Purpose. To elucidate the impact of right atrial (RA) pressure on early mortality after transjugular intrahepatic portosystemic shunt (TIPS).
Material and methods. In this single institution retrospective study, 125 patients (M:F = 75:50, mean age 55 years) who underwent TIPS with recorded intra-procedural RA pressures between 1999-2012 were studied. Demographic (age, gender), liver disease (Child-Pugh, Model for End Stage Liver Disease or MELD score), and procedure (indication, urgency, Stent type, portosystemic gradient or PSG reduction, baseline and post-TIPS RA pressure) data were identified, and the influence of these parameters on 30- and 90-day mortality was assessed using binary logistic regression.
Results. TIPS were created for variceal hemorrhage (n = 55) and ascites (n = 70). Hemodynamic success rate was 99% (124/125) and mean PSG reduction was 13 mmHg. 30- and 90-day mortality rates were 18% (19/106) and 28% (29/106). Baseline and final RA pressure were significantly associated with 30- (12 vs. 15 mmHg, P = 0.021; 18 vs. 21 mmHg, P = 0.035) and 90-day (12 vs. 14 mmHg, P = 0.022; 18 vs. 20 mmHg, P = 0.024) survival on univariate analysis. Predictive usefulness of RA pressure was not confirmed in multivariate analyses. Area under receiver operator characteristic (AUROC) curve analysis revealed good pre- and post-TIPS RA pressure predictive capacity for 30- (0.779, 0.810) and 90-day (0.813, 0.788) mortality among variceal hemorrhage patients at 14.5 and 21.5 mm Hg thresholds.
Conclusion. Intra-procedural RA pressure may have predictive value for early post-TIPS mortality. Pre-procedure consideration and optimization of patient cardiac status may enhance candidate selection, risk stratification, and clinical outcomes, particularly in variceal hemorrhage patients.
Transjugular intrahepatic portosystemic shunt (TIPS) creation alleviates portal hypertension by diverting blood from the portal venous system into the systemic circulation, reducing hepatic sinusoidal pressure, and increasing effective circulatory volume.1 The immediate hemodynamic consequences of portosystemic shunting include increased cardiac preload, elevated pulmonary wedge pressures, raised cardiac output, and increased stroke volume.2–5 While these changes are typically well tolerated in the early post-procedure period with further cardiac adaptation over time,2,5 patients with limited cardiac reserve may suffer acute pulmonary edema, the unmasking of underlying cardiomyopathy, or cardiac failure immediately after TIPS.3,6,7 Accordingly, pre-procedure echocardiographic evaluation is routinely performed on at-risk patients, and elevated right atrial (RA) and pulmonary artery pressures constitute relative contraindications to TIPS creation.8 Given the well-documented association between elevated baseline RA pressure and mortality in other conditions,9 it is conceivable that RA pressure elevation may negatively impact early survival of patients after TIPS creation. However, data describing the relationship of RA pressure to TIPS clinical outcomes are limited, and there is currently no cutoff RA pressure that represents an absolute threshold for high-risk patients. With this in mind, the current study was undertaken to elucidate the impact of RA pressure on early mortality in patients undergoing TIPS creation.
Materials and MethodsThis retrospective study was conducted in compliance with the Health Insurance Portability and Accountability Act, and the institutional review board at our institution granted approval with waiver of consent for inclusion. All patients provided written informed consent for TIPS procedures, which were created within medical standard of care for various indications.
Clinical setting and study designBetween November 1999 and July 2012, consecutive patients with liver cirrhosis who underwent successful TIPS creation at a single tertiary care, academic university affiliated hospital situated in a large metropolitan area were identified and selected for retrospective study. Patients were identified through review of our hospital’s Picture Archiving and Communication System (PACS).
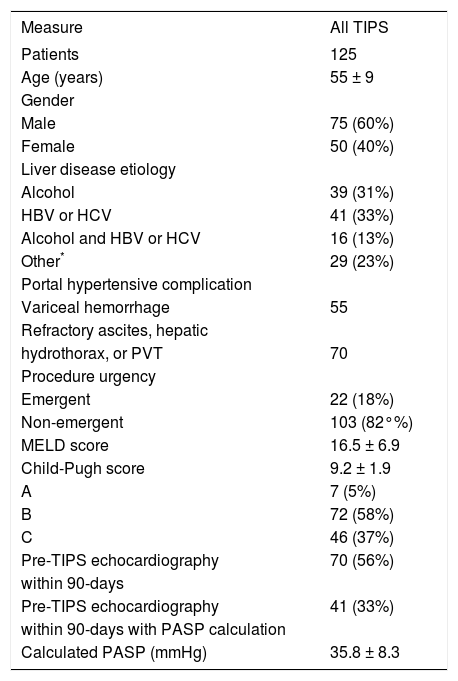
Patients and liver diseaseTwo hundred forty six patients who underwent technically successful TIPS creation were identified for potential inclusion in this retrospective study. Of these, 200 patients with recorded pre- and post-TIPS intra-procedural RA pressures were selected for analysis. Patients lacking 30- or 90-day clinical follow-up were then excluded from analysis. Thus, 125 patients were included in the final study cohort. Patient demographics, liver disease characteristics, and clinical presentation data of the study cohort are summarized in table 1. Variceal bleeding was confirmed by upper endoscopy. Resistant or refractory ascites and hepatic hydrothorax were not adequately controlled by conventional therapy, including dietary sodium restriction, fluid restriction, and diuretic therapy. Patients with portal vein thrombus underwent TIPS to prevent clot propagation in order to maintain liver transplant candidacy, and were not necessarily symptomatic (e.g. bleeding, ascites) in any way. Emergent cases included those with acute hemorrhage, hemodynamic instability (lowest recorded supine mean arterial blood pressure within 24 h before TIPS creation ≤ 65 mm Hg) and/or vasopressor requirement within 24 h of TIPS creation.10 The remaining cases were classified as non-emergent. 7/125 (6%) TIPS were performed in previously transplanted patients. Of note, nearly two-thirds of the study cohort underwent pre-procedure echocardiographic evaluation within 3-months prior to TIPS, and pulmonary artery systolic pressure (PASP) was calculated in 41/125 (33%) of patients. The calculated mean PASP among these patients was less than the 40-50 mm Hg threshold commonly used as a screening cutoff for portopulmonary hypertension diagnosis.11
Patient demographics and liver disease characteristics.
| Measure | All TIPS |
|---|---|
| Patients | 125 |
| Age (years) | 55 ± 9 |
| Gender | |
| Male | 75 (60%) |
| Female | 50 (40%) |
| Liver disease etiology | |
| Alcohol | 39 (31%) |
| HBV or HCV | 41 (33%) |
| Alcohol and HBV or HCV | 16 (13%) |
| Other* | 29 (23%) |
| Portal hypertensive complication | |
| Variceal hemorrhage | 55 |
| Refractory ascites, hepatic | |
| hydrothorax, or PVT | 70 |
| Procedure urgency | |
| Emergent | 22 (18%) |
| Non-emergent | 103 (82°%) |
| MELD score | 16.5 ± 6.9 |
| Child-Pugh score | 9.2 ± 1.9 |
| A | 7 (5%) |
| B | 72 (58%) |
| C | 46 (37%) |
| Pre-TIPS echocardiography | 70 (56%) |
| within 90-days | |
| Pre-TIPS echocardiography | 41 (33%) |
| within 90-days with PASP calculation | |
| Calculated PASP (mmHg) | 35.8 ± 8.3 |
HBV: hepatitis B virus. HCV: hepatitis C virus. PVT: portal vein thrombosis. MELD: Model for End Stage Liver Disease. TIPS: transjugular intrahepatic portosystemic shunt. PASP: pulmonary artery systolic pressure.
The technique for TIPS placement has been previously described.10 Procedures were performed in the Interventional Radiology (IR) suite using general anesthesia. Right jugular venous access was gained with dilation to a 10 French sheath. The sheath was advanced into the RA and pressure measurement was performed. A 5 French catheter was used to engage the right hepatic vein. After hepatic venography and pressure measurement, wedged hepatic venography was performed. Next, a Rösch-Uchida transjugular liver access set (Cook Medical Co., Bloomington IN) was used to access the right portal vein. After portal vein catheterization and direct portal vein pressure measurement, balloon dilation of the hepatic parenchymal tract was performed. Next, direct portography was performed. Subsequently, 10 mm Viatorr covered stent-grafts (W.L. Gore & Associates, Flagstaff AZ) were deployed across the liver tract. If the distal shunt fell short of the hepatic vein to inferior vena cava (IVC) junction, additional stents were utilized to extend the shunt. Balloon angioplasty was performed using a 7-10 mm balloon. After measurement of portal and RA pressures, shunt venography was performed. Coil embolization of gastroesophageal varices was performed following TIPS creation in cases of variceal hemorrhage at the discretion of the primary operator. Selective catheterization of the coronary vein or gastroesophageal varix was performed using a 5 French catheter or microcatheter. Embolization was then performed using 0.035 inch or 0.018 inch metallic coils. Repeat portal and RA pressures were obtained after embolization.
Post-procedure care and clinical follow-upFollowing TIPS procedures, patients were monitored in an intensive care unit. Immediate post-procedure clinical follow-up was performed while patients remained hospitalized following TIPS. Subsequent clinical follow-up was in the outpatient Hepatology clinic. Patients were monitored for evidence of shunt dysfunction using Doppler ultrasound at 1-month, 3-months, and subsequent 6-month intervals post-procedure.
Measured outcomesThe primary outcome measures of this study were 30- and 90-day overall mortality and the impact of intra-procedural pre- and post-TIPS RA pressures on patient survival outcomes. Secondary outcome measures included the effect of demographic factors, liver disease scores, and other procedure parameters on patient survival outcomes, TIPS hemodynamic success, and procedure-related complications. Patient mortality was identified through electronic medical record review and was confirmed using the United States Social Security Death Index. TIPS hemodynamic success was defined as reduction in the portosystemic pressure gradient (PSG) to an absolute value ≤ 12 mmHg. Procedure-related complications were classified according to the Society of Interventional Radiology (SIR) Standards of Practice Committee classification of complications.12 Post-TIPS hepatic encephalopathy was defined by development of new mental status changes (confusion) or alterations in level of consciousness. Presence of hepatic encephalopathy was determined clinically by the patient’s hepatologist and was graded according to the West Haven classification system.13
Statistical analysisDescriptive statistics were used to characterize demographic features of the study population. Comparisons for continuous variables were performed by the independent samples t-test. Comparisons for categorical data were performed using Pearson’s χ2 test. Multivariate binary logistic regression analysis was used to assess the influence of demographic factors (age, gender), liver disease scores (Child-Pugh, Model for End Stage Liver Disease or MELD score), and procedure parameters (urgency, indication, PSG reduction) including RA pressures- on patient transplant free survival at 30- and 90-days post-procedure. A significance level of P ≤ 0.10 in univariate analysis was used as a cutoff to include a variable in multivariate analysis. Area under receiver operator characteristic (AUROC) curve analysis was utilized to determine numerical thresholds for RA pressure predictive capacity for survival outcome at various time points based on optimal sensitivity and specificity values. AUROC curves were compared using the method of DeLong, et al.14 Statistical analysis was performed utilizing commercially available software packages (SPSS version 18; SPSS Inc., Chicago IL). P-values ≤ 0.05 were considered statistically significant.
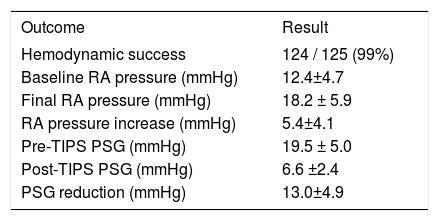
ResultsTIPS procedures and procedure-related complicationsTIPS procedure results are summarized in table 2. Variceal embolization was performed in 24/55 (44%) bleeding patients. Thirty day procedure-related adverse events included hepatic encephalopathy in 42/125 (34%) patients, and hepatic ischemia or infarction in 9/125 (7%) patients. Encephalopathy was predominantly mild, with 24/42 (57%) patients categorized as grade 1, 12/42 (29%) patients classified as grade 2 or 3, and 6/42 (14%) patients classified as grade 4. Notably, there were no cases of TIPS related cardiac failure.
TIPS results.
| Outcome | Result |
|---|---|
| Hemodynamic success | 124 / 125 (99%) |
| Baseline RA pressure (mmHg) | 12.4±4.7 |
| Final RA pressure (mmHg) | 18.2 ± 5.9 |
| RA pressure increase (mmHg) | 5.4±4.1 |
| Pre-TIPS PSG (mmHg) | 19.5 ± 5.0 |
| Post-TIPS PSG (mmHg) | 6.6 ±2.4 |
| PSG reduction (mmHg) | 13.0±4.9 |
TIPS: transjugular intrahepatic portosystemic shunt. RA: right atrial. PSG: portosystemic gradient.
Four and seven patients underwent liver transplantation within 30- and 90-days, respectively, and were therefore censored from the survival analysis. Transplant free 30- and 90-day patient survival rates were 84% (102/121) and 75% (88/118). Causes of death included gastrointestinal hemorrhage (n = 7), sepsis (n = 6), liver failure (n = 4), and unspecified (n = 13). Of the 13 patients with an unspecified cause of death, seven (54%) underwent echocardiography within 90 days prior to TIPS, and none had a prior clinical diagnosis of heart failure. The mean PASP among these seven patients was 37.8 ± 8.6 mmHg. Three of these seven had PASP values (41, 41, and 52 mmHg) within or above the 40-50 mmHg threshold range commonly used as a screening cutoff for portopulmonary hypertension diagnosis;11 as none underwent confirmatory right heart catheterization, the presence of portopulmonary hypertension could not be ruled out, and it is unknown whether post-TIPS exacerbation contributed to death.
Results of 30-day univariate and multivariate analyses are presented in table 3, and results of 90-day univariate and multivariate analyses are presented in table 4. Univariate analysis revealed that baseline RA pressure and final RA pressure were significantly associated with survival at 30- and 90-days, as were Child-Pugh score and MELD score. However, only MELD score was confirmed as independently significant on multivariate analysis for both time points. No other parameters (age, gender, procedure indication, procedure urgency, and PSG reduction) showed a statistically significant relationship with survival.
Analysis of prognostic factors affecting 30-day mortality after TIPS.
| Factor | Living | Dead | Univariate analysis | Multivariate analysis |
|---|---|---|---|---|
| Patient age (years) | 55 | 57 | 0.302 | - |
| Patient gender | 0.876 | - | ||
| Male | 61 | 11 | ||
| Female | 41 | 8 | ||
| Child-Pugh score | 8.9 ± 1.7 | 10.5 ± 2.1 | < 0.001 | 0.324 |
| MELD score | 14.8 ± 5.0 | 23.7 ± 9.3 | < 0.001 | 0.001 |
| Procedure urgency | 0.261 | - | ||
| Emergent | 16 | 5 | ||
| Non-emergent | 86 | 14 | ||
| Procedure indication | 0.398 | - | ||
| Bleeding | 43 | 10 | ||
| Other | 59 | 9 | ||
| PSG reduction (mmHg) | 13.0 ± 4.9 | 13.3 ± 7.1 | 0.796 | - |
| Baseline RA pressure (mmHg) | 11.9 ± 4.3 | 14.7 ± 6.4 | 0.021 | 0.618 |
| Final RA pressure (mmHg) | 17.7 ± 5.2 | 20.8 ± 7.8 | 0.035 | 0.511 |
| RA pressure increase (mmHg) | 5.8 ± 3.9 | 6.2 ± 5.1 | 0.703 | - |
TIPS: transjugular intrahepatic portosystemic shunt. MELD: Model for End Stage Liver Disease. PSG: portosystemic gradient. RA: right atrial.
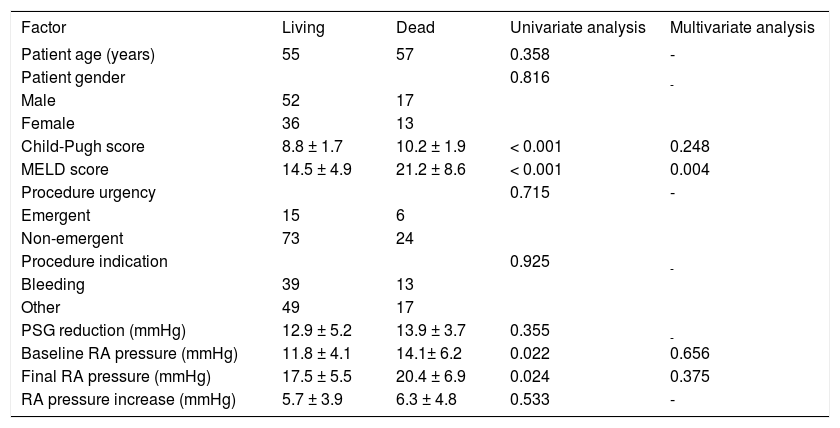
Analysis of prognostic factors affecting 90-day mortality after TIPS.
| Factor | Living | Dead | Univariate analysis | Multivariate analysis |
|---|---|---|---|---|
| Patient age (years) | 55 | 57 | 0.358 | - |
| Patient gender | 0.816 | - | ||
| Male | 52 | 17 | ||
| Female | 36 | 13 | ||
| Child-Pugh score | 8.8 ± 1.7 | 10.2 ± 1.9 | < 0.001 | 0.248 |
| MELD score | 14.5 ± 4.9 | 21.2 ± 8.6 | < 0.001 | 0.004 |
| Procedure urgency | 0.715 | - | ||
| Emergent | 15 | 6 | ||
| Non-emergent | 73 | 24 | ||
| Procedure indication | 0.925 | - | ||
| Bleeding | 39 | 13 | ||
| Other | 49 | 17 | ||
| PSG reduction (mmHg) | 12.9 ± 5.2 | 13.9 ± 3.7 | 0.355 | - |
| Baseline RA pressure (mmHg) | 11.8 ± 4.1 | 14.1± 6.2 | 0.022 | 0.656 |
| Final RA pressure (mmHg) | 17.5 ± 5.5 | 20.4 ± 6.9 | 0.024 | 0.375 |
| RA pressure increase (mmHg) | 5.7 ± 3.9 | 6.3 ± 4.8 | 0.533 | - |
TIPS: transjugular intrahepatic portosystemic shunt. MELD: Model for End Stage Liver Disease. PSG: portosystemic gradient. RA: right atrial.
Subset analysis of the variceal hemorrhage patient cohort revealed that baseline RA pressure (12.3 vs. 17.4 mmHg, P = 0.002), final RA pressure (17.6 vs. 24.2 mmHg, P = 0.001), Child-Pugh score (8.3 vs. 11.2, P < 0.001), and MELD score (13.9 vs. 30.5, P < 0.001) were significantly associated with survival at 30-days post-TIPS; none of these factors were independently significant on multivariate analysis. At 90-days post-TIPS, baseline RA pressure (11.9 vs. 17.0, P = 0.001), final RA pressure (17.3 vs. 23.2 mmHg, P = 0.002), Child-Pugh score (8.3 vs. 10.5, P < 0.001), and MELD score (13.8 vs. 26.5, P < 0.001) were significantly associated with survival; MELD score was confirmed as independently significant on multivariate analysis (P = 0.031).
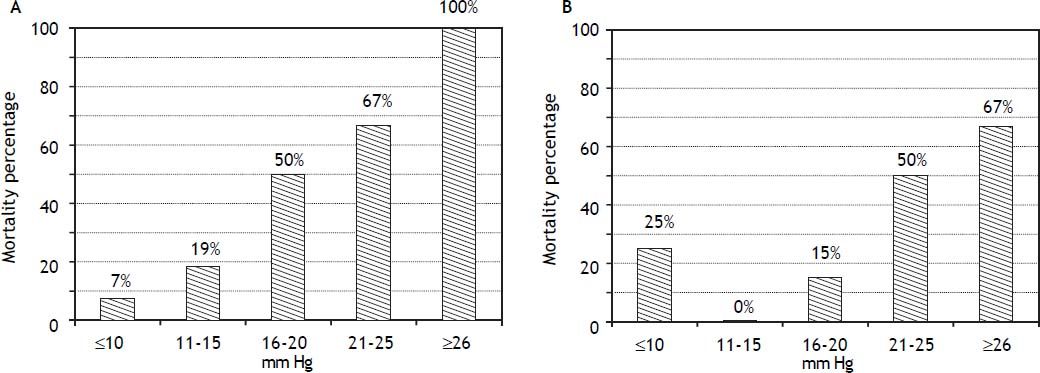
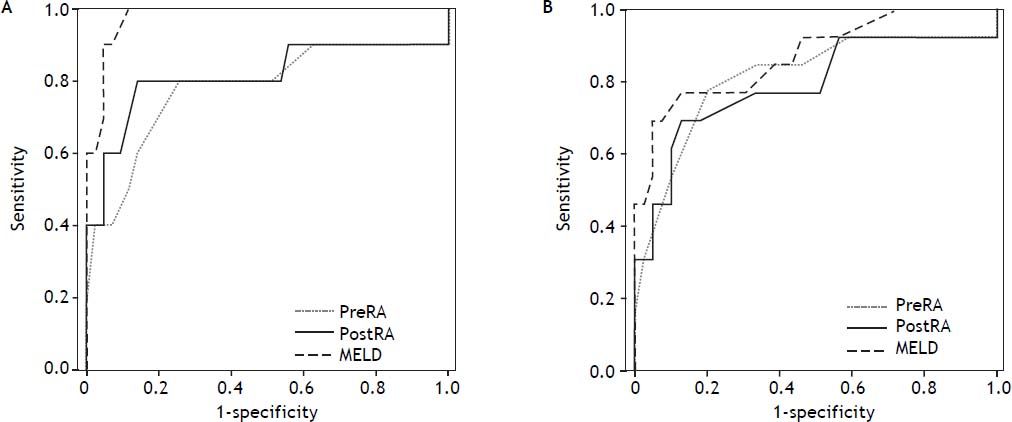
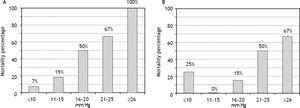
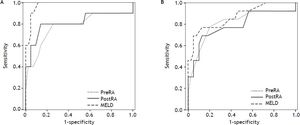
RA pressure and TIPS mortalityBased on significant univariate association, further analysis of the relationship of intra-procedural RA pressure measurements and TIPS survival outcomes was performed, with particular attention to variceal hemorrhage patients. Figure 1 demonstrates the 90-day mortality risk among variceal hemorrhage patients based on incremental baseline and final RA pressures, and figure 2 shows predictive capacity of baseline and final RA pressures for survival based on AUROC curves.
AUROC curves demonstrate relative performance of MELD score, baseline RA pressure, and final RA pressure in patients with variceal hemorrhage at 30-days (A) and 90-days (B) after TIPS creation. At 30- and 90-days, both baseline (0.779, 95% CI 0.582-0.977; 0.810, 95% CI 0.651-0.969) and final (0.813, 95% CI 0.615-1.00; 0.788, 95% CI 0.620-0.956) RA pressures demonstrate good predictive capacity for survival.
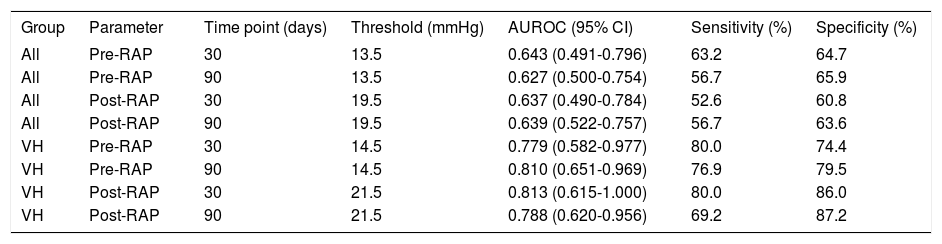
The predictive capacities of RA pressures for postTIPS mortality are presented in table 5, and indicate poor predictive capacity of RA pressure for 30-and 90-day post-TIPS mortality among all patients studied. However, the predictive capacity of RA pressure for 30- and 90-day post-TIPS mortality among variceal hemorrhage patients was good, suggesting utility to this measure in this particular patient population. In the variceal hemorrhage cohort, mortality in patients with baseline RA pressure ≤ 14.5 vs. ≥ 14.5 mmHg was 6 vs. 42% (P = 0.035) at 30-days and 9 vs. 56% (P < 0.001) at 90-days. In patients with final RA pressure ≤ 21.5 vs. ≥ 21.5 mmHg, mortality was 5 vs. 57% (P < 0.001) at 30-days and 11 vs. 64% (P < 0.001) at 90-days.
Predictive capacity of RAP for post-TIPS mortality.
| Group | Parameter | Time point (days) | Threshold (mmHg) | AUROC (95% CI) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| All | Pre-RAP | 30 | 13.5 | 0.643 (0.491-0.796) | 63.2 | 64.7 |
| All | Pre-RAP | 90 | 13.5 | 0.627 (0.500-0.754) | 56.7 | 65.9 |
| All | Post-RAP | 30 | 19.5 | 0.637 (0.490-0.784) | 52.6 | 60.8 |
| All | Post-RAP | 90 | 19.5 | 0.639 (0.522-0.757) | 56.7 | 63.6 |
| VH | Pre-RAP | 30 | 14.5 | 0.779 (0.582-0.977) | 80.0 | 74.4 |
| VH | Pre-RAP | 90 | 14.5 | 0.810 (0.651-0.969) | 76.9 | 79.5 |
| VH | Post-RAP | 30 | 21.5 | 0.813 (0.615-1.000) | 80.0 | 86.0 |
| VH | Post-RAP | 90 | 21.5 | 0.788 (0.620-0.956) | 69.2 | 87.2 |
RAP: right atrial pressure. TIPS: transjugular intrahepatic portosystemic shunt. AUROC: area under receiver operator curve. VH: variceal hemorrhage.
In comparison to RA pressure prognostic capability, the discriminative capacity of MELD score was 0.978 (95% CI 0.945-1.000) and 0.871 (95% CI 0.751-0.991) at 30- and 90-days, respectively. MELD score was statistically superior to baseline RA pressure for prediction of 30-day survival (P = 0.041), but no other statistically significant difference was present between AUROC curves.
DiscussionThe cardiovascular manifestations of decompensated liver cirrhosis include splanchnic arterial vasodilation, hyper dynamic circulation, and elements of systolic and diastolic cardiac dysfunction.15 These derangements may be exacerbated by TIPS, which shunts splanchnic blood and vasodilators into the central circulation, causing an acute increase in cardiac preload and further systemic vasodilation.4,16 Although these changes typically normalize within six months to one year,2,17 patients with diminished cardiac reserve have limited adaptive capacity and may be at particular risk during the interim. Identification of such high-risk patients is therefore paramount in order to refine selection criteria and implement supportive measures.
RA pressure, which is associated with survival outcomes in conditions in other settings,9 is routinely measured by IRs in order to calculate the PSG, an established indicator of TIPS hemodynamic success. In examining outcomes in 125 patients, we found that intra-procedural RA pressure elevation was associated with early post-TIPS mortality on univariate analysis. Patients with a baseline RA pressure exceeding 14 mmHg or a final RA pressure exceeding 20 mmHg had approximately twice the mortality of those with pressures below these thresholds. This finding was more pronounced in the subset of patients undergoing TIPS for variceal hemorrhage, in which elevated RA pressure was associated with a five to ten-fold increase in mortality and was very predictive of both 30-and 90-day survival outcomes on AUROC analysis. Although limited sample size may have precluded multivariate confirmation of these findings (evaluation in larger cohorts may prove useful), our data highlight the utility of pre-TIPS cardiac assessment, and may suggest a role for baseline RA pressure measurement in prognosticating early survival outcomes. Baseline and final RA pressures exceeding 14 and 20-21 mmHg, respectively, may serve as a cutoff for pre- and post-TIPS identification of high risk patients who would derive particular benefit from careful monitoring and optimization of cardiac and fluid status.
The use of the RA as a reference point for calculation of PSG is controversial, as its intra-thoracic position may yield an inaccurate measurement of transhepatic pressure.18 In patients with RA pressure lower than IVC pressure, use of the former for calculation of PSG may prompt excess dilation of the TIPS in order to achieve satisfactory gradient reduction. This may consequently increase the risk of shunt-related adverse events such as encephalopathy or liver failure, and may account for a portion of such events in our cohort. Nonetheless, the RA has potential benefits over the hepatic vein -which is subject to artifactually increased pressures due to portal venous inflow following TIPS creation- and the IVC, which lacks a standardized location for obtaining pressure measurements,18 but has been utilized in landmark TIPS investigations.19–21 One study advocates against the use of RA pressure for PSG measurement; however, this recommendation is made on the basis of data derived from non-TIPS cases and thus cannot be extrapolated to TIPS patients.22 Though not ideal, measurement of the PSG from the main portal vein to the RA remains a widely utilized method for TIPS hemodynamic assessment and is supported by current SIR clinical practice guidelines,23 although not advocated by the Cardiovascular and Interventional Radiology Society of Europe.24 Our data support the potential utility of RA pressure measurement for its role in identification of high-risk patients and its potential predictive capacity for early survival outcomes in variceal bleeders. However, given the controversy surrounding the proper location for PSG determination and the potential differences in prognostic capacity, further investigation into the issue is clearly warranted.
There are several limitations to this investigation. First, this study was retrospective and non-randomized in nature, and is subject to the inherent weaknesses of non-prospective studies. Second, our investigation represents the experience of a single institution and includes a relatively small sample size, as insufficient right atrial pressure data resulted in exclusion of nearly half of the potential cases. Third, because patients in this study were accrued over a decade-long period, technical differences in TIPS creation and improvements in medical care during the study period may have contributed to differences in clinical outcomes over time. Fourth, the inclusion of deaths due to all causes may have confounded survival analysis. Fifth, PSG was measured immediately following TIPS creation without delayed pressure measurement, although the effects of deep sedation during general anesthesia may introduce variability to PSG measurements.25
In summary, increased RA pressure may be a prognostic factor for early mortality following TIPS creation, particularly in variceal hemorrhage patients. Lower baseline and post-TIPS RA pressures may be associated with improved survival, but larger prospective studies are required for confirmation. Patient cardiac function and fluid status should thus be optimized prior to TIPS procedures, and IR practitioners should consider patient right heart function to enhance patient selection and risk stratification.
Abbreviations- •
IR: Interventional radiology.
- •
MELD: Model for End Stage Liver Disease.
- •
PACS: picture archiving and communication system.
- •
PASP: pulmonary artery systolic pressure.
- •
PSG: portosystemic pressure gradient.
- •
RA: right atrial.
- •
SIR: Society of Interventional Radiology.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
None.