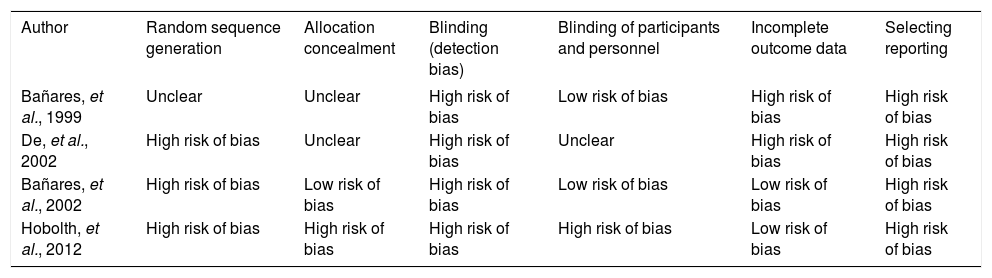
Assessment of risk of bias in trialsRandom sequence generation was reported in three studies, and the allocation concealment was reported in only one trial (Table 2). The four trials used blinded assessment, but only one used blinding of participants and personnel.17 Selective reporting of outcome measures was not registered in any trial.
Assessment of risk of bias summary for each included study.
| Author | Random sequence generation | Allocation concealment | Blinding (detection bias) | Blinding of participants and personnel | Incomplete outcome data | Selecting reporting |
|---|---|---|---|---|---|---|
| Bañares, et al., 1999 | Unclear | Unclear | High risk of bias | Low risk of bias | High risk of bias | High risk of bias |
| De, et al., 2002 | High risk of bias | Unclear | High risk of bias | Unclear | High risk of bias | High risk of bias |
| Bañares, et al., 2002 | High risk of bias | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | High risk of bias |
| Hobolth, et al., 2012 | High risk of bias | High risk of bias | High risk of bias | High risk of bias | Low risk of bias | High risk of bias |
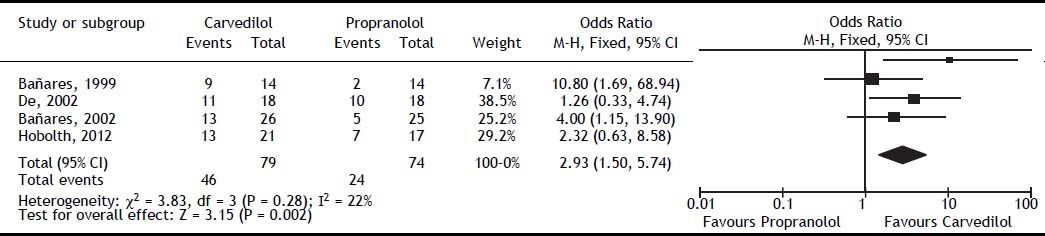
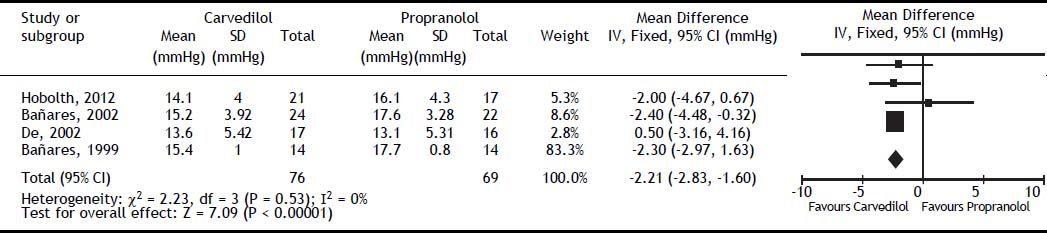
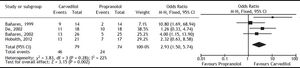
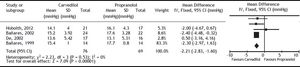
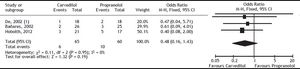
Carvedilol was superior to propranolol in reducing HVPG by ≥ 20% from the baseline value or to ≤ 12 mmHg (OR: 2.93; 95% CI: 1.50 to 5.74, I2 = 22%, P = 0.002) (Figure 2). The percentage of patients achieving this objective was 60% with carvedilol versus 35% with propranolol, with a number needed to treat of 4. The magnitude of reduction in HVPG was also greater with carvedilol (MD: -2.21; 95% CI: -2.83 to -1.60, I2 = 0%, P < 0.00001) (Figure 3).
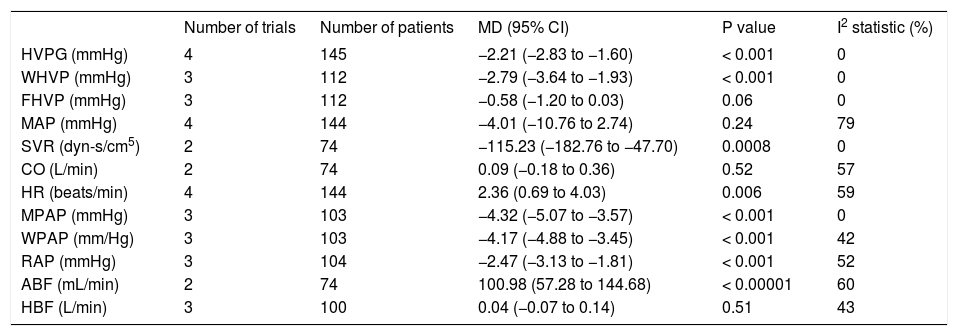
All haemodynamics parameters recorded are summarised in Table 3. The wedged hepatic venous pressure decreased significantly (MD: -2.79; 95% CI: −3.64 to −1.93, P < 0.00001), but the free hepatic venous pressure was not different (MD: -0.58; 95% CI: −1.20 to 0.03, P = 0.06).
Haemodynamics analyses from randomised clinical trials comparing efficiency of carvedilol vs. propranolol on portal hypertension in cirrhotic patients.
| Number of trials | Number of patients | MD (95% CI) | P value | I2 statistic (%) | |
|---|---|---|---|---|---|
| HVPG (mmHg) | 4 | 145 | −2.21 (−2.83 to −1.60) | < 0.001 | 0 |
| WHVP (mmHg) | 3 | 112 | −2.79 (−3.64 to −1.93) | < 0.001 | 0 |
| FHVP (mmHg) | 3 | 112 | −0.58 (−1.20 to 0.03) | 0.06 | 0 |
| MAP (mmHg) | 4 | 144 | −4.01 (−10.76 to 2.74) | 0.24 | 79 |
| SVR (dyn-s/cm5) | 2 | 74 | −115.23 (−182.76 to −47.70) | 0.0008 | 0 |
| CO (L/min) | 2 | 74 | 0.09 (−0.18 to 0.36) | 0.52 | 57 |
| HR (beats/min) | 4 | 144 | 2.36 (0.69 to 4.03) | 0.006 | 59 |
| MPAP (mmHg) | 3 | 103 | −4.32 (−5.07 to −3.57) | < 0.001 | 0 |
| WPAP (mm/Hg) | 3 | 103 | −4.17 (−4.88 to −3.45) | < 0.001 | 42 |
| RAP (mmHg) | 3 | 104 | −2.47 (−3.13 to −1.81) | < 0.001 | 52 |
| ABF (mL/min) | 2 | 74 | 100.98 (57.28 to 144.68) | < 0.00001 | 60 |
| HBF (L/min) | 3 | 100 | 0.04 (−0.07 to 0.14) | 0.51 | 43 |
HVPG: hepatic venous pressure gradient. WHVP: wedged hepatic venous pressure. FHVP: free hepatic venous pressure. ABF: azygos blood flow. HBF: hepatic blood flow. MAP: mean arterial pressure. HR: heart rate. CO: cardiac output. MPAP: mean pulmonary artery pressure. WPAP: wedge pulmonary artery pressure (mmHg). RAP: right atrial pressure. SVR: systemic vascular resistance. MD: mean difference. CI: confidence interval.
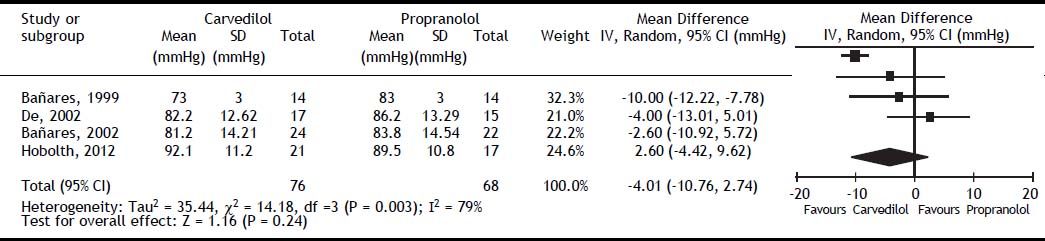
All studies reported mean arterial pressure (MAP), the overall effect on MAP did not differ between groups (MD: -4.01; 95% CI: −10.76 to 2.74, I2 = 79%, P = 0.24) (Figure 4). Systemic vascular resistance (SVR) and cardiac output (CO) were reported only in the studies by Bañares, et al.14,16 The first study by Bañares, et αl.14 reported a significant decrease in SVR, but the second study did not.16 Our meta-analysis showed a greater reduction in SVR in the carvedilol group (MD: −115.23; 95% CI: −182.76 to −47.70, P = 0.0008). For CO, results from individual studies and the overall meta-analysis did not differ between groups (MD: 0.09; 95% CI: -0.18 to 0.36, P = 0.52). Heart rate was reported in all studies and was higher with carvedilol (MD: 2.36; 95% CI: 0.69 to 4.03, P = 0.006).
Three studies14–16 included mean pulmonary arterial pressure (MPAP), right arterial pressure (RAP), and wedge pulmonary arterial pressure (WPAP). Carvedilol decreased MPAP (MD: −4.32; 95% CI: − 5.07 to −3.57, P < 0.00001), RAP (MD: −2.47; 95% CI: −3.13 to −1.81, P < 0.00001), and WPAP (MD: −4.17; 95% CI: −4.88 to −3.45, P< 0.00001).
Finally, the hepatic14–17 and azygos14–16 blood flow was evaluated. Hepatic blood flow was not different (MD: 0.04; 95% CI: -0.07 to 0.14, P = 0.51), but the azygos blood flow was increased in the carvedilol group (MD: 100.98; 95% CI: 57.28 to 144.68, P < 0.00001).
The heterogeneity was low for all outcomes, except for mean artery pressure.
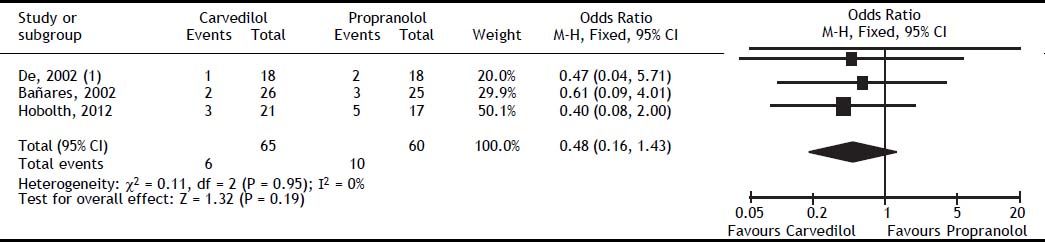
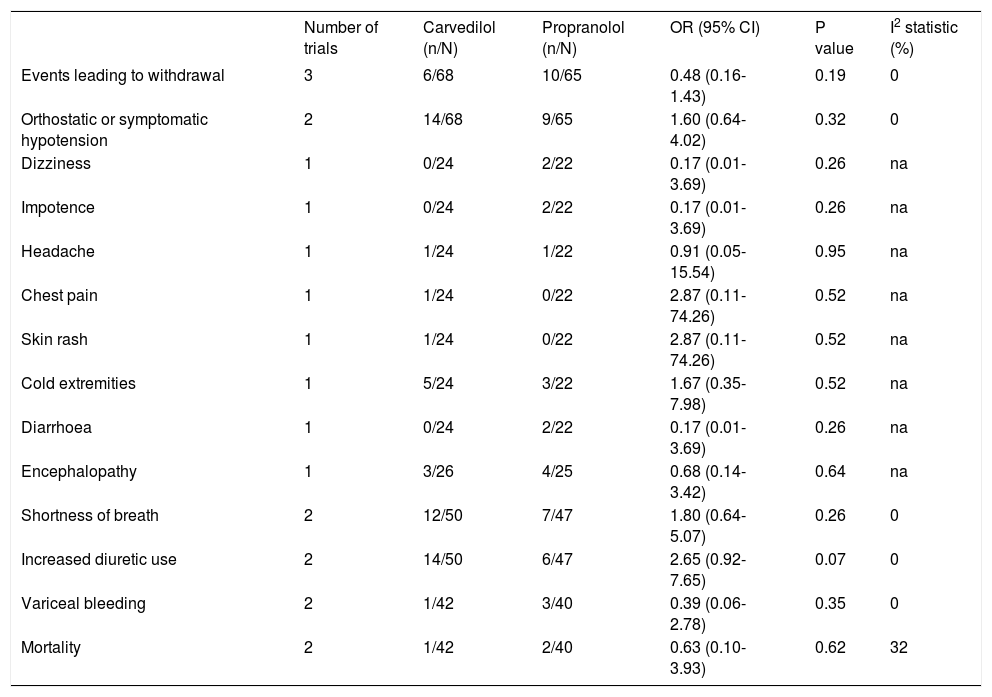
Adverse eventsAdverse events leading to withdrawal were not different between the groups (OR: 0.48; 95% CI: 0.16–1.43, I2 = 0%, P = 0.19) (Figure 5). The rate of orthostatic or symptomatic hypotension did not differ between groups (OR: 1.60; 95% CI: 0.64-4.02, P = 0.32) (Table 4). Other adverse effects such as dizziness, impotence, headache, chest pain, skin rash, cold extremities, diarrhoea, and encephalopathy were evaluated by one trial,17 which showed no differences between carvedilol and propranolol (Table 4).
Summary of adverse events reported from randomised clinical trials comparing efficiency of carvedilol vs. propranolol on portal hypertension in cirrhotic patients.
| Number of trials | Carvedilol (n/N) | Propranolol (n/N) | OR (95% CI) | P value | I2 statistic (%) | |
|---|---|---|---|---|---|---|
| Events leading to withdrawal | 3 | 6/68 | 10/65 | 0.48 (0.16-1.43) | 0.19 | 0 |
| Orthostatic or symptomatic hypotension | 2 | 14/68 | 9/65 | 1.60 (0.64-4.02) | 0.32 | 0 |
| Dizziness | 1 | 0/24 | 2/22 | 0.17 (0.01-3.69) | 0.26 | na |
| Impotence | 1 | 0/24 | 2/22 | 0.17 (0.01-3.69) | 0.26 | na |
| Headache | 1 | 1/24 | 1/22 | 0.91 (0.05-15.54) | 0.95 | na |
| Chest pain | 1 | 1/24 | 0/22 | 2.87 (0.11-74.26) | 0.52 | na |
| Skin rash | 1 | 1/24 | 0/22 | 2.87 (0.11-74.26) | 0.52 | na |
| Cold extremities | 1 | 5/24 | 3/22 | 1.67 (0.35-7.98) | 0.52 | na |
| Diarrhoea | 1 | 0/24 | 2/22 | 0.17 (0.01-3.69) | 0.26 | na |
| Encephalopathy | 1 | 3/26 | 4/25 | 0.68 (0.14-3.42) | 0.64 | na |
| Shortness of breath | 2 | 12/50 | 7/47 | 1.80 (0.64-5.07) | 0.26 | 0 |
| Increased diuretic use | 2 | 14/50 | 6/47 | 2.65 (0.92-7.65) | 0.07 | 0 |
| Variceal bleeding | 2 | 1/42 | 3/40 | 0.39 (0.06-2.78) | 0.35 | 0 |
| Mortality | 2 | 1/42 | 2/40 | 0.63 (0.10-3.93) | 0.62 | 32 |
OR: odds ratio. CI: confidence interval. na: not applicable.
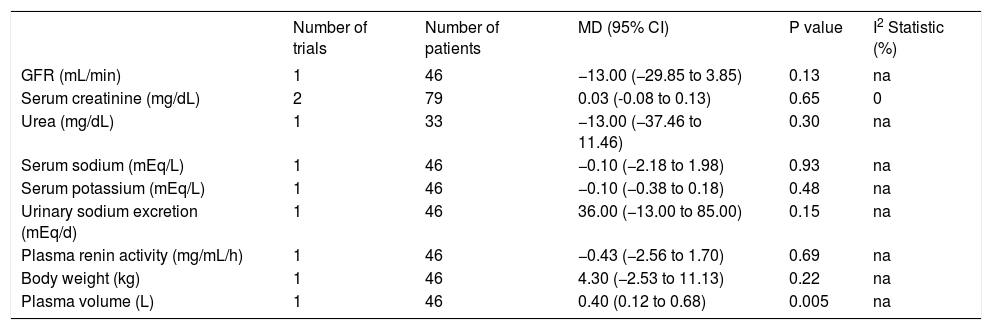
Renal function, including glomerular filtration rate; serum concentrations of creatinine, urea, sodium, and potassium; urinary sodium excretion; plasma renin activity; and body weight did not differ between the treatments (Table 5). Bañares, et al.16 found a higher plasma volume in the carvedilol group (MD: 0.40; 95% CI: 0.12 to 0.68, P = 0.005), and two studies16,17 reported a tendency toward increased diuretic consumption in the carvedilol group (OR: 2.65; 95% CI: 0.92 to 7.65, P = 0.07).
Renal function after carvedilol or propranolol treatment for portal hypertension in cirrhotic patients.
| Number of trials | Number of patients | MD (95% CI) | P value | I2 Statistic (%) | |
|---|---|---|---|---|---|
| GFR (mL/min) | 1 | 46 | −13.00 (−29.85 to 3.85) | 0.13 | na |
| Serum creatinine (mg/dL) | 2 | 79 | 0.03 (-0.08 to 0.13) | 0.65 | 0 |
| Urea (mg/dL) | 1 | 33 | −13.00 (−37.46 to 11.46) | 0.30 | na |
| Serum sodium (mEq/L) | 1 | 46 | −0.10 (−2.18 to 1.98) | 0.93 | na |
| Serum potassium (mEq/L) | 1 | 46 | −0.10 (−0.38 to 0.18) | 0.48 | na |
| Urinary sodium excretion (mEq/d) | 1 | 46 | 36.00 (−13.00 to 85.00) | 0.15 | na |
| Plasma renin activity (mg/mL/h) | 1 | 46 | −0.43 (−2.56 to 1.70) | 0.69 | na |
| Body weight (kg) | 1 | 46 | 4.30 (−2.53 to 11.13) | 0.22 | na |
| Plasma volume (L) | 1 | 46 | 0.40 (0.12 to 0.68) | 0.005 | na |
GFR: glomerular filtration rate. MD: mean difference. CI: confidence interval. na: not applicable.
Finally, variceal bleeding and mortality were reported in two trials,15,17 and these did not differ between treatments (Table 4).
This systematic review and meta-analysis analysed the current head-to-head randomised trials comparing carvedilol versus propranolol for portal hypertension in cirrhotic patients. Portal pressure decreased more with carvedilol compared with propranolol treatment. A higher percentage of patients showed a reduction in HVPG by ≥ 20% from the baseline value or to ≤ 12 mmHg after carvedilol than after propranolol administration. Analysis of adverse events showed no significant differences between carvedilol and propranolol. The results of this meta-analysis suggest that carvedilol could be an alternative for primary and secondary variceal bleeding prophylaxis in selected cirrhotic patients.
Differences in the specific doses of carvedilol and propranolol used to treat portal hypertension might have contributed to the differences between the studies. Studies with higher doses of carvedilol14,16 were those that showed statistically significant differences in their favour. This agrees with the previous finding by Bañares, et al. that the haemodynamic effect of carvedilol is dose dependent.14 However, some studies have suggested that lower doses of carvedilol (12.5 mg/d) provide a good portal pressure-reducing effect with less systemic vasodilation.20,21 Previously, two meta-analysis abstracts comparing carvedilol vs. pro-pranolol in portal hypertension were published.22,23 Razon-Gonzalez, et al. find that carvedilol is superior to propranolol in reducing HVPG (−8.36, 95% CI: −9.43 to −7.28, p < 0.00001), but MAP is significantly reduced in carvedilol group (-8.62, 95% CI: -9.63 to -7.61, P < 0.00001). Sinagra, et al. reported chronic effect of carvedilol vs. propranolol in HVPG reduction (−6.79, 95% CI: −12.04 to −1.54), but severe hypotension and need for increasing diuretics was reported with carvedilol.
In clinical practice, the findings of our meta-analysis may benefit patients who are unresponsive to treatment with propranolol because a higher percentage of patients, about 25%, reached the target HVPG reduction after carvedilol administration. In this concern, the greater therapeutic potential of carvedilol over propranolol has recently been demonstrated in a pragmatic design.24
In regard to adverse events, we did not find significant differences in the meta-analysis. However this should be considered carefully in clinical practice or future clinical trials. Acute administration of carvedilol seems to cause significant systemic vasodilatation, and could worsen the hemodynamics in the cirrhotic patients.
We found some limitations in this review. A low number of patients were considered in each trial and the results for some haemodynamics were obtained from a minimum number of patients. Another limitation is that there was no standardisation of the carvedilol and propranolol doses, so the ranges were broad and we find some heterogeneity between the studies. Finally, the studies were conducted with short follow-up periods; thus, there is no information from long-term comparisons and no data on clinical outcomes such as the long-term adverse effects, variceal bleeding, and mortality.
Future studies are needed with a larger number of patients and long-term monitoring, directed at clinical outcomes, mainly variceal bleeding and mortality.
ConclusionThis systematic review and meta-analysis showed limited evidence suggesting that carvedilol is more effective than propranolol for improving the haemo-dynamic response in cirrhotic patients with portal hypertension. However the effect on adverse events is not clear and should be assessed carefully.
Abbreviations- •
CO: cardiac output.
- •
HPVG: hepatic vein pressure gradient.
- •
MAP: mean arterial pressure.
- •
MD: mean difference.
- •
MPAP: mean pulmonary arterial pressure.
- •
NSBB: non-selective beta blocker.
- •
RAP: right arterial pressure.
- •
SVR: systemic vascular resistance.
- •
WPAP: wedge pulmonary arterial pressure
This research received no specific grant from any funding agency in the public, commercial or not-forprofit sectors.