A 28-year-old female presented with upper quadrant abdominal pain. The patient did use oral contraceptives, her BMI was 35 kg/m2 and serum AFP level was within normal limit. A palpable liver mass was noted and CT demonstrated a pedonculated 10 cm tumor under the right liver lobe. A resection was performed.
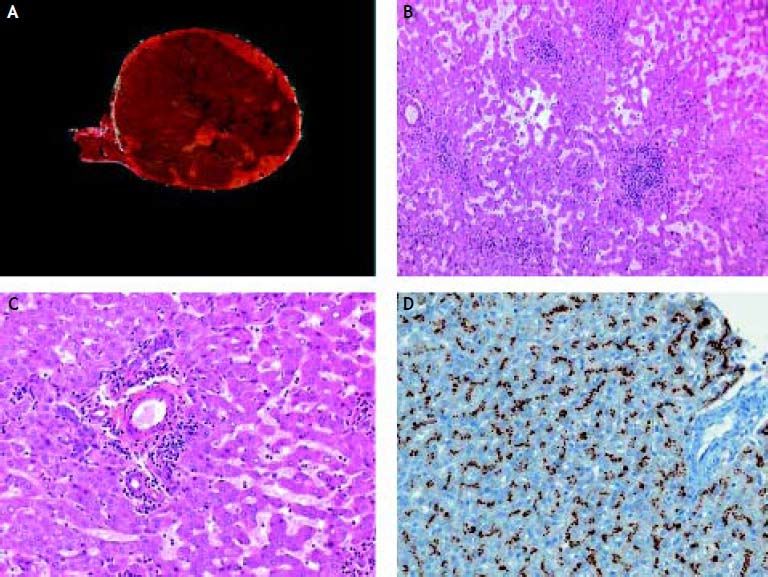
Image: The resected specimen showed a solitary well-circumscribed but not encapsulated tumor (Figure 1A). On light microscope, the tumor was made of normal hepatocytes organized in trabeculae often dissociated by dilated sinusoids (Figure 1B). No portal tract or central vein was noted within the tumor. Small aggregates made of unpaired arteries embedded by collagen fibers and inflammatory cells, mainly lymphocytes were present within the tumor (Figure 1С). Hepatocytes display a strong immunostaining with anti-SAA antibody (Figure 1D). No dysplastic or tumoral area was noted within the tumor. The peritumoral liver was normal.
Diagnosis: telangiectatic/inflammatory hepatocellular adenoma.
Hepatocellular adenoma (HCA) is a rare, benign liver neoplasm strongly associated with use of oral contraceptive (OC) in females, androgen steroid therapy in male or with rare genetic metabolic diseases.1 HCAs are usually solitary, well-delineated, sometimes encapsulated, with a fleshy appearance. Histologically, HCA consists of a proliferation of benign hepatocytes arranged in a trabecular pattern, without any residual lobular organisation. Small thin and unpaired vessels are usually found throughout the tumor.
Molecular studies have recently gained insights in the physiopathology of HCA resulting in the categorization of HCA into 3 main subtypes with specific histological and molecular features.2 These molecular alterations allowed to develop specific immunohistochemical markers for each tumor type that can be used as surrogate of molecular testing.3
The first group of HCA displays biallelic mutations of the TCF1 gene inactivating the hepatocyte nuclear factor 1α (HNF1a) transcription factor.4 Histologically, it is characterized by marked steatosis, absence of cytological abnormalities or inflammatory infiltrates. Whereas, HNF1a mutations are somatic in most cases, patients with inherited mutation in one allele of HNF1a may develop maturityonset diabetes of the young type 3 (MODY3) and are predisposed to have familial liver adenomatosis when the second allele is inactivated. Lack of Liver Fatty Acid Binding Protein (LFABP) expression, a protein positively regulated by HNF1 a, serve as surrogate for characterization of HNF1 α-mutated HCA by immunohistochemistry.
The second group of HCA displays β-catenin-activating mutations and is characterized by increased risk for malignant transformation into HCC. These HCA are mostly encountered in male patients and frequently show significant cell atypias and pseudoglandular formations.2 This HCA subtype display abnormal nuclear staining of β-catenin in tumoral hepatocytes and a strong cytoplasmic immunostaining with glutamine synthetase, a β-catenin-targeted gene.
The third group of HCA is the telangiectatic/inflammatory adenoma subgroup characterized by sinusoidal dilation and/or peliotic changes, the presence of few and short fibrous tracts around small arteries, sometimes accompanied by inflammatory infiltrates.5 These HCA are more frequent in patients with overweight and with inflammatory syndrome. Indeed, inflammatory HCA display positive immunostaining with acute phase inflammatory proteins such as serum amyloid A (SAA) and CRP. In approximately 60% of these HCA, IL6 signaling pathway is activated in relation with mutations in the IL6ST gene that encodes the signaling coreceptor gp130.6
Lastly, β-catenin mutations may be observed in some telangiectatic/inflammatory HCA whereas gp130 activation and HNF1 a inactivation (steatotic subtype) are mutually exclusive. Finally, a small group of HCA remain “unclassified” since they do not display any specific morphological or genotypical features.
Due to the potential risk for complications (haemorrhage, malignant degeneration), surgical resection is required for HCA larger than 5 cm in diameter and for all HCAs in males whatever their size. Small lesions with a low risk of complication could be initially observed after cessation of OC.7