Introduction. Standard treatment for patients with chronic hepatitis C genotype 1 (CHC G-1) infection includes pegylated interferon plus ribavirin (PEG-RBV) for 48 weeks. Shorter treatment regimen would be more acceptable due to lower cost and fewer side-effects. We aimed to compare the efficacy of 36 week PEG-RBV therapy with standard 48 week therapy in CHC G-1 patients who achieve complete early virological response (cEVR).
Material and methods. Consecutive treatment-naïve patients with CHC G-1 were treated with pegylated interferon a2b (1.5 μg/kg/week) or α2a (180 μg/week) and weight based ribavirin. Patients who achieved cEVR at 12 weeks [undetectable HCV RNA irrespective of RVR (rapid virological response)] were randomized into- group A (48 weeks therapy) and group B (36 weeks therapy). Primary end-point was achievement of sustained virological response (SVR) at 24 weeks of follow up.
Results. Out of the total 166 patients started on treatment, 112 (69.3%) achieved cEVR, and were randomized into group A (n = 59) and group B (n = 53). Fifty-five (93.2%) patients in group A and 50 (94.3%) in group B completed therapy. The overall SVR rate in group A was 79.6% (47/59) and group B was 84.9% (45/53) (p = 0.622). SVR rates in the two groups were comparable in all patient sub-groups according to factors like viral load (≤ or > 400,000 IU/mL), RVR (achieved/not achieved), age (≤ or > 40 years), body mass index (≤ or > 27) and cirrhosis (present/absent).
Conclusion. In CHC G-1 patients who achieve cEVR, 36 weeks PEG-RBV therapy is as effective as standard 48 weeks therapy, irrespective of other host or virological factors.
Standard treatment regimen for patients with chronic hepatitis C (CHC) genotype 1 (G-1) infection includes pegylated interferon plus ribavirin (PEG-RBV) for 48 weeks.1 Although newer therapeutic options have been recently approved for the treatment of CHC,2 their use is limited due to high cost, side-effects and availability. Even the PEG-RBV therapy is associated with significant cost and side effects. Therefore, efforts have been made to keep the total drug dosage to the minimal but without compromising the treatment efficacy. This can be achieved by either using a lower dose or by reducing the total duration of therapy. In a large randomized trial, Hadziyannis, et al.3 reported that the sustained virological response (SVR) rate in CHC G-1 patients was significantly higher in patients treated for 48 weeks as compared to 24 weeks. However, a retrospective analysis of this trial showed that among patients who achieve rapid virological response (RVR) at week 4, the SVR rate was 89-90% after 24 or 48 weeks of treatment.4 Later, few groups prospectively tested the efficacy of an abbreviated 24 weeks PEG-RBV therapy in CHC G-1 patients but found it to be effective in a selected sub-group of patients who have low viral load (LVL) and who achieve RVR at 4 weeks.4–7 In all other patients, 48 weeks therapy is still recommended. We hypothesized that in patients who achieve virological response at 12 weeks, giving an additional 24 weeks of therapy (i.e. total 36 weeks therapy) may be sufficient to achieve a similar SVR rate as with standard 48 week therapy. In this study, we aimed to compare the efficacy of 36 week PEG-RBV therapy with standard 48 week therapy in patients with CHC G 1 infection patients who achieve complete early virological response (cEVR). To the best of our knowledge, no study till date has evaluated the efficacy of 36 weeks therapy for genotype 1 patients.
Material and MethodsThis prospective study included consecutive treatment naïve CHC genotype 1 patients who were treated with PEG-RBV therapy in the gastroenterology out-patient department in our institute between January 2006 and December 2013. Inclusion criteria were- age 18-65 years, hepatitis C virus (HCV) ribo-nucleic acid (RNA) positivity, genotype 1 and treatment naïve patients. Patients with compensated cirrhosis (Child-Pugh class A) were eligible for the study. Exclusion criteria wereco-infections with HBV or HIV viruses, history of previous antiviral or immuno-modulatory therapy, decompensated liver disease, active alcohol or drug abuse, major neuro-psychiatric illness, uncontrolled thyroid disorder or uncontrolled diabetes, autoimmune diseases, advanced cardio-pulmonary disease, renal failure, hemoglobin < 12 g/dL (females) or < 13 g/dL (males), platelet count < 90,000/mm3 or neutrophil count < 1,500/mm3 were not offered anti-viral treatment. Written informed consent was taken from the patients and the study was approved by Institutional Ethics Committee. The study was conducted according to the guidelines of the Declaration of Helsinki.
A detailed history and clinical examination was undertaken. Baseline laboratory work-up included haemogram, liver and renal function tests, thyroid function tests, prothrombin time index (PTI), fasting blood sugar, α-feto protein and ultrasound abdomen. Increased alanine aminotransferase (ALT) was defined as ALT levels > 40 IU/L. Cirrhosis was diagnosed on the basis of clinical, laboratory, radiological, endoscopic and/or histological (Metavir grade F4) or transient elastography (TE) criteria. In the initial phase of the study, liver biopsy was performed in all patients, but later, it was replaced by TE after its availability beyond the year 2010 at our institute. TE was performed using Fibroscan (Echosens, Paris, France) and results were expressed as Kilo Pascals. TE measurement was considered valid if 10 measurements were obtained with a success rate of > 60% and an interquartile range < 30% of the median. TE value > 11.7 was taken as the cut-off for cirrhosis (≥ F4).8 IL28B genotype testing (SNPs rs12979860 and rs8099917) was performed in all patients beyond the year 2011. The CC genotype was taken as the favorable genotype for rs12979860, while CT and TT were taken as unfavorable. For rs8099917, TT was favorable while the TG and GG were unfavorable genotypes.
All the patients were tested for HBV and HIV co-infections. Work-up for autoimmune and metabolic diseases was done, where indicated. Anti-HCV antibody positivity was confirmed by ELISA (ELISCAN HCV; RFCL limited, Dehradun, India). HCV RNA was quantified by real time polymerase chain reaction technology (COBAS Taqman HCV TEST 2.0; Roche Diagnostics Corporation, Indiapolis, IN, USA). Low viral load was defined as HCV RNA < 400,000 IU/mL. HCV genotype was also determined in all patients.
Patients were treated with pegylated interferon α2b (PEG-Intron, Schering Corp, Kenilworth, NJ, USA) (1.5 μg/kg per week) or pegylated interferon α2a (Pegasys, Hoffmann-LaRoche) (180 μg per week) subcutaneously plus ribavirin 800-1,200 mg/ day orally, according to body weight (< 65 kg, 800 mg/day; 65-85 kg, 1,000 mg/day; > 85 kg, 1,200 mg/ day).
After 12 weeks of treatment, patients were assessed for the presence of EVR. Patients who achieved cEVR (defined as undetectable HCV RNA in serum at 12 weeks, regardless of the 4 week RNA level1,9 were randomized into 2 groups- group A (48 weeks therapy) and group B (36 weeks therapy). Randomization was performed using sealed opaque envelopes. Patients who did not achieve cEVR were not included in this study. However, treatment was continued in these patients according to standard guidelines.1 Treatment was withdrawn if viral load did not decrease by at least 2 log10 IU/mL after 12 weeks of therapy.
All patients were then followed for additional 24 weeks after the end of treatment to assess SVR. Treatment was considered to be ‘complete’ if the patient received > 80% dose of both the treatment drugs for > 80% of the recommended duration. Dose adjustments for adverse effects were made according to standard protocols. Antidepressants and growth factors (erythropoietin or granulocyte colony stimulating factor) were used based on clinical condition of the patient or laboratory abnormalities.10
Treatment efficacy was assessed by HCV RNA monitoring at weeks 12, 24, 36 and 48 of treatment, and 24 weeks after the end of treatment. In addition, HCV RNA at 4 weeks for assessment of RVR was performed after the year 2007.
Safety and tolerability were assessed with patientreported adverse events, physical examination and laboratory evaluation (hematology at 2 and 4 weeks, then every 4 weeks and whenever required; liver biochemistry every 4 weeks and thyroid-stimulating hormone levels every 12 weeks during treatment).
Study outcome measures and endpointsThe primary outcome measure was an undetectable HCV RNA at 24 weeks of follow-up (SVR) after end of treatment. The secondary outcome measures were the proportion of patients who achieved end-of-treatment response (ETR).
The primary endpoint of the study was completion of 24 weeks of follow up after completion of full recommended therapy (36 or 48 weeks). Secondary end-points were- premature withdrawal of treatment due to severe side effects, or patient being lost to follow up.
Statistical analysisAll data were analyzed using SPSS v. 14.0 software package. Continuous data were expressed as median (range) and compared using student’s t-test. Categorical data were expressed as number/proportion of subjects with a specific feature, and compared using χ2 test. Analysis was performed on an ‘intention-to-treat’ basis. Univariate analysis was performed to compare various host and viral factors predicting SVR in the two groups. A two-tailed p value of < 0.05 was required for statistical significance.
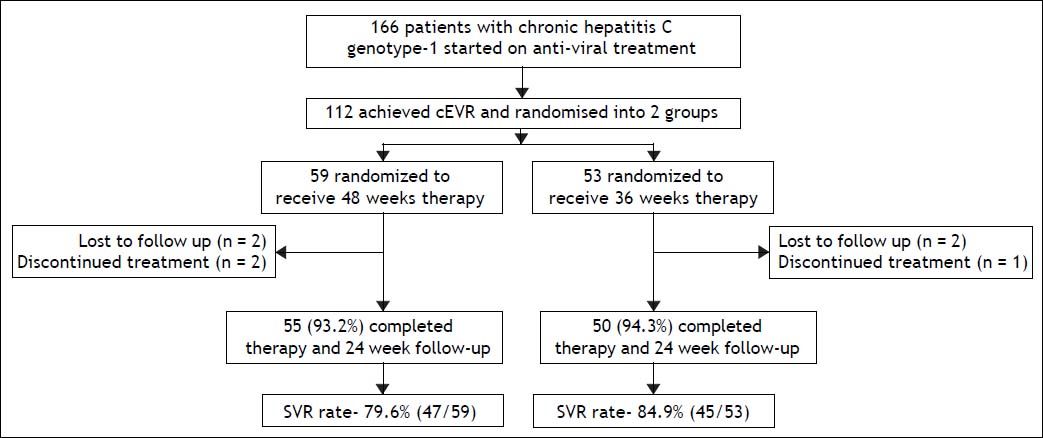
ResultsA total of 166 patients with chronic hepatitis C genotype 1 infection were started on PEG-RBV combination treatment. After 12 weeks of treatment, all patients were assessed for the achievement of EVR. One hundred and twelve patients (67.5%) achieved cEVR, 42 (25.3%) achieved partial EVR, 8 (4.8%) did not achieve EVR, and 4 (2.4%) were lost to follow up. Of the 112 patients who achieved cEVR, 59 patients were randomized to group A (standard 48 weeks therapy), and 53 patients to group B (36 weeks therapy). Flow of patients in the study is shown in figure 1.
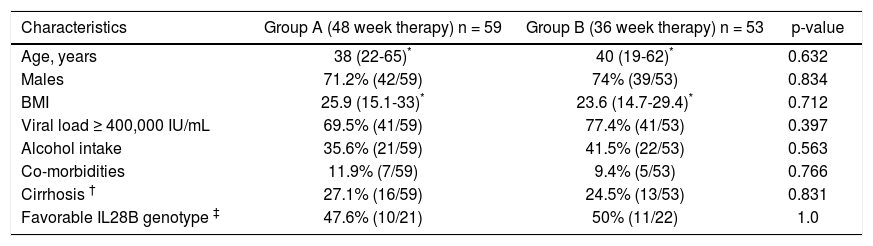
Baseline characteristics of the two groups were comparable (Table 1). Majority of the patients were males, with a median age of 38-40 years, and a median BMI of 23.6-25.9 kg/m2. Almost three-quarter of the patients had viral load ≥ 400,000 IU/mL. About 25% of the patients had presence of advanced fibrosis/cirrhosis. Of the patients tested for IL28B phenotype, about half had favorable phenotype.
Baseline characteristics of patients.
| Characteristics | Group A (48 week therapy) n = 59 | Group B (36 week therapy) n = 53 | p-value |
|---|---|---|---|
| Age, years | 38 (22-65)* | 40 (19-62)* | 0.632 |
| Males | 71.2% (42/59) | 74% (39/53) | 0.834 |
| BMI | 25.9 (15.1-33)* | 23.6 (14.7-29.4)* | 0.712 |
| Viral load ≥ 400,000 IU/mL | 69.5% (41/59) | 77.4% (41/53) | 0.397 |
| Alcohol intake | 35.6% (21/59) | 41.5% (22/53) | 0.563 |
| Co-morbidities | 11.9% (7/59) | 9.4% (5/53) | 0.766 |
| Cirrhosis † | 27.1% (16/59) | 24.5% (13/53) | 0.831 |
| Favorable IL28B genotype ‡ | 47.6% (10/21) | 50% (11/22) | 1.0 |
In group A, 55 out of 59 patients (93.2%) completed 48 weeks of therapy, 2 (3.4%) were lost to followup and treatment had to be stopped in 2 (3.4%) patients due to severe adverse events. In group B, 50 out of 53 patients (94.3%) completed 36 weeks of therapy, 2 (3.8%) were lost to follow-up and treatment had to be stopped in 1 (1.9%) patient due to severe adverse events.
According to the intention-to-treat analysis, the end-of-treatment response was 93.2% (55/59) in group A and 94.3% (50/53) in group B (p = 1.0). The relapse rates in group A and B were 13.6% (8/ 59) and 9.4% (5/53) respectively. The SVR rate in group A was similar to than in group B [79.6% (47/ 59) and 84.9% (45/53) respectively; p = 0.622]. According to the per-protocol analysis, the SVR rate in group A and B were 85.5% (47/55) and 90% (45/50) respectively (p = 0.562)
Post hoc analysis of patients was performed to assess the effect of RVR on SVR. In group A, HCV viral load estimation at 4 weeks was performed in 45 patients. Out of these 27 (60%) patients achieved RVR while 18 did not. Of the RVR positive patients, 92.5% (25/27) achieved SVR, while of the RVR negative patients, 72.2% (13/18) achieved SVR (p = 0.097). In group B, HCV viral load estimation at 4 weeks was performed in 39 patients. Out of these 22 (56.4%) patients had achieved RVR while 17 did not. Of the RVR positive patients, 100% (22/22) achieved SVR, while of the RVR negative patients, 70.6% (12/ 17) achieved SVR (p = 0.011). Only 13.1% (8/61) patients in group A and 7.3% (4/55) in group B had both low viral load and also achieved RVR.
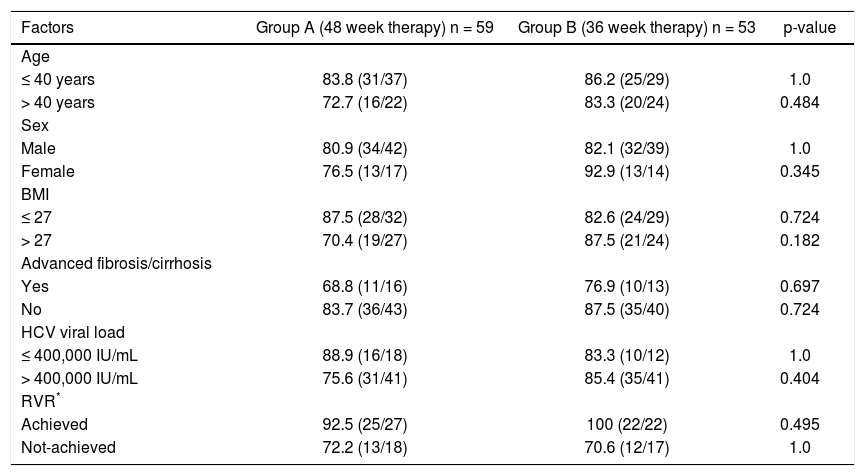
Univariate analysis including various factors like viral load (≤ or > 400,000 IU/mL), RVR (achieved/ not achieved), age (≤ or > 40 years), body mass index (≤ or > 27) and advanced fibrosis (present/absent) was performed to assess the influence of these factors on SVR in the two groups (Table 2). However, the SVR rates in the two groups were found to be comparable in all patient sub-groups.
Univariate analysis of various host and viral factors predicting sustained virological response.
| Factors | Group A (48 week therapy) n = 59 | Group B (36 week therapy) n = 53 | p-value |
|---|---|---|---|
| Age | |||
| ≤ 40 years | 83.8 (31/37) | 86.2 (25/29) | 1.0 |
| > 40 years | 72.7 (16/22) | 83.3 (20/24) | 0.484 |
| Sex | |||
| Male | 80.9 (34/42) | 82.1 (32/39) | 1.0 |
| Female | 76.5 (13/17) | 92.9 (13/14) | 0.345 |
| BMI | |||
| ≤ 27 | 87.5 (28/32) | 82.6 (24/29) | 0.724 |
| > 27 | 70.4 (19/27) | 87.5 (21/24) | 0.182 |
| Advanced fibrosis/cirrhosis | |||
| Yes | 68.8 (11/16) | 76.9 (10/13) | 0.697 |
| No | 83.7 (36/43) | 87.5 (35/40) | 0.724 |
| HCV viral load | |||
| ≤ 400,000 IU/mL | 88.9 (16/18) | 83.3 (10/12) | 1.0 |
| > 400,000 IU/mL | 75.6 (31/41) | 85.4 (35/41) | 0.404 |
| RVR* | |||
| Achieved | 92.5 (25/27) | 100 (22/22) | 0.495 |
| Not-achieved | 72.2 (13/18) | 70.6 (12/17) | 1.0 |
Data are expressed as percentage (number).
Adverse event profile was similar to that reported with PEG-RBV treatment.10,16 A vast majority of patients [93.2% (55/59) in group A and 88.7% (47/ 53) in group B; p = 0.513] reported adverse events during the study period. Important adverse events were:
- •
Fatigue: 88.1% (52/59) vs. 84.9% (45/53).
- •
Weight loss: 64.4% (38/59) vs. 60.1% (32/53).
- •
Anemia: 35.5% (21/59) vs. 35.8% (19/53).
- •
Depression: 25.4% (15/59) vs. 20.7% (11/53).
- •
Breathlessness: 22% (13/59) vs. 18.8% (10/53).
- •
Thyroid dysfunction: 13.6% (8/59) vs. 9.4% (5/ 53).
- •
Neutropenia: 11.8% (7/59) vs. 9.4% (5/53) and
- •
Thrombocytopenia: 8.5% (5/59) vs. 9.4% (5/53) in groups A and B respectively.
Therapy had to be discontinued due to severe adverse events in 3.4% patients in group A and 1.9% patient in group B (p = 1.0). Ribavirin dose modification was required in 37.3% (22/59) and 35.8% (19/53) patients (p = 1.0); and PEG-IFN dose modification in 11.8% (7/59) and 11.3% (6/53) patients (p = 1.0) in group A and B respectively.
DiscussionPatients with chronic hepatitis C genotype 1 virus infection have been traditionally treated with 48 weeks of PEG-RBV combination therapy, because of the ‘difficult to cure’ nature of this genotype. SVR rate with the standard 48 week therapy has varied from 37-52% in studies from western world,4,11–15 while we recently reported 57% SVR rate among CHC G-1 patients treated in ‘real-life’ setting in northern India.16 Nowadays, the focus has been shifted to response guided therapy depending on on-treatment virological response rather than fixed duration treatment. Ours is the first study which compared the efficacy of a shorter 36 weeks PEG-RBV combination therapy with the standard 48 weeks therapy in genotype 1 patients who achieve cEVR, and found it to have equal efficacy irrespective of other host or virological factors like age, BMI, advanced fibrosis, viral load and RVR.
Pegylated IFN and ribavirin therapy for CHC is associated with significant side-effects and high cost. Because of both of these factors, a large number of patients with CHC are either not able to initiate the therapy or have to stop the treatment prematurely. As a result, the goal for clinicians is to find the most effective therapy with the shortest treatment duration. The shorter regimen would definitely be more acceptable to the patients, with fewer side-effects and fewer withdrawals from therapy. Initial studies by Jensen, et al.4 and Zeuzem, et al.17 reported that abbreviated therapy for 24 weeks is a reasonable option for CHC G-1 patients who have LVL (< 600,000 IU/mL) and achieve RVR. Later, in a study by Ferenci, et al.,5 all patients with CHC G-1 who achieved RVR were treated for 24 weeks and a SVR rate of 78.8% was observed. However, the SVR rate was significantly lower in patients with HCV RNA > 800,000 IU/mL (66.7%) compared to those with < 400,000 IU/mL (81.3%). A limitation of this study was the lack of a control arm receiving standard treatment. In another multicenter randomized trial in Asian patients, Liu, et al.6 reported that the SVR rates with 24 week therapy in G-1 patients were similar to that with 48 week therapy only in patients with HCV RNA level < 800,000 IU/mL and those achieving RVR. In a recent study, Lin, et al.7 reported baseline HVL as an important predictor of relapse in G-1 patients receiving abbreviated therapy even if they achieved RVR. Thus, 24 weeks therapy in genotype 1 patients appears to be a good option but only in patients who have LVL and who achieve RVR. However, in real life practice, only a small fraction of patients will be fulfilling these criteria to qualify for 24 weeks therapy. In the study by Ferenci, et al.,5 only 9.4% (52/552) of the patients had both LVL and achieved RVR. Similarly, in the present study, if we had followed these criteria, only 10% of our patients would have qualified for 24 weeks therapy. On the other hand, a greater percentage of CHC G-1 patients would benefit from abbreviated therapy of 36 weeks using our novel criteria (which is independent of the baseline viral load and RVR), as 34-64% patients are known to achieve cEVR in various trials.18
Ours is the first study which has evaluated the usefulness of HCV RNA level after 12 weeks of PEG-RBV therapy as a criterion for abbreviated (36 weeks) therapy in CHC G-1 patients. Earlier data suggests the use of HCV RNA level after 12 weeks as a criterion for stoppage of treatment in CHC G-1 patients because of its high negative predictive value.1 In patients with less than 2 log10 IU/mL decrease in HCV RNA after 12 weeks, treatment should be stopped at week 12, as the SVR rate in these patients with standard treatment duration is less than 2%. In patients with delayed virological response (DVR) (defined as a more than 2 log10 decline in HCV RNA concentration but a detectable HCV RNA level at week 12 and an undetectable HCV RNA level at week 24),2 treatment may be prolonged for 72 weeks.
In the present study, we did not consider RVR as a criterion for offering a shorter duration (36 weeks) therapy to the patients. In post-hoc analysis, we found that although the SVR rates were lower in patients who did not achieve RVR compared to patients who did, these were not significantly different among the 2 treatment groups. Thus, the shorter duration therapy was as effective as the standard 48 weeks therapy in patients who achieve cEVR, irrespective of the RVR status.
On the basis of RVR and EVR, we propose to classify the patient into 3 sub-groups. First sub-group of patients who achieve RVR and cEVR can be called ‘quick responders’. These patients had exceptionally good SVR rates (93-100%) regardless of the treatment duration (36 weeks or 48 weeks). Therefore, they should be treated for 36 weeks only, while a further sub-group of these patients having LVL should be treated for 24 weeks only, as supported by recent literature.2,4–7 The second sub-group of patients who do not achieve RVR but achieve cEVR can be called as ‘average responders’. The SVR rate in these patients was about 70%, regardless of the treatment duration (36 weeks or 48 weeks). Therefore, they are also eligible for abbreviated therapy, as prolongation of therapy beyond 36 weeks did not increase the SVR rate. Both these sub-groups have 25% reduction in treatment cost and duration, and also suffer from fewer adverse events. The third subgroup of patients who do not achieve RVR or cEVR but have DVR can be labeled as ‘slow responders’ and should be considered for prolonged therapy. However, we did not address this issue in our study.
Our study has few limitations. First, the pattern of IL28B polymorphisms was determined only in about half of the patients, as its importance as a predictor of response to treatment emerged only recently, when this trial was already underway. So the effect of IL28B on SVR could not be determined. Whether the pattern of IL28B polymorphism would affect the response rate of shorter duration therapy needs to be explored in future studies. Second, the total sample size in our study was relatively small.
To conclude, our study suggests that HCV genotype 1 patients who achieve cEVR can be successfully treated with PEG-RBV therapy of 36 weeks duration without compromising the efficacy. Important observation is that factors like viral load, RVR, age, BMI and advanced fibrosis do not influence the SVR rates as is the case with 24 weeks therapy. Therefore, a larger proportion of patients would be eligible for abbreviated therapy using our criterion. However, our results need to be confirmed in larger controlled trials.
Abbreviations- •
ALT: alanine aminotransferase.
- •
cEVR: complete early virological response.
- •
CHC: chronic hepatitis C.
- •
ETR: end-of-treatment response.
- •
G-1: genotype 1.
- •
HCV: hepatitis C virus.
- •
LVL: low viral load.
- •
PEG-RBV: pegylated interferon plus ribavirin.
- •
PTI: prothrombin time index.
- •
RNA: ribo-nucleic acid.
- •
RVR: rapid virological response.
- •
SVR: sustained virological response.
- •
TE: transient elastography.
We acknowledge the help from Dr. Jaskaran Sethi in data collection, and Dr. Suresh K. Sharma in statistical analysis.
Financial SupportDepartment of Gastroenterology, D.M.C. and Hospital, Ludhiana.