Background and Aim. Hepatitis E virus (HEV) infection represents a risk for mortality in pregnant women. The seroepidemiology of HEV infection in rural pregnant women in the Americas is largely unknown. The aim of the study was to determine the seroepidemiology of anti-HEV IgG antibodies in rural pregnant women in Durango, Mexico.
Materials and methods. The presence of anti-HEV IgG antibodies was determined in 439 pregnant women in rural Durango, Mexico using an enzyme-linked immunoassay. Seroprevalence association with socio-demographic, clinical and behavioral characteristics of the women was also investigated.
Results. Twenty five (5.7%; 95% CI: 3.88-8.27) of the 439 women (mean age: 24.53 ± 6.1 years) had anti-HEV antibodies. Multivariate analysis showed that HEV seropositivity was associated with increasing age (OR = 1.11; 95% CI: 1.03-1.20; P = 0.004), consumption of unpasteurized cow milk (OR = 5.37; 95% CI: 1.17-24.63; P = 0.03), and overcrowding at home (OR = 2.36; 95% CI: 1.13-4.92; P = 0.02). In contrast, the variables educational level, occupation, socio-economic status, foreign travel, consumption of untreated water and raw or undercooked meat, and raising animals did not show associations with HEV seropositivity. Exposure to HEV was associated with the number of deliveries but not with the number of cesarean sections or miscarriages.
Conclusions. This is the first report of seroprevalence and contributing factors for HEV infection in rural pregnant women in the Americas, and of an association of the consumption of unpasteurized cow milk with HEV exposure. Results of this study should be useful for designing optimal preventive measures against HEV infection.
Hepatitis E virus (HEV) is a single-stranded RNA virus.1 Infections with HEV occur in developing countries as well as in developed countries.2–5 HEV is transmitted predominantly by the fecal-oral route, usually through contaminated water.1 The clinical spectrum of HEV infections varies from asymptomatic to severe hepatitis that may lead to fulminant hepatic failure.6 Hepatitis E occurs frequently in epidemic outbreaks and as sporadic hepatitis.1 Hepatitis E often affects young adults and is particularly severe in pregnant women7–9 and in persons with pre-existing alcoholic liver disease.10 Infection with HEV may also lead to extra-hepatic manifestations.11 Hepatitis E is considered a zoonosis,12 and HEV is pathogenic for some domestic and wild animals.13
The seroepidemiology of HEV infection in rural pregnant women in Mexico is largely unknown. Many pregnant women in rural Mexico live in suboptimal sanitary conditions that may favor transmission of HEV including poor availability of drinkable water, poor disposal of excretes, and soil flooring and overcrowding at home. In addition, a large number of rural pregnant women have contact with a variety of animals. Infections with HEV may lead to mortality in pregnant women.8,9 However, the immunological status (presence of IgG anti-HEV) and correlates of HEV seropositivity in pregnant women in rural Mexico is unknown. Such information is needed to determine the magnitude of the HEV infection as a public health problem in our region and for an optimal planning of preventive measures against HEV infection. Hepatitis E is unrecognized in rural pregnant women in Mexico since there are not diagnostic tests available for HEV infection in rural practice. Therefore, we sought to determine the seroprevalence of anti-HEV IgG antibodies in rural pregnant women in Durango, Mexico. In addition, this study was aimed to determine socio-demographic, clinical, and behavioral characteristics of the rural pregnant women associated with HEV seropositivity.
Material and MethodsEthics statementThe present study used serum samples and data from a previous study,14 in such previous study the purpose and procedures of the survey were explained to all pregnant women. In addition, a written informed consent was obtained from all participants and from the next of kin of minor participants. This survey was approved by the ethical committees of the Mexican Social Security Institute and the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado in Durango City.
Study design and study populationThrough a cross sectional study using serum samples from a Toxoplasma gondii serosurvey,14 439 pregnant women living in rural areas of Durango State, Mexico were studied. Serum samples were originally used to determine the seroepidemiology of Toxoplasma gondii in rural pregnant women and were collected from August 2007 to February 2008. All pregnant women studied attended public prenatal care clinics in 9 rural communities: (5 de mayo, Colonia Hidalgo, Guadalupe Victoria, Santa Clara, Vicente Guerrero, Canatlán, Nuevo Ideal, Santiago Papasquiaro, and Rodeo). Inclusion criteria for voluntary participation of the women were pregnant women of any age, any month of pregnancy, and residing in rural Durango State.
General epidemiological characteristics of pregnant womenSocio-demographic, behavioral, and clinical characteristics were obtained from all pregnant women with the aid of a standardized questionnaire. Socio-demographic items included age, birth place, residence, educational and socio-economic levels, housing conditions and occupation. Housing conditions were obtained by using the Bronfman’s criteria15 and allowed us to assess crowding and sanitation. Briefly, five variables were evaluated: number of persons in the house, number of rooms in the house, material of the floor of the house (ceramic, concrete, soil), availability of drinkable water (within the house, out of the house, street), and form of elimination of excretes (flush toilet, latrine). A woman was considered as living in a crowded house when 1.6 to 3.5 persons per room live in her home. A woman was considered as living in an overcrowded house when more than 3.5 persons per room live in her home. In addition, educational level (years of education) of the head of the family was obtained. Behavioral items included animal contacts, foreign travel, contact with soil (gardening or agriculture), eating away from home (in restaurants or fast food outlets), consumption of raw or under-cooked meat, type of meat consumed (pork, lamb, beef, goat, boar, chicken, turkey, rabbit, deer, squirrel, horse, fish, snake, or other), consumption of dried or cured meat (chorizo, ham, sausages or salami), consumption of unpasteurized milk, or untreated water, and consumption of unwashed raw vegetables or fruits. Clinical data included gestational age, number of pregnancies, deliveries, cesarean sections and miscarriages, and history of blood transfusions or transplants.
Laboratory testsSera of all rural pregnant women were analyzed for anti-HEV IgG antibodies by a commercially available enzyme immunoassay “HEV-IgG ELISA” kit (Diagnostic Automation Inc., Calabasas, CA). This kit has been previously used in seroepidemiological studies.16,17 Sensitivity of the kit has been tested against World Health Organization (WHO) reference standard serum for HEV IgG by the manufacturer. This kit has routinely obtained a detection limit of 0.3125 WHO units/mL. In addition, this kit has a sensitivity of 99.8% and a specificity of 99.8%, and its lowest detection titer was 1:5120 using the WHO HEV antibody standard. The assays were performed following the instructions of the manufacturer. As a quality control measure, randomly selected sera of 3 anti-HEV IgG negative and 3 anti-HEV IgG positive participants were retested and results remained negative and positive, respectively.
Statistical analysisThe statistical analysis was performed using the software Epi Info version 3.5.4 and SPSS version 15.0. For calculation of the sample size, a reference seroprevalence of 10.5%18 as the expected frequency for the factor under study, 15000 as the population size from which the sample was selected, an absolute error of 3%, and a 95% confidence level were considered. The result of the sample size calculation was 391 women. We calculated the sample size from a population of 15,000 because such value was the approximated annual number of pregnant women in rural areas in Durango. We used the Pearson’s Chisquared test and the two-tailed Fisher’s exact test (when values were less than 5) for comparison of frequencies among groups. Bivariate and multivariate analyses were used to determine the association between the socio-demographic and behavioral characteristics of the pregnant women and HEV seropositivity. Clinical data was not included in the multivariate analysis. As a criterion for inclusion of variables in the multivariate analysis, we considered variables with a P value equal to or less than 0.20 obtained in the bivariate analysis. Odds ratios (OR) and 95% confidence intervals (CI) were calculated by regression analysis using the Enter method. The Hosmer-Lemeshow goodness of fit test was used to assess the fitness of the regression model. The statistical significance was set at P < 0.05.
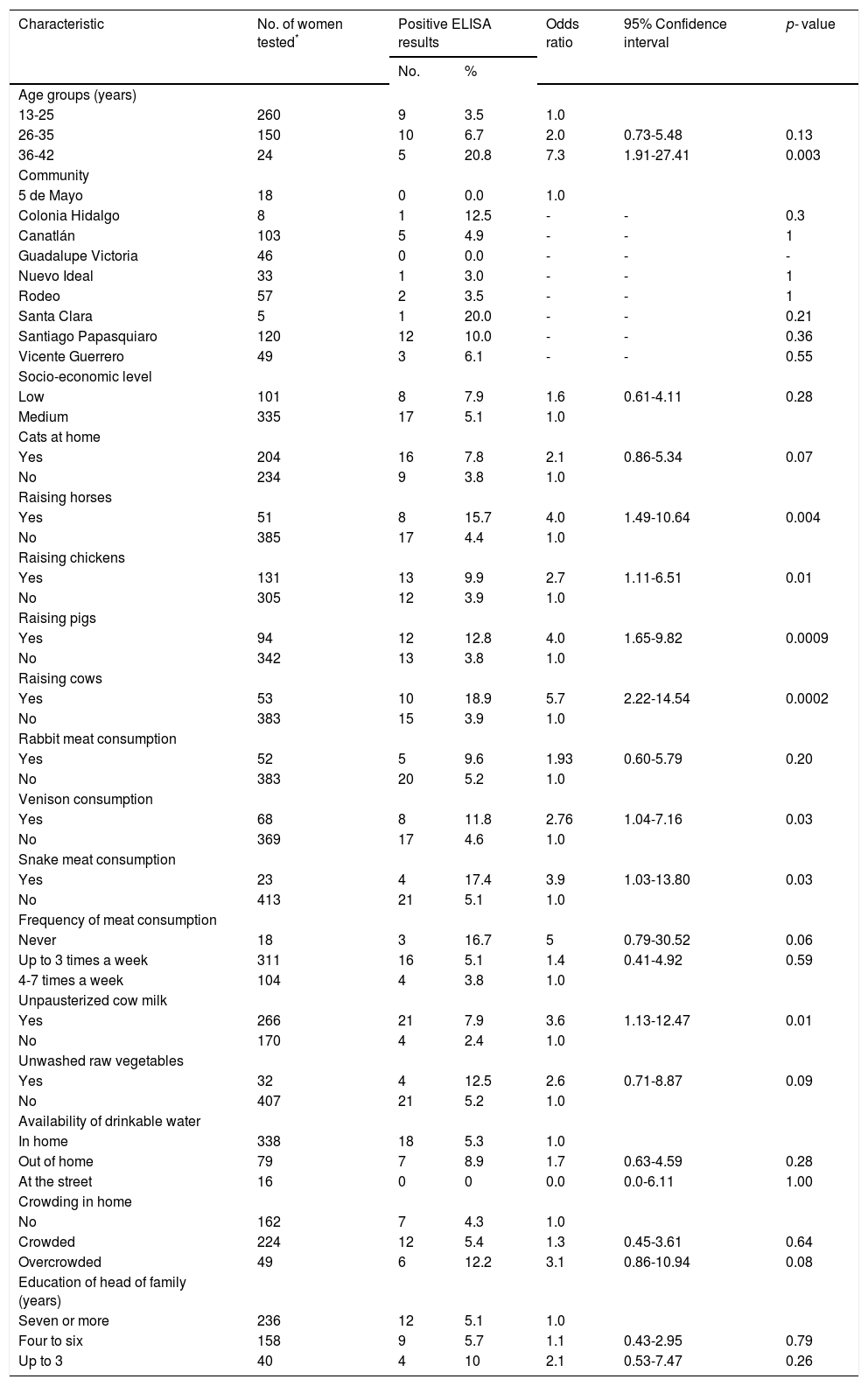
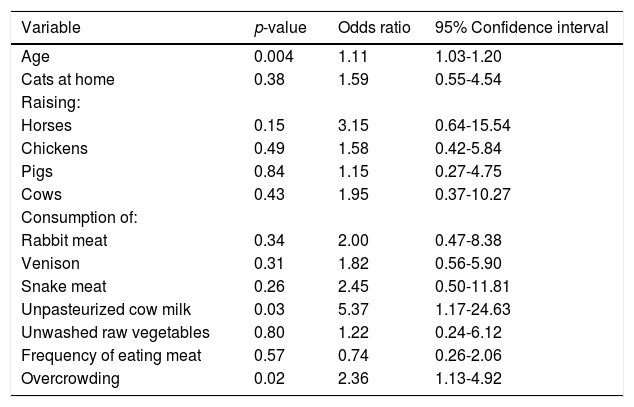
ResultsTwenty five (5.7%; 95% CI: 3.88-8.27) of the 439 rural pregnant women studied had anti-HEV IgG antibodies. A selection of socio-demographic and behavioral characteristics of the pregnant women studied and their correlation with HEV seropositivity are shown in Table 1. Four hundred and thirty eight women were born in Mexico and 1 in the USA; their mean age was 24.53 ± 6.1 years (range 13-42 years). Of the socio-demographic characteristics of the women studied, the variables age and crowding at home had P values < 0.20 by bivariate analysis. Other socio-demographic characteristics including community of residence, educational level, occupation, socio-economic status, availability of water at home, type of floorings at home and education of the head of the family had P values > 0.20 by bivariate analysis. Of the behavioral characteristics studied, 11 variables showed P values equal to or lower than 0.20 by bivariate analysis: cats at home, raising horses, chickens, pigs and cows, consumption of venison and meat from rabbit and snake, frequency of meat consumption, consumption of unpasteurized cow milk, and unwashed raw vegetables (Table 1). Other behavioral characteristics including foreign travel, consumption of unwashed raw fruits, or untreated water, eating away from home, consumption of raw or undercooked meat, consumption of dried or cured meat, and contact with soil showed P values > 0.20 in the bivariate analysis. Further analysis using logistic regression of socio-demographic and behavioral characteristics of rural pregnant women showed that HEV exposure was positively associated with increasing age (OR = 1.11; 95% CI: 1.03-1.20; P = 0.004), consumption of unpasteurized cow milk (OR = 5.37; 95% CI: 1.17-24.63; P = 0.03), and overcrowding at home (OR = 2.36; 95% CI: 1.13-4.92; P = 0.02) (Table 2). The result of the Hosmer-Lemeshow test (P = 0.81) indicated an acceptable fit of our regression model.
Bivariate analysis of exposure variables and seroprevalence of HEV infection in rural pregnant women.
| Characteristic | No. of women tested* | Positive ELISA results | Odds ratio | 95% Confidence interval | p- value | |
|---|---|---|---|---|---|---|
| No. | % | |||||
| Age groups (years) | ||||||
| 13-25 | 260 | 9 | 3.5 | 1.0 | ||
| 26-35 | 150 | 10 | 6.7 | 2.0 | 0.73-5.48 | 0.13 |
| 36-42 | 24 | 5 | 20.8 | 7.3 | 1.91-27.41 | 0.003 |
| Community | ||||||
| 5 de Mayo | 18 | 0 | 0.0 | 1.0 | ||
| Colonia Hidalgo | 8 | 1 | 12.5 | - | - | 0.3 |
| Canatlán | 103 | 5 | 4.9 | - | - | 1 |
| Guadalupe Victoria | 46 | 0 | 0.0 | - | - | - |
| Nuevo Ideal | 33 | 1 | 3.0 | - | - | 1 |
| Rodeo | 57 | 2 | 3.5 | - | - | 1 |
| Santa Clara | 5 | 1 | 20.0 | - | - | 0.21 |
| Santiago Papasquiaro | 120 | 12 | 10.0 | - | - | 0.36 |
| Vicente Guerrero | 49 | 3 | 6.1 | - | - | 0.55 |
| Socio-economic level | ||||||
| Low | 101 | 8 | 7.9 | 1.6 | 0.61-4.11 | 0.28 |
| Medium | 335 | 17 | 5.1 | 1.0 | ||
| Cats at home | ||||||
| Yes | 204 | 16 | 7.8 | 2.1 | 0.86-5.34 | 0.07 |
| No | 234 | 9 | 3.8 | 1.0 | ||
| Raising horses | ||||||
| Yes | 51 | 8 | 15.7 | 4.0 | 1.49-10.64 | 0.004 |
| No | 385 | 17 | 4.4 | 1.0 | ||
| Raising chickens | ||||||
| Yes | 131 | 13 | 9.9 | 2.7 | 1.11-6.51 | 0.01 |
| No | 305 | 12 | 3.9 | 1.0 | ||
| Raising pigs | ||||||
| Yes | 94 | 12 | 12.8 | 4.0 | 1.65-9.82 | 0.0009 |
| No | 342 | 13 | 3.8 | 1.0 | ||
| Raising cows | ||||||
| Yes | 53 | 10 | 18.9 | 5.7 | 2.22-14.54 | 0.0002 |
| No | 383 | 15 | 3.9 | 1.0 | ||
| Rabbit meat consumption | ||||||
| Yes | 52 | 5 | 9.6 | 1.93 | 0.60-5.79 | 0.20 |
| No | 383 | 20 | 5.2 | 1.0 | ||
| Venison consumption | ||||||
| Yes | 68 | 8 | 11.8 | 2.76 | 1.04-7.16 | 0.03 |
| No | 369 | 17 | 4.6 | 1.0 | ||
| Snake meat consumption | ||||||
| Yes | 23 | 4 | 17.4 | 3.9 | 1.03-13.80 | 0.03 |
| No | 413 | 21 | 5.1 | 1.0 | ||
| Frequency of meat consumption | ||||||
| Never | 18 | 3 | 16.7 | 5 | 0.79-30.52 | 0.06 |
| Up to 3 times a week | 311 | 16 | 5.1 | 1.4 | 0.41-4.92 | 0.59 |
| 4-7 times a week | 104 | 4 | 3.8 | 1.0 | ||
| Unpausterized cow milk | ||||||
| Yes | 266 | 21 | 7.9 | 3.6 | 1.13-12.47 | 0.01 |
| No | 170 | 4 | 2.4 | 1.0 | ||
| Unwashed raw vegetables | ||||||
| Yes | 32 | 4 | 12.5 | 2.6 | 0.71-8.87 | 0.09 |
| No | 407 | 21 | 5.2 | 1.0 | ||
| Availability of drinkable water | ||||||
| In home | 338 | 18 | 5.3 | 1.0 | ||
| Out of home | 79 | 7 | 8.9 | 1.7 | 0.63-4.59 | 0.28 |
| At the street | 16 | 0 | 0 | 0.0 | 0.0-6.11 | 1.00 |
| Crowding in home | ||||||
| No | 162 | 7 | 4.3 | 1.0 | ||
| Crowded | 224 | 12 | 5.4 | 1.3 | 0.45-3.61 | 0.64 |
| Overcrowded | 49 | 6 | 12.2 | 3.1 | 0.86-10.94 | 0.08 |
| Education of head of family (years) | ||||||
| Seven or more | 236 | 12 | 5.1 | 1.0 | ||
| Four to six | 158 | 9 | 5.7 | 1.1 | 0.43-2.95 | 0.79 |
| Up to 3 | 40 | 4 | 10 | 2.1 | 0.53-7.47 | 0.26 |
Results of the multivariate regression analysis.
| Variable | p-value | Odds ratio | 95% Confidence interval |
|---|---|---|---|
| Age | 0.004 | 1.11 | 1.03-1.20 |
| Cats at home | 0.38 | 1.59 | 0.55-4.54 |
| Raising: | |||
| Horses | 0.15 | 3.15 | 0.64-15.54 |
| Chickens | 0.49 | 1.58 | 0.42-5.84 |
| Pigs | 0.84 | 1.15 | 0.27-4.75 |
| Cows | 0.43 | 1.95 | 0.37-10.27 |
| Consumption of: | |||
| Rabbit meat | 0.34 | 2.00 | 0.47-8.38 |
| Venison | 0.31 | 1.82 | 0.56-5.90 |
| Snake meat | 0.26 | 2.45 | 0.50-11.81 |
| Unpasteurized cow milk | 0.03 | 5.37 | 1.17-24.63 |
| Unwashed raw vegetables | 0.80 | 1.22 | 0.24-6.12 |
| Frequency of eating meat | 0.57 | 0.74 | 0.26-2.06 |
| Overcrowding | 0.02 | 2.36 | 1.13-4.92 |
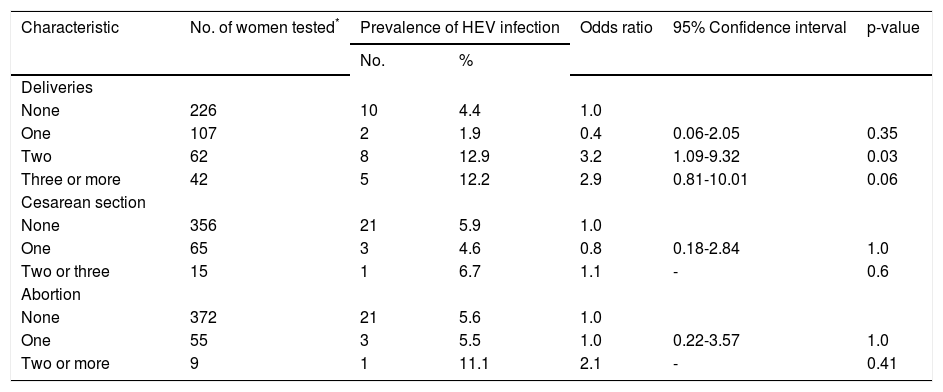
Of the clinical characteristics studied, seropositivity to HEV was associated with the number of pregnancies and deliveries (Table 3). In contrast, seropositivity to HEV was not associated with transplantation, blood transfusion, or the number of cesarean sections and miscarriages.
Bivariate analysis of clinical data and infection with HEV in rural pregnant women in Durango, Mexico.
| Characteristic | No. of women tested* | Prevalence of HEV infection | Odds ratio | 95% Confidence interval | p-value | |
|---|---|---|---|---|---|---|
| No. | % | |||||
| Deliveries | ||||||
| None | 226 | 10 | 4.4 | 1.0 | ||
| One | 107 | 2 | 1.9 | 0.4 | 0.06-2.05 | 0.35 |
| Two | 62 | 8 | 12.9 | 3.2 | 1.09-9.32 | 0.03 |
| Three or more | 42 | 5 | 12.2 | 2.9 | 0.81-10.01 | 0.06 |
| Cesarean section | ||||||
| None | 356 | 21 | 5.9 | 1.0 | ||
| One | 65 | 3 | 4.6 | 0.8 | 0.18-2.84 | 1.0 |
| Two or three | 15 | 1 | 6.7 | 1.1 | - | 0.6 |
| Abortion | ||||||
| None | 372 | 21 | 5.6 | 1.0 | ||
| One | 55 | 3 | 5.5 | 1.0 | 0.22-3.57 | 1.0 |
| Two or more | 9 | 1 | 11.1 | 2.1 | - | 0.41 |
We found a 5.7% seroprevalence of HEV exposure in rural pregnant women in Durango, Mexico. The seroprevalence of HEV exposure found in rural pregnant women in the present study cannot be compared with other seroprevalences of HEV exposure in Mexico since there is not any previous survey in pregnant women. There have been only two previous studies about HEV exposure in Mexico: in a national survey a mean seroprevalence of 10.5% was found in young adults and children,18 and a 6.3% seroprevalence was found in residents in the central Mexican state of Hidalgo.19 There are few reports about the seroprevalence of HEV infection in pregnant women in the Americas. In a study in pregnant women living in the USA/Mexico border, 0.4% of women living in the USA side had antibodies to HEV while 1.6% of women living in the Mexican side had such antibodies.20 In a study in Caracas, Venezuela, antibodies against HEV were found in 1.6% of urban pregnant women.21 Similarly, in Rio de Janeiro, Brazil, a low seroprevalence (1%) of anti-HEV IgG antibodies was found in pregnant women (22). Therefore, rural pregnant women in Durango have a higher seroprevalence of HEV infection than pregnant women in the USA/ Mexico border, Venezuela and Brazil. However, comparison of such seroprevalences should be taken with care since we studied rural pregnant women while other researchers studied urban pregnant women. To the best of our knowledge, our study is the first report of the seroprevalence of HEV infection in rural pregnant women in the Americas. Concerning the seroprevalence of HEV infection in pregnant women in other parts of the world, the seroprevalence found in our study is lower than the 33.67% seroprevalence of anti-HEV IgG antibodies found in 300 asymptomatic healthy primigravidae from north India23 and the 11.6% to 25% seroprevalence in pregnant women in Burkina-Faso,24 Egypt,25 Gabon,26 and Tunisia.27 In contrast, our seroprevalence is comparable to the 6.6% seroprevalence of anti-HEV IgG antibodies found in pregnant women infected with HIV or HTLV-1 in Gabon, Africa,28 and the 3.6% seroprevalence of HEV infection in pregnant women in Madrid, Spain.29
With respect to socio-demographic and behavioral characteristics associated with HEV seropositivity in the rural pregnant women studied, we found that HEV exposure was positively associated with increasing age, and overcrowding at home. The increase of HEV seroprevalence with age found in the present study is consistent with other reports in pregnant women,27 and other populations.18,19 Similarly, the association of overcrowding at home with exposure to HEV found in our study agrees with those found in other studies.27,28 Remarkably, multivariate analysis showed an association of HEV sero-positivity with consumption of unpasteurized cow milk. To the best of our knowledge, such positive association has not been reported. In addition, we are not aware of any report about the HEV sero-prevalence in consumers of unpasteurized cow milk in our region or elsewhere. Antibodies to HEV have been reported in cows25,31,32 including milk cows,33 but it is unknown whether HEV is present in milk. Further research to determine the presence of HEV antigen and viral RNA in milk is needed. In addition, the presence of infecting HEV should be determine in milk. If milk directly obtained from the cows does not contain infecting HEV then contamination of cow milk with HEV might occur after milking. The use of unwashed containers for cow milk collection or the use of contaminated water for washing the containers is possible. In addition, milking is usually performed by hand in rural Mexico, therefore, milking with contaminated hands might contaminate milk. Furthermore, milk might be adulterated with contaminated water. Some milk sellers might adulterate milk with water to increase the volume and their earnings. It is unclear what type of water is used for such purpose but it might be untreated water. Milk sellers in our region usually handle milk (transfer milk with a glass from their containers to the client container) with bare and likely unwashed hands, and measures to avoid contamination of the glass are not suitable. Therefore, the poor hygiene practices in handling cow milk from the stall to the consumers’ table may contribute to explain the link of HEV seropositivity with consumption of unpasteurized cow milk. Thus, contamination of milk with human feces might occur as also occur with water. Further research should be conducted to elucidate the association of HEV exposure and consumption of unpasteurized cow milk. Several of the risk factors evaluated in the present study are interrelated; for instance, a low education may be related with low hygiene practices including eating of unwashed raw vegetables, and keeping cattle with drinking unpasteurized milk. However, the multivariate analysis allowed us to identified independent variables associated with HEV exposure. Other putative risk factors associated with HEV infection including low socio-economic status23,30 and low educational level18 were not associated with HEV infection in the rural pregnant women studied. Exposure to HEV has been linked to consumption of contaminated water.1,7 However, in the present study we did not find any evidence of such transmission in rural pregnant women in Durango. Sero-positivity to HEV was not associated with consumption of untreated water or poor availability of drinkable water in our study. Exposure to HEV has been found higher in urban than in rural pregnant women in studies in India and Africa.23,26 Therefore, it is likely that the magnitude of HEV infection may be higher in urban than in rural pregnant women in our region too. Further studies on the epidemiology of HEV infection in pregnant women in Mexico are needed.
Concerning the clinical characteristics in the pregnant women studied, seropositivity to HEV was associated with the number of pregnancies and deliveries but no with transplantation, blood transfusion, or the number of cesarean sections and miscarriages. It is not clear why HEV seroprevalence increased with the number of deliveries. We are not aware of any report of such association in the medical literature. The seroprevalence of HEV infection increases with age, and the older the women the higher the number of deliveries. Therefore, the association of HEV exposure with the increasing number of pregnancies and deliveries can be explained by the concomitant increase of HEV seroprevalence with age. We were unable to obtain further information about the type of deliveries (normal or abnormal). However, it raises the question whether women with more number of deliveries are more frequently exposed to risk factors for HEV infection i.e. contaminated materials including blood, medical instruments, infected patients or other unknown factors than women with less number of deliveries. It is not clear why HEV seroprevalence was associated with deliveries but not with cesarean sections. Hygiene practices during these obstetric procedures might be different. Cesarean sections are always performed in hospitals while deliveries are performed in small clinics, or even at home particularly in rural communities in Mexico. Therefore, it is possible that differences in hygiene practices might have contributed for HEV exposure. However, further research to elucidate the potential role of obstetric procedures in HEV exposure is needed.
Clear variability in sensitivity among various kits for detection of anti-HEV IgG antibodies has been reported.34,35 In a study performed by Bendall, et al.,34 the performance of two commercially available (Genelabs and Wantai) kits for detection of anti-HEV IgG antibodies was compared. They found a lower detection limit (0.25 WHO units/mL) with the Wantai kit than with the Genelabs kit (2.5 WHO units/mL). The detection limit (0.3125 WHO units/ mL) of the Diagnostic Automation kit used in the present study is comparable with the reported detection limit (0.25 WHO units/mL) of the Wantai kit.34
ConclusionsThis is the first report of seroprevalence and contributing factors for HEV infection in rural pregnant women in the Americas, and of an association of the consumption of unpasteurized cow milk with HEV exposure. Results of this study should be useful for designing optimal preventive measures against HEV infection.
ABBREVIATIONS- •
CI: confidence interval.
- •
ELISA: enzyme linked immunosorbent assay.
- •
HEV: hepatitis E virus.
- •
IgG: immunoglobulin G.
- •
OR: Odds ratio.
- •
RNA: ribonucleic acid.
- •
SPSS: statistical package for the Social Sciences.
This study was financially supported by Universidad Juárez del Estado de Durango. Mexico.