Hepatitis E virus (HEV) superinfection is a common excerbating event in patients with chronic hepatitis B, but the impact on the long-term prognosis is not clear. This study investigates the specific role of HEV superinfection in the long-term outcome of hepatitis B virus (HBV) patients with liver cirrhosis.
Patients and MethodsA retrospective, observational cohort study was conducted using clinical, laboratory, and survival data collected from patients suffering from hepatitis B cirrhosis with or without HEV superinfection. Disease progression and mortality rates were analyzed.
ResultsAfter a two-year follow-up, HEV superinfection was identified in 27 of 811 patients. The transplantation-free mortality was significantly increased (51.9% vs. 14.3%, p< 0.001) in HEV superinfection compared to that in hepatitis B cirrhosis patients without HEV superinfection. Logistic regression analysis demonstrated that elderly people were independent host risk factors for hepatitis B cirrhosis patients with HEV superinfection before and after propensity score matching (PSM). Moreover, HEV superinfection was a risk factor for patients with hepatitis B cirrhosis with new acute decompensation (AD) and acute-on-chronic liver failure (ACLF) during hospitalization. A multivariate Cox proportional hazards regression model demonstrated that acute HEV co-infection is associated with two-year mortality (hazard ratio [HR]: 2.49; 95% CI: 1.40–4.43; p= 0.002; and HR: 5.79; 95% CI: 1.87–17.87; p= 0.002) in patients with hepatitis B cirrhosis before and after PSM.
ConclusionsElder patients with hepatitis B cirrhosis are susceptible to HEV superinfection, accelerating disease progression and increasing long-term mortality in hospitalized patients with HBV-related decompensated liver cirrhosis.
Hepatitis E, induced by hepatitis E virus (HEV), is a significant global threat [1]. Despite hepatitis E vaccination, HEV infections have affected approximately one-third of the global population, resulting in an estimated 3.3 million symptomatic cases annually. In contrast, hepatitis E has an approximately 3.3% mortality rate due to viral hepatitis [1–3]. Hepatitis E is usually a self-limiting episode of hepatitis among immunocompetent individuals. However, pregnant women and some immunosuppressed patients with uncontrolled chronic diseases, post-transplantation, hematological complications, and other malignancies have high mortality rates [2].
The prevalent use of hepatitis B vaccine has reduced the morbidity ratio of chronic hepatitis B in recent decades. Research in serological epidemiology disclosed that HEV superinfection in patients with chronic hepatitis B (CHB) ranged from 2.8% to 17.6% [4]. It has been reported that HEV infection of hepatocytes or superinfection on HBV-infected hepatocytes arised host innate immune response and the RNA sequencing indicated the enriching of host defense transcriptional program (for instance, the type I IFN signaling pathway and cytoplasmic PRR signaling pathway) and metabolic reshaping [5,6]. Nonetheless, the concomitant with acute HEV that increases the clinical progression of patients with chronic hepatitis B remains controversial [4,7-10]. In Hong Kong, HEV infection has increased the rate of liver decompensation (10% vs. 3%, p= 0.13) and mortality (6.5% vs. 3%, p= 0.17) in chronic HBV carriers. However, the results did not indicate any statistical significance [7]. Furthermore, acute HEV superinfection does not increase the poor outcome of chronic hepatitis B virus infection; nonetheless, it could elevate the clinical progression and mortality in cirrhosis [9,10]. According to Tai-Chung Tseng et al., a one-year mortality rate was significantly increased in patients with cirrhosis and HEV infection (2.4% vs. 35.7%) compared to HEV superinfection without cirrhosis [7]. However, few studies analyzed patients with HBV cirrhosis with acute decompensation (AD), using a relatively short follow-up period [9,10].
Given the poor clinical outcomes of cirrhosis in patients with HBV and HEV superinfection, the current retrospective study aimed to assess the role of HEV superinfection from various sources on the long-term prognosis of HBV cirrhosis in hospitalized patients.
2Patients and Methods2.1PatientsBetween February 2014 and March 2015, patients with CHB and cirrhosis were enrolled retrospectively at the Department of Infectious Diseases, the First Affiliated Hospital, School of Medicine, Zhejiang University. Cirrhosis was diagnosed in patients with CHB based on liver biopsy, endoscopic or radiologic examination, clinical features of thrombocytopenia, gastroesophageal varices, or ascites [11,12]. Patients with CHB with cirrhosis were diagnosed with AD if they suffered from one of the following complications: ascites, encephalopathy, severe liver injury, or infection [12]. HEV superinfection diagnosis was defined as anti-HEV immunoglobulin M antibodies (HEV-IgM) seroconversion or HEV RNA-positive during the follow-up period. HEV RNA was tested for acute or chronic HEV infection in patients who had been HEV-IgM positive for more than three months. Patients over 60 years of age were considered elderly. AD was defined as cirrhosis with acute development of ascites (within less than two weeks), gastrointestinal hemorrhage, hepatic encephalopathy, and infection requiring hospitalization or appearing during hospitalization [13,14]. Acute liver injury was defined as a total bilirubin level >85 mmol/L and an international normalized ratio >1.5 within one month following the onset of the disease [12]. Based on EASL-CLIF criteria, ACLF was diagnosed as follows: kidney failure (creatinine ≥2.0 mg/dL (176 µmol/L), or in need of renal replacement therapy); two or more organ failures; one organ failure with the presence of kidney dysfunction 1.5 mg/dL ≤ creatinine < 2 mg/dL (132–176 µmol/L) and/or mild-to-moderate HE. Organ failure was diagnosed as follows: liver failure with serum bilirubin ≥12 mg/dL (204 µmol/L); coagulation failure with INR ≥2.5 or platelet count ≤20 × 109/L; kidney failure with creatinine ≥2.0 mg/dL (176 µmol/L) or in need of renal replacement therapy; circulation failure with mean arterial pressure (MAP) < 70 mmHg despite adequate fluid resuscitation and need for vasoactive agents; lung failure with PaO2/FiO2 ≤ 200, SpO2/FiO2 ≤ 214, or in need of mechanical ventilation; cerebral failure with HE grade III or IV [12,13]. As previously described, gastrointestinal hemorrhage and encephalopathy were diagnosed [12,13,15].
Patients were excluded based on the following criteria: (1) under the age of 18; (2) pregnancy; (3) human immunodeficiency virus (HIV), Epstein-Barr virus, human cytomegalovirus, or HCV co-infection; (4) severe comorbidities such as severe trauma, chronic heart or lung diseases, and cerebral hemorrhage or infarction; (5) hepatocellular carcinoma (HCC) and liver transplantation [15,16].
2.2HEV antibody detection, HEV-RNA, and other serum indexesThe clinic biochemical parameters were tested in the laboratory of the First Affiliated Hospital, Zhejiang University, School of Medicine. According to the manufacturer's instructions, enzyme-linked immunosorbent assay (ELISA) was used to detect HBsAg, HBsAb, and anti-HBc (Acon Biotech Co. Hangzhou, China), and Qiagen PRC was utilized to detect HBV DNA (Hilden, Germany). The lower limit of HBV DNA detection was 103 copies/mL, and the lower limit of quantitation of HBsAg was 0.05 IU/mL. At the time of enrollment, the patients were tested for hepatitis E antibodies (IgG or Ig M-HEV). Reverse-transcription-polymerase chain reaction (RT-PCR) was employed to test for HEV RNA, and ELISA was used to test for anti-HEV immunoglobulin (Ig) M and IgG (Wantai, Beijing, China). HEV superinfection was defined as anti-HEV immunoglobulin M antibodies (HEV-IgM) seroconversion or HEV RNA positivity.
2.3Data collectionBaseline information was collected, including demographics, clinical history, physical examination findings, laboratory measurements, complications, and prognosis with AD and ACLF. Survival was recorded at 28-day, 90-day, one-year, and two-year periods after enrollment. The primary clinical endpoint was determined as the incidence of death. Prognostic information was obtained from the patients after being discharged from the hospital. To avoid bias, two doctors reviewed the recorded data; one reviewed the data, and the other double-checked the database against the data collected to ensure accuracy and consistency. The data of epidemiological and demographic characteristics were confirmed by contacting patients or their family members by telephone. According to the diagnosis of HEV superinfection, all patients with hepatitis B cirrhosis were categorized into two groups: those with HEV superinfection and those without. If a follow-up of a patient was lost, the data collected were discarded.
2.4Statistical analysesThe data were analyzed using SPSS (version 26.0; SPSS, Inc., Chicago, IL, USA). The mean ± standard deviation (SD), the median with inter-quartile values, numbers, or percentages for variables, were calculated. Chi-square or Fisher's exact tests were used to compare categorical variables, and Kruskal–Wallis or Mann–Whitney U tests were employed to compare quantitative variables. Logistic regression analysis was performed to identify and compare HEV co-infection between groups and verify the correlation of HEV infection with disease progression. The transplant-free survival in each subgroup was calculated using the Kaplan-Meier method and compared using the log-rank test. Before and after propensity score matching (PSM), baseline characteristics of infected and non-infected HEV patients were compared. We performed PSM to adjust for differences in baseline characteristics of hepatitis B cirrhosis, including a history of hypertension and diabetes upon admission, complications with AD, and ACLF on admission by matching HEV non-infection with similar HEV co-infection in patients within a 1:1 ratio with 0.1 calipers of standard deviation. A multivariate logistic regression model was used to analyze the risk factors for host susceptibility to acute HEV co-infection, new AD, and ACLF during hospitalization. Violin charts of clinical detection indices, the cumulative incidence of hepatitis B cirrhotic patients between the two groups, and a forest map for regression analysis were performed using GraphPad Prism for Windows (version 8.0.0, GraphPad Software, San Diego, California, USA). A multivariate Cox proportional hazard model was used to analyze risk factors for 28-day, 90-day, one-year, and two-year mortality. For the multivariate analysis, the entry and removal probability for stepwise analysis was set at 0.05 and 0.10, respectively, and variables with P< 0.05 were kept in the final model. In all analyses, the significance level was set at p< 0.05.
2.5Ethical statementsWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of the First Affiliated Hospital, Zhejiang University, School of Medicine (2015IIT157).
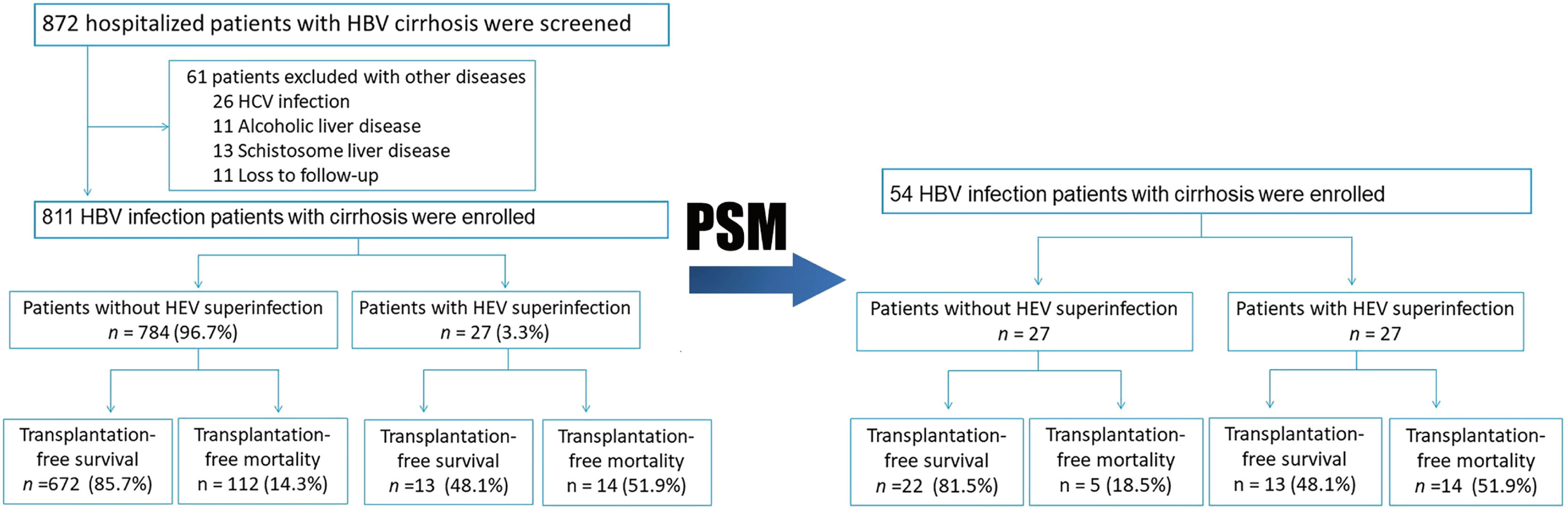
3Results3.1Patients with and without HEV superinfectionA total of 872 hospitalized patients with HBV cirrhosis were examined. A total of 811 subjects were enrolled in the study, while 61 were excluded due to other diseases (Fig. 1). In this study, the follow-up period is 710 (Quartile: 250–720) days. The observation period after study entry is 16 (Quartile: 8–22) days. After a two-year follow-up, 27 (3.3%) patients have HEV superinfection, including 24 HEV IgM positive and 3 HEV RNA positive. Transplant-free mortality is significantly higher in hepatitis B cirrhosis patients with HEV superinfection at one year (40.7% vs. 12.6%, p< 0.001) and two-year (51.9% vs. 14.3%, p< 0.001) compared to patients with hepatitis B cirrhosis without HEV infection (Figs. 1 and 4; Table 2).
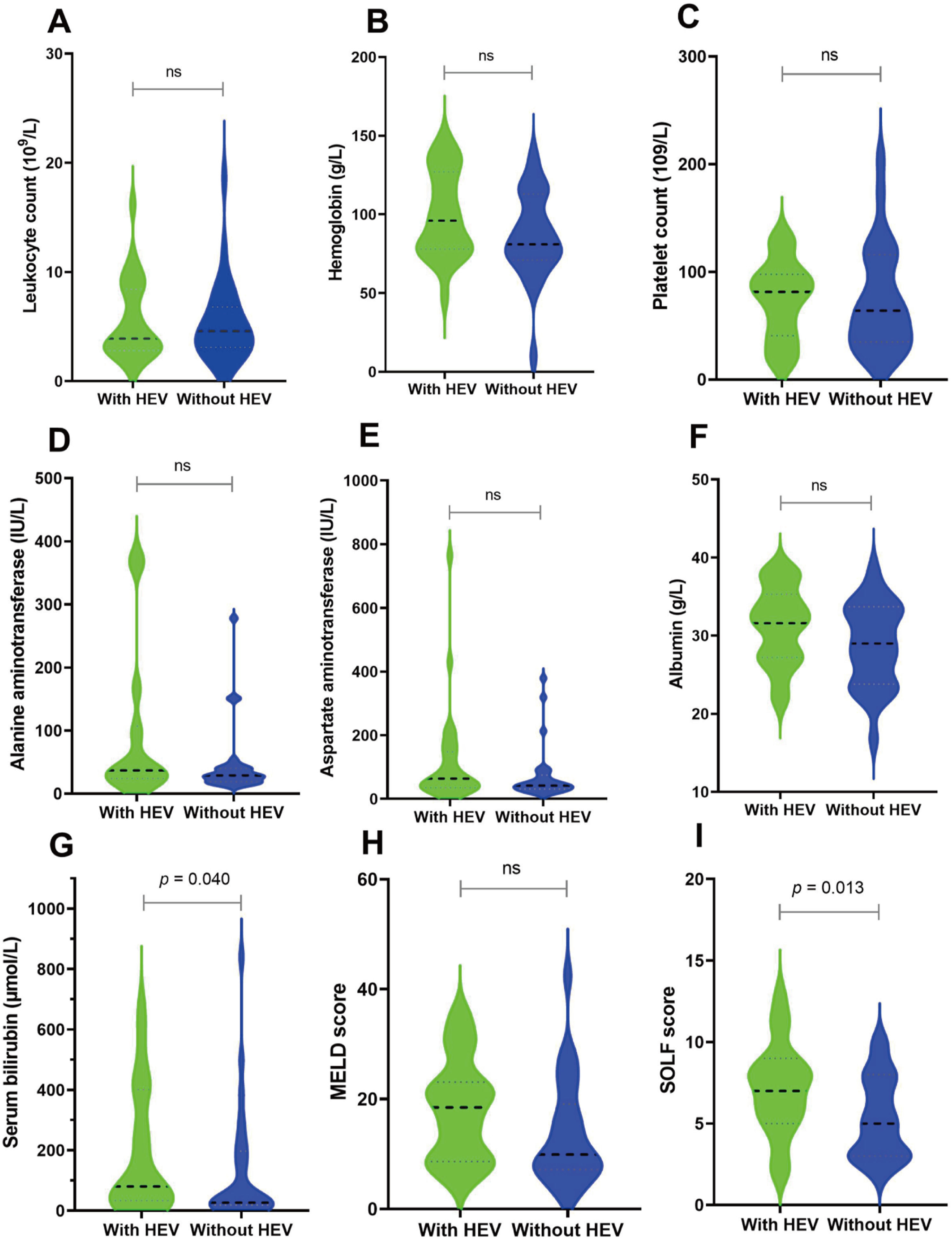
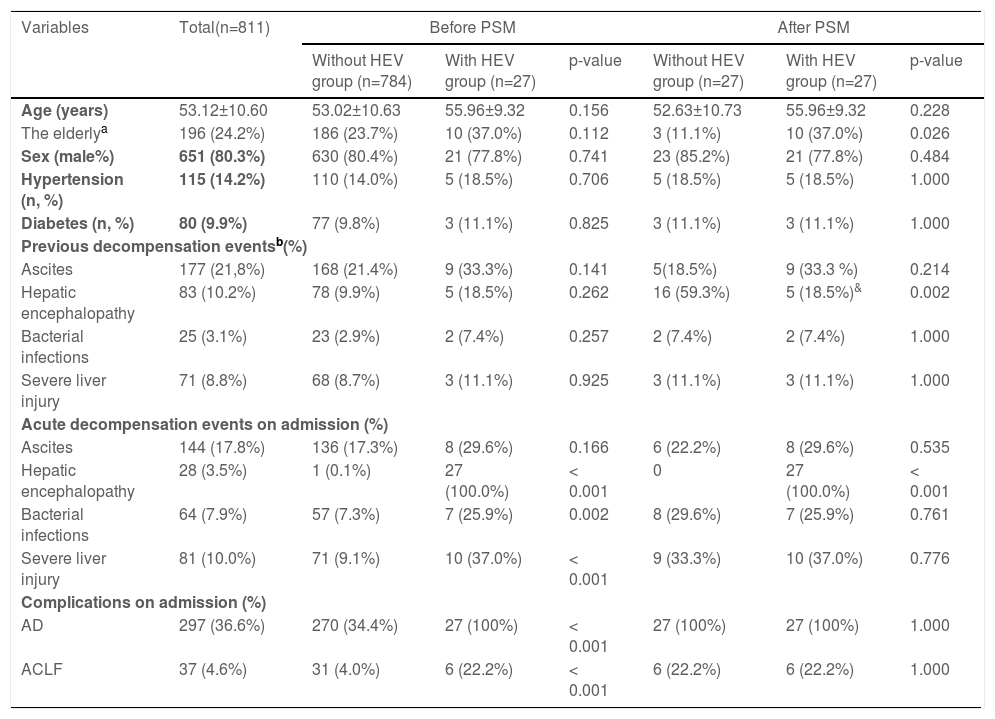
No significant differences existed between patients with HEV superinfection (n = 27) and those without HEV superinfection (n = 784) with respect to age (55.96±9.32 vs. 53.02±10.63), gender (male, 77.8% vs. 80.4%), history of hypertension (18.5% vs. 14.0%), history of diabetes (11.1% vs. 9.8%), and previous decompensation events. Patients with HEV superinfection experience an increased incidence of acute decompensation on admission with hepatic encephalopathy (100% vs. 0.1%, p< 0.001), bacterial infections (25.9% vs. 7.3%, p= 0.002), severe liver injury (37.0% vs. 9.1%, p< 0.001), complications with AD (100% vs. 34.4%, p< 0.001), and ACLF (22.2% vs. 4.0%, p< 0.001) on admission (Table 1). Comparing patients with hepatitis B cirrhosis without HEV superinfection to those with HEV superinfection show lower platelet count (74.50±35.94 vs. 91.42±65.80, p= 0.030), higher serum bilirubin (median: 80 vs. 26, p< 0.001), INR (median: 1.57 vs. 1.26, p< 0.001), AD during hospitalization (100% vs. 57.3%, p< 0.001), ACLF during hospitalization (22.2% vs. 5.4%, p 0.001) as well as MELD score (18.04±9.16 vs. 10.65±7.58, p< 0.001) and SOLF score (7.15±2.78 vs. 3.89±2.49, p< 0.001) during hospitalization (Table 2).
Baseline characteristics of enrolled patients with hepatitis B virus of cirrhosis with and without HEV infections.
| Variables | Total(n=811) | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|---|
| Without HEV group (n=784) | With HEV group (n=27) | p-value | Without HEV group (n=27) | With HEV group (n=27) | p-value | ||
| Age (years) | 53.12±10.60 | 53.02±10.63 | 55.96±9.32 | 0.156 | 52.63±10.73 | 55.96±9.32 | 0.228 |
| The elderlya | 196 (24.2%) | 186 (23.7%) | 10 (37.0%) | 0.112 | 3 (11.1%) | 10 (37.0%) | 0.026 |
| Sex (male%) | 651 (80.3%) | 630 (80.4%) | 21 (77.8%) | 0.741 | 23 (85.2%) | 21 (77.8%) | 0.484 |
| Hypertension (n, %) | 115 (14.2%) | 110 (14.0%) | 5 (18.5%) | 0.706 | 5 (18.5%) | 5 (18.5%) | 1.000 |
| Diabetes (n, %) | 80 (9.9%) | 77 (9.8%) | 3 (11.1%) | 0.825 | 3 (11.1%) | 3 (11.1%) | 1.000 |
| Previous decompensation eventsb(%) | |||||||
| Ascites | 177 (21,8%) | 168 (21.4%) | 9 (33.3%) | 0.141 | 5(18.5%) | 9 (33.3 %) | 0.214 |
| Hepatic encephalopathy | 83 (10.2%) | 78 (9.9%) | 5 (18.5%) | 0.262 | 16 (59.3%) | 5 (18.5%)& | 0.002 |
| Bacterial infections | 25 (3.1%) | 23 (2.9%) | 2 (7.4%) | 0.257 | 2 (7.4%) | 2 (7.4%) | 1.000 |
| Severe liver injury | 71 (8.8%) | 68 (8.7%) | 3 (11.1%) | 0.925 | 3 (11.1%) | 3 (11.1%) | 1.000 |
| Acute decompensation events on admission (%) | |||||||
| Ascites | 144 (17.8%) | 136 (17.3%) | 8 (29.6%) | 0.166 | 6 (22.2%) | 8 (29.6%) | 0.535 |
| Hepatic encephalopathy | 28 (3.5%) | 1 (0.1%) | 27 (100.0%) | < 0.001 | 0 | 27 (100.0%) | < 0.001 |
| Bacterial infections | 64 (7.9%) | 57 (7.3%) | 7 (25.9%) | 0.002 | 8 (29.6%) | 7 (25.9%) | 0.761 |
| Severe liver injury | 81 (10.0%) | 71 (9.1%) | 10 (37.0%) | < 0.001 | 9 (33.3%) | 10 (37.0%) | 0.776 |
| Complications on admission (%) | |||||||
| AD | 297 (36.6%) | 270 (34.4%) | 27 (100%) | < 0.001 | 27 (100%) | 27 (100%) | 1.000 |
| ACLF | 37 (4.6%) | 31 (4.0%) | 6 (22.2%) | < 0.001 | 6 (22.2%) | 6 (22.2%) | 1.000 |
Data are expressed as mean ± SD, median (Q1–Q3), or number (percentage). Comparisons between groups were performed using Student's t-test, Mann-Whitney U test, or chi-square test. Comparisons of cumulative transplant-free mortality were made using the log-rank test.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; Diabetes, diabetes mellitus that requires treatment; HBV, hepatitis B virus; INR, international normalized ratio; MELD score, a model for end-stage liver disease score; PSM, propensity score matching.
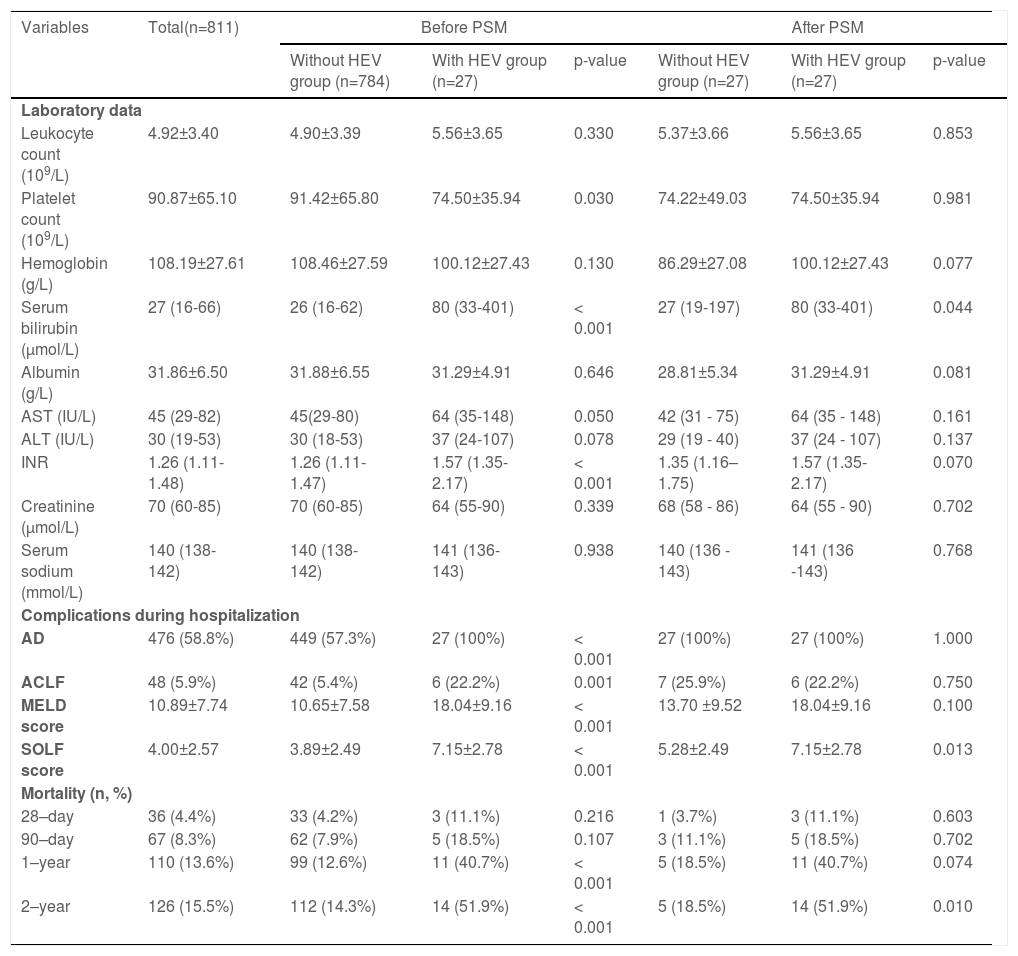
Clinic changes and prognosis for hepatitis B patients suffering from cirrhosis with and without HEV co-infections.
| Variables | Total(n=811) | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|---|
| Without HEV group (n=784) | With HEV group (n=27) | p-value | Without HEV group (n=27) | With HEV group (n=27) | p-value | ||
| Laboratory data | |||||||
| Leukocyte count (109/L) | 4.92±3.40 | 4.90±3.39 | 5.56±3.65 | 0.330 | 5.37±3.66 | 5.56±3.65 | 0.853 |
| Platelet count (109/L) | 90.87±65.10 | 91.42±65.80 | 74.50±35.94 | 0.030 | 74.22±49.03 | 74.50±35.94 | 0.981 |
| Hemoglobin (g/L) | 108.19±27.61 | 108.46±27.59 | 100.12±27.43 | 0.130 | 86.29±27.08 | 100.12±27.43 | 0.077 |
| Serum bilirubin (µmol/L) | 27 (16-66) | 26 (16-62) | 80 (33-401) | < 0.001 | 27 (19-197) | 80 (33-401) | 0.044 |
| Albumin (g/L) | 31.86±6.50 | 31.88±6.55 | 31.29±4.91 | 0.646 | 28.81±5.34 | 31.29±4.91 | 0.081 |
| AST (IU/L) | 45 (29-82) | 45(29-80) | 64 (35-148) | 0.050 | 42 (31 - 75) | 64 (35 - 148) | 0.161 |
| ALT (IU/L) | 30 (19-53) | 30 (18-53) | 37 (24-107) | 0.078 | 29 (19 - 40) | 37 (24 - 107) | 0.137 |
| INR | 1.26 (1.11-1.48) | 1.26 (1.11-1.47) | 1.57 (1.35-2.17) | < 0.001 | 1.35 (1.16–1.75) | 1.57 (1.35-2.17) | 0.070 |
| Creatinine (μmol/L) | 70 (60-85) | 70 (60-85) | 64 (55-90) | 0.339 | 68 (58 - 86) | 64 (55 - 90) | 0.702 |
| Serum sodium (mmol/L) | 140 (138-142) | 140 (138-142) | 141 (136-143) | 0.938 | 140 (136 - 143) | 141 (136 -143) | 0.768 |
| Complications during hospitalization | |||||||
| AD | 476 (58.8%) | 449 (57.3%) | 27 (100%) | < 0.001 | 27 (100%) | 27 (100%) | 1.000 |
| ACLF | 48 (5.9%) | 42 (5.4%) | 6 (22.2%) | 0.001 | 7 (25.9%) | 6 (22.2%) | 0.750 |
| MELD score | 10.89±7.74 | 10.65±7.58 | 18.04±9.16 | < 0.001 | 13.70 ±9.52 | 18.04±9.16 | 0.100 |
| SOLF score | 4.00±2.57 | 3.89±2.49 | 7.15±2.78 | < 0.001 | 5.28±2.49 | 7.15±2.78 | 0.013 |
| Mortality (n, %) | |||||||
| 28–day | 36 (4.4%) | 33 (4.2%) | 3 (11.1%) | 0.216 | 1 (3.7%) | 3 (11.1%) | 0.603 |
| 90–day | 67 (8.3%) | 62 (7.9%) | 5 (18.5%) | 0.107 | 3 (11.1%) | 5 (18.5%) | 0.702 |
| 1–year | 110 (13.6%) | 99 (12.6%) | 11 (40.7%) | < 0.001 | 5 (18.5%) | 11 (40.7%) | 0.074 |
| 2–year | 126 (15.5%) | 112 (14.3%) | 14 (51.9%) | < 0.001 | 5 (18.5%) | 14 (51.9%) | 0.010 |
Data are expressed as mean ± SD, median (Q1–Q3), or number (percentage). Comparisons between groups were performed using Student's t-test, Mann-Whitney U test, or chi-square test. Comparisons of cumulative transplant-free mortality were made using the log-rank test.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; Diabetes, diabetes mellitus requiring treatment; HBV, hepatitis B virus; INR, international normalized ratio; MELD score, a model for end-stage liver disease score; PSM, propensity score matching.
Subsequently, we matched 27 patients with HEV superinfection cirrhosis 1:1. After PSM, as displayed in Table 1, some baseline characteristics have changed compared with patients with hepatitis B cirrhosis without HEV superinfection. The incidence of HEV superinfection was significantly higher for elderly patients with HBV cirrhosis than for younger (37.0% vs. 11.1%, p= 0.026); some preceding decompensation events and AD events have changed. As displayed in Table 2, after PSM, compared with non-HEV superinfection, HEV superinfection has higher serum bilirubin (median: 80 vs. 27, p= 0.044) and SOLF score (7.15±2.78 vs. 5.28±2.49, p= 0.013) (Table 2 and Fig. 2).
Before and after PSM, the clinical outcomes of HEV superinfection in hepatitis B patients with cirrhosis. HBV cirrhosis patients were categorized into two groups “without active HEV infection” (shown as “without HEV”) and “with active HEV infection” (displayed as “with HEV”). Different clinical and biochemical parameters including leukocyte count (A), hemoglobin (B), platelet count (C), ALT (D), AST (E), albumin (F), serum total bilirubin (G), MELD score (H) and SOLF score (I) were compared.
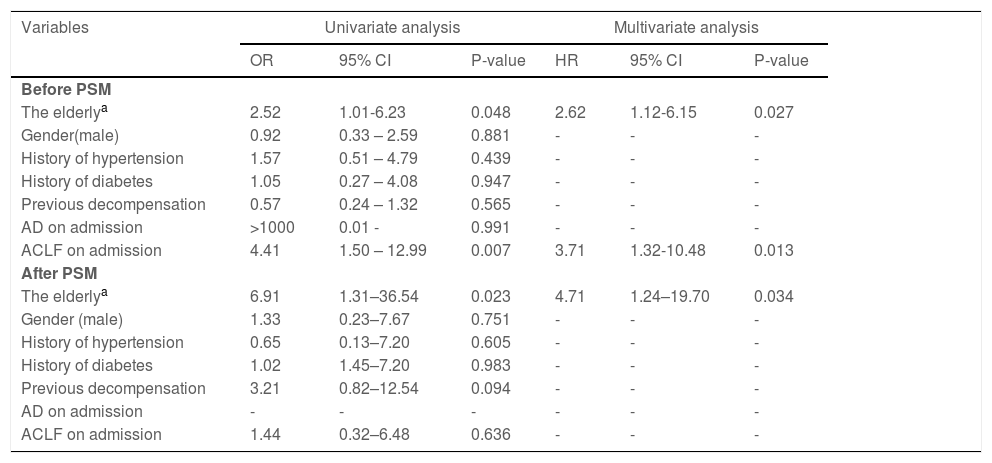
We analyzed the host susceptibility factors associated with HEV superinfection in patients with hepatitis B cirrhosis, including gender, age, history of hypertension, history of diabetes, previous decompensation events, acute decompensation, and ACLF on admission. The elderly participants (odds ratio [OR] = 2.62, 95% CI: 1.12–6.15, p= 0.027) and ACLF (OR = 3.71, 95% CI: 1.32–10.48, p= 0.013) on admission have host sensitivity factors associated with HEV superinfection prior to PSM. Following PSM, the risk factors were altered, and increasing age (OR = 4.71, 95% CI: 1.24–19.70, p= 0.034) is the only independent risk factor for hepatitis B cirrhosis patients with acute HEV co-infection (Table 3).
Host susceptibility factors associated with HEV superinfection in hepatitis B cirrhosis patients before and after PSM.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | HR | 95% CI | P-value | |
| Before PSM | ||||||
| The elderlya | 2.52 | 1.01-6.23 | 0.048 | 2.62 | 1.12-6.15 | 0.027 |
| Gender(male) | 0.92 | 0.33 – 2.59 | 0.881 | - | - | - |
| History of hypertension | 1.57 | 0.51 – 4.79 | 0.439 | - | - | - |
| History of diabetes | 1.05 | 0.27 – 4.08 | 0.947 | - | - | - |
| Previous decompensation | 0.57 | 0.24 – 1.32 | 0.565 | - | - | - |
| AD on admission | >1000 | 0.01 - | 0.991 | - | - | - |
| ACLF on admission | 4.41 | 1.50 – 12.99 | 0.007 | 3.71 | 1.32-10.48 | 0.013 |
| After PSM | ||||||
| The elderlya | 6.91 | 1.31–36.54 | 0.023 | 4.71 | 1.24–19.70 | 0.034 |
| Gender (male) | 1.33 | 0.23–7.67 | 0.751 | - | - | - |
| History of hypertension | 0.65 | 0.13–7.20 | 0.605 | - | - | - |
| History of diabetes | 1.02 | 1.45–7.20 | 0.983 | - | - | - |
| Previous decompensation | 3.21 | 0.82–12.54 | 0.094 | - | - | - |
| AD on admission | - | - | - | - | - | - |
| ACLF on admission | 1.44 | 0.32–6.48 | 0.636 | - | - | - |
AD, acute decompensation; CI, confidence interval; ACLF, acute-on-chronic liver failure; HE, hepatic encephalopathy; OR, odds ratio; PSM, propensity score matching; UGH, upper gastrointestinal bleeding. Statistical analysis was performed using logistic regression analysis. For HEV superinfection, the variables entered into the multivariate analysis were gender, elderly patients, history of hypertension, history of diabetes, previous decompensation, AD, ACLF on admission.
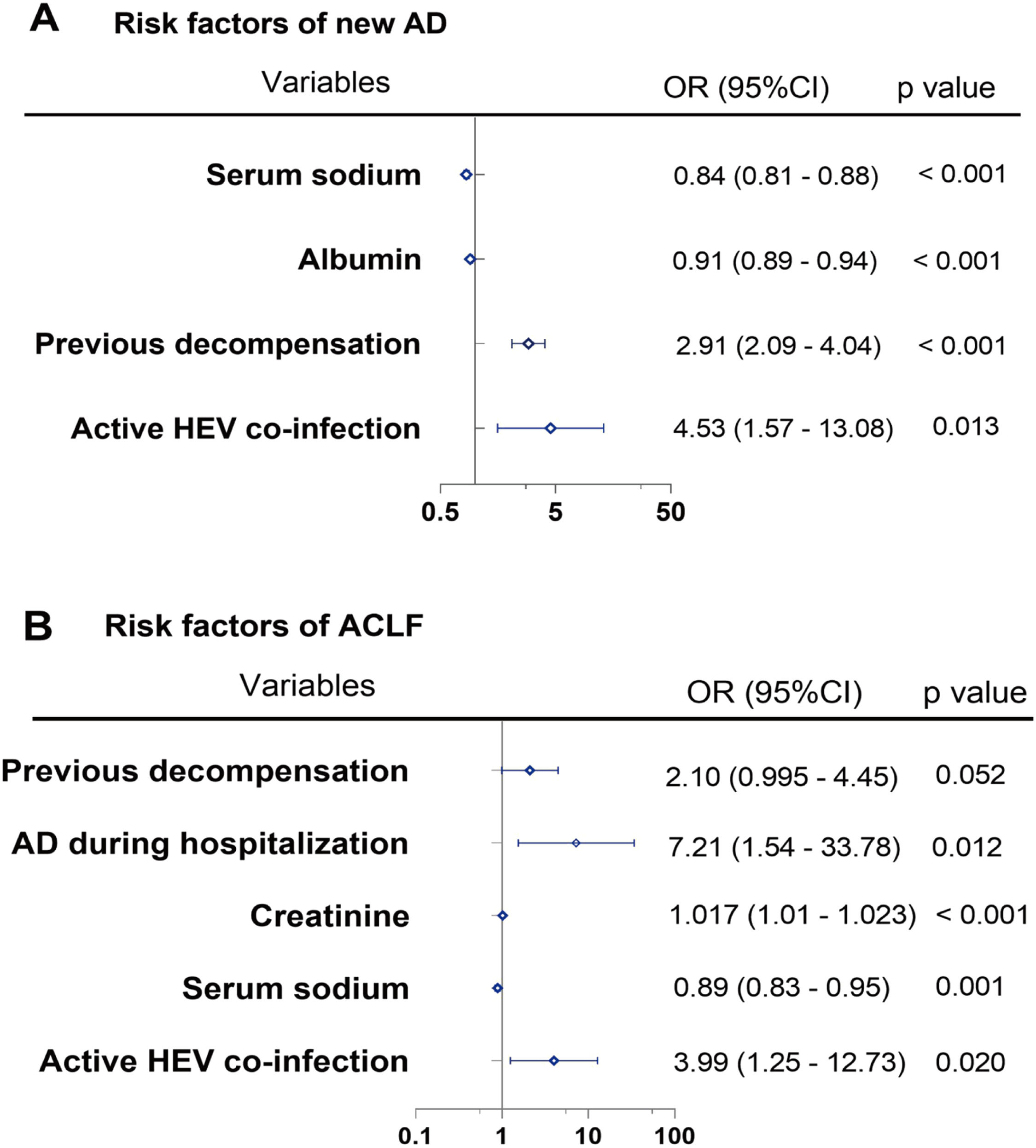
We analyzed risk factors associated with new AD during hospitalization in patients with hepatitis B cirrhosis, including variables such as gender, elderly patients, history of hypertension, history of diabetes, previous decompensation, AD on admission, albumin, creatinine, and serum sodium using multivariate analysis. HEV superinfection (OR = 4.53, 95% CI: 1.57–13.08, p= 0.013), previous decompensation (OR = 2.91, 95% CI: 2.09–4.04, p< 0.001), albumin (OR = 0.91, 95% CI: 0.89–0.94, p< 0.001), and serum sodium (OR = 0.84, 95% CI: 0.81–0.88, p< 0.001) are correlated with new AD during hospitalization. Additionally, HEV superinfection (OR = 3.99, 95% CI: 1.25–12.73, p= 0.020), serum sodium (OR = 0.89, 95% CI: 0.83–0.95, p= 0.001), creatinine (OR = 1.017, 95% CI: 1.01–1.023, p< 0.001), and AD during hospitalization (OR = 7.21, 95% CI: 1.54–33.78, p= 0.012) are associated with ACLF during hospitalization (Fig. 3).
3.4Overall risk factors for mortality in patients with hepatitis B cirrhosisAfter a two-year follow-up, 28-day, 90-day, one-year, and two-year mortality rates were 4.4%, 8.3%, 13.6%, and 15.5%, respectively. Compared with patients with HEV non-infection with hepatitis B cirrhosis, mortality rates of one year (40.7% vs. 12.6%, p< 0.001) and two years (51.9% vs. 14.3%, p< 0.001) are higher in the patients with hepatitis B cirrhosis with HEV superinfection (Table 2).
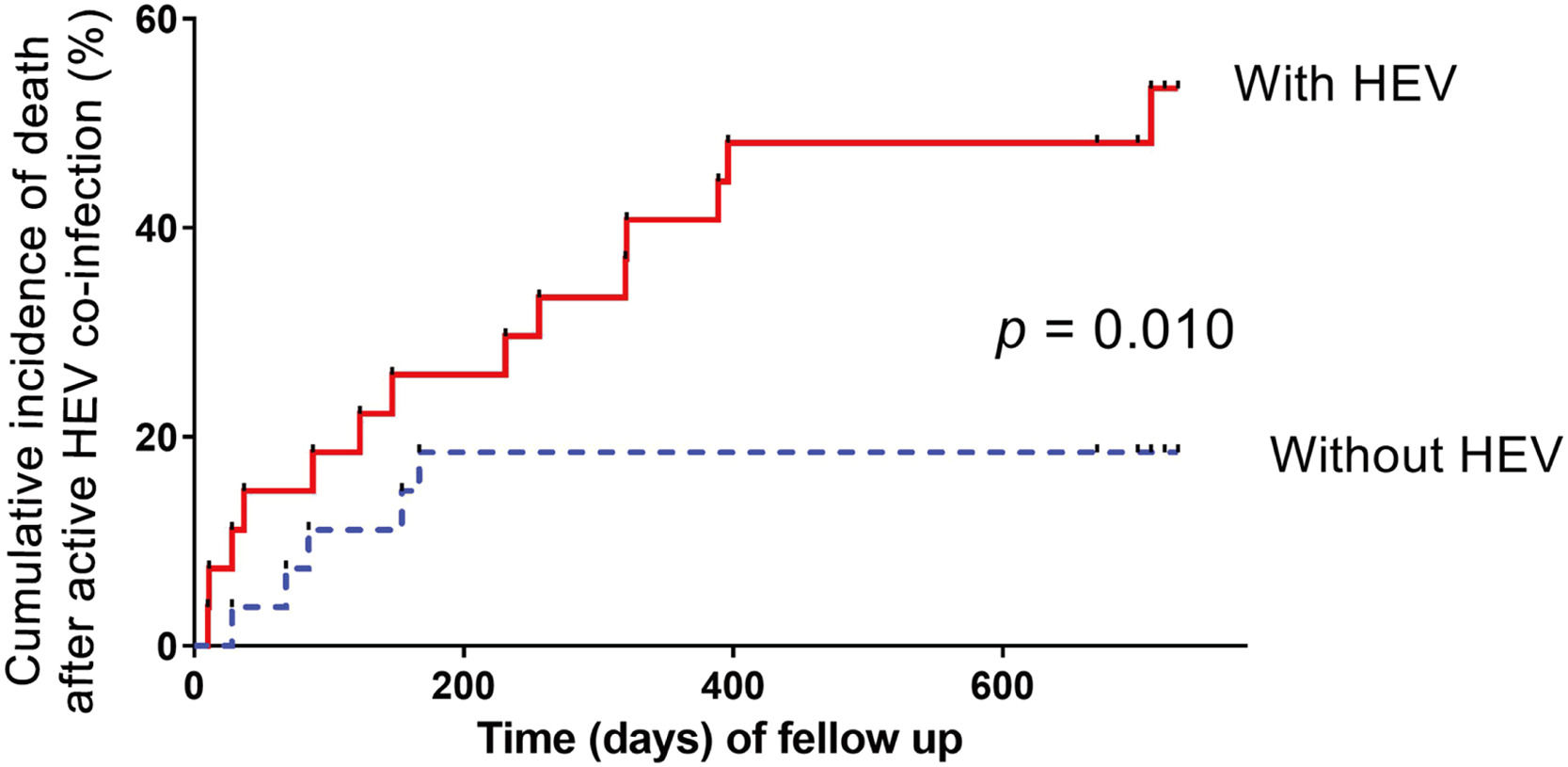
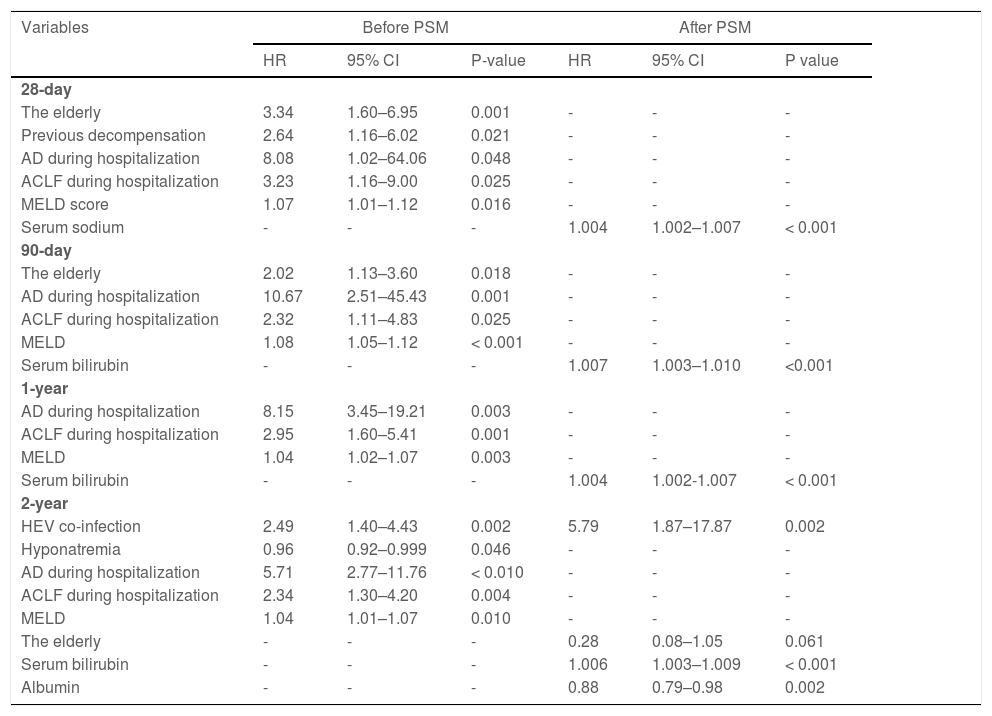
The multivariate Cox proportional hazards regression model demonstrates that severe HEV superinfection is linked to two-year mortality (hazard ratio [HR]: 2.49; 95% CI: 1.40–4.43; p= 0.002) in patients with hepatitis B cirrhosis (Table 4). With a two-year follow-up, the cumulative incidence of death after HEV superinfection is significantly increased (p< 0.001) compared to the absence of HEV superinfection in patients with hepatitis B cirrhosis (Fig. 4). After PSM, severe HEV co-infection is also correlated with two-year mortality (HR: 5.79; 95% CI: 1.87–17.87; p= 0.002) in patients with hepatitis B cirrhosis (Table 4).
Risk factors associated with mortality in hepatitis B virus patients suffering from cirrhosis after PSM.
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P value | |
| 28-day | ||||||
| The elderly | 3.34 | 1.60–6.95 | 0.001 | - | - | - |
| Previous decompensation | 2.64 | 1.16–6.02 | 0.021 | - | - | - |
| AD during hospitalization | 8.08 | 1.02–64.06 | 0.048 | - | - | - |
| ACLF during hospitalization | 3.23 | 1.16–9.00 | 0.025 | - | - | - |
| MELD score | 1.07 | 1.01–1.12 | 0.016 | - | - | - |
| Serum sodium | - | - | - | 1.004 | 1.002–1.007 | < 0.001 |
| 90-day | ||||||
| The elderly | 2.02 | 1.13–3.60 | 0.018 | - | - | - |
| AD during hospitalization | 10.67 | 2.51–45.43 | 0.001 | - | - | - |
| ACLF during hospitalization | 2.32 | 1.11–4.83 | 0.025 | - | - | - |
| MELD | 1.08 | 1.05–1.12 | < 0.001 | - | - | - |
| Serum bilirubin | - | - | - | 1.007 | 1.003–1.010 | <0.001 |
| 1-year | ||||||
| AD during hospitalization | 8.15 | 3.45–19.21 | 0.003 | - | - | - |
| ACLF during hospitalization | 2.95 | 1.60–5.41 | 0.001 | - | - | - |
| MELD | 1.04 | 1.02–1.07 | 0.003 | - | - | - |
| Serum bilirubin | - | - | - | 1.004 | 1.002-1.007 | < 0.001 |
| 2-year | ||||||
| HEV co-infection | 2.49 | 1.40–4.43 | 0.002 | 5.79 | 1.87–17.87 | 0.002 |
| Hyponatremia | 0.96 | 0.92–0.999 | 0.046 | - | - | - |
| AD during hospitalization | 5.71 | 2.77–11.76 | < 0.010 | - | - | - |
| ACLF during hospitalization | 2.34 | 1.30–4.20 | 0.004 | - | - | - |
| MELD | 1.04 | 1.01–1.07 | 0.010 | - | - | - |
| The elderly | - | - | - | 0.28 | 0.08–1.05 | 0.061 |
| Serum bilirubin | - | - | - | 1.006 | 1.003–1.009 | < 0.001 |
| Albumin | - | - | - | 0.88 | 0.79–0.98 | 0.002 |
ACLF, acute-on-chronic liver failure; CI, confidence interval; HR, hazard ratio; MELD score, a model for end-stage liver disease score; PSM, propensity score matching. Statistical analysis was performed using Cox proportional hazard model. With regard to mortality, the variables entered into the multivariate Cox proportional hazard model include gender, the elderly, hypertension complications, diabetes complications, previous decompensation, AD during hospitalization, ACLF during hospitalization, MELD score, hemoglobin, serum bilirubin, albumin, serum sodium, and HEV superinfection.
Because of the difference in incidence of death compared between HBV DNA-positive and -negative groups, the samples were then divided into HBV DNA positive and negative groups. Positive HEV superinfection does not differ between the two groups (37.0% vs. 25.9%, p= 0.379). A subgroup analysis was performed of the cirrhotic patients with HBV DNA negative group through multivariate Cox proportional hazards regression model, indicating that HEV superinfection (HR: 7.69; 95% CI: 1.52–38.92; p= 0.014), albumin (HR: 0.85; 95% CI: 0.74–0.98; p= 0.026), and serum bilirubin (HR: 1.009; 95% CI: 1.004–1.013; p< 0.001) correspond with two-year mortality. Furthermore, in HBV DNA positive group, HEV superinfection (HR: 4.87; 95% CI: 0.94–25.32; p= 0.060) corresponds with two-year mortality. These findings indicated that HEV superinfection was associated with exacerbating long-term outcome irrespective of HBV DNA status.
4DiscussionHepatitis E remains prevalent among HBV-infected people and has a high mortality rate in patients with underlying chronic liver diseases, particularly cirrhosis [2,8,9,17]. In a retrospective cohort study, we investigated the clinical outcomes of patients with hepatitis B cirrhosis after HEV superinfection. The incidence of HEV superinfection in patients with hepatitis B cirrhosis was 3.3%, higher than that in patients without cirrhosis (0.2%–2%) [9]. Additionally, the transplant-free mortality rate in patients with HEV superinfection (51.9%) was substantially higher than those without HEV superinfection (14.3%) in patients with hepatitis B cirrhosis. Meanwhile, elderly patients with cirrhosis and AD were prone to HEV superinfection. HEV superinfection accelerates disease progression and increases long-term mortality in patients with hepatitis B liver cirrhosis, especially during the end stages.
According to the reported end-stage liver disease, increasing age was the risk factor for superinfection HEV in hepatitis B patients with cirrhosis [8,17]. We discovered that increasing age is the only host susceptibility factor. Professor Hoan N.X. et al. reported that HEV superinfection in chronic hepatitis B might aggravate the course of underlying conditions, including increased liver enzymes and bilirubin, decreased albumin levels, prothrombin, and platelet counts [8]. Moreover, patients with end-stage liver disease with severe hepatitis E were generally older, had lower levels of ALB, PLT, and ALT, and had an increased mortality rate and liver-related mortality rate compared to those without the liver disease [17]. The present study presented no difference in platelet count after PSM, although the platelet count level was lower than before PSM. Meanwhile, serum bilirubin levels and SOLF score increased in active HEV superinfection.
HEV superinfection accelerates disease progression in patients with chronic HBV infection, and we found that it is a risk factor associated with new AD and ACLF during hospitalization in patients with hepatitis B cirrhosis [2,8-10].
We found adverse outcomes with HEV superinfection in decompensated hepatitis B cirrhosis but not in compensated cirrhotic hepatitis B. According to previous reports of HEV superinfection, patients with end-stage liver disease have lower all-cause and liver-related mortality rates than those without end-stage liver disease [17]. The mortality rate was significantly higher in patients with cirrhosis with HEV than in HEV superinfection without cirrhosis [9]. However, only patients with compensated liver cirrhosis were included. In our study, many patients with cirrhosis were enrolled, which validates the finding that, in patients with CHB with liver cirrhosis, HEV superinfection is associated with a higher mortality rate than those without, especially in patients with cirrhosis and AD. Poor outcomes of HEV superinfection in elderly patients with decompensated cirrhosis with HBV infection may be due to either more aggressive immune- or inflammatory-mediated activation of cell death with decompensation cirrhotic liver status, which may increase the collateral injury of cirrhotic liver when infected with HEV [1,8, 9].
The hepatitis E vaccine has recently demonstrated high short- and long-term protective efficacy in China; however, the World Health Organization (WHO) does not recommend vaccination exclusively during outbreaks of hepatitis [3,17,18]. Due to the high rate of decompensation and mortality in patients with cirrhosis with HBV infection with HEV superinfection, general vaccination should be recommended for patients with hepatitis B cirrhosis.
It is important to acknowledge the limitations of the current investigation. First, our retrospective study enrolled hospitalized superinfected patients with HEV and followed up on the mortality for two years post-discharge. However, the study did not analyze the characteristics of non-hospitalized patients with HEV superinfection, which may overestimate the risk of HEV in patients with cirrhosis and AD. Second, we may have overestimated the risk of HEV-related disease progression, considering that some asymptomatic patients superinfected with HEV were not rechecked because they had hepatitis E antibody negative. Third, we assessed liver function every three months in patients with compensated hepatitis B cirrhosis during follow-up, and some active HEV superinfected patients might have been missed. Fourthly, HEV genotypes are responsible for different levels of hepatic aggression and maybe lead to different progress of HEV superinfection in HBV patients, but in our study, we did not further distinguish the genotype of HEV, which may be a confounding factor interfering with the clinical outcome of HEV superinfection. Finally, the number of cases recruited after PSM was insufficient, and the study included only patients from one center. Despite the limitations, we can provide insights into the severity and poor outcomes of HEV co-infection in hepatitis B cirrhosis. To identify the variants for predicting poor outcomes associated with HEV infection in hepatitis B cirrhosis, more work with a large prospective cohort study is required.
5ConclusionsIn the present study, we investigated the specific role of HEV superinfection in the long-term outcome of HBV patients with liver cirrhosis. And our findings showed that HEV superinfection accelerated disease progression and increased long-term mortality in patients with hepatitis B liver cirrhosis, especially in patients with end-stage of liver cirrhosis.
Author contributionsHZ, YS, and JFS conceived and designed the study; WYY, XY, XZ, and JHH collected and analyzed the data; ZH and WYY drafted the manuscript; YS and JFS revised the manuscript. All authors approved the final version of the manuscript, including the authorship list.
FundingThis work was supported by The National Key Research and Development Program of China (No.2021YFC2301800), the National Natural Science Foundation of China (Grant No. 81870425 and 81971982) and the Youth Talent Fund Project of Zhejiang Traditional Chinese Medicine Science and Technology Plan (2020ZQ016)
Data availability statementThe data behind a research project can be accessed by Prof. Y. Shi and J. Sheng.
This paper has been proofread and edited for language clarity and grammar by Freescience Editorial Team.