A 46-year old man with a chronic hepatitis C virus infection received triple therapy with ribavirin, pegylated interferon and telaprevir. The patient also received simvastatin. One month after starting the antiviral therapy, the patient was admitted to the hospital because he developed rhabdomyolysis. At admission simvastatin and all antiviral drugs were discontinued because toxicity due to a drug-drug interaction was suspected. The creatine kinase peaked at 62,246 IU/L and the patient was treated with intravenous normal saline. The patient’s renal function remained unaffected. Fourteen days after hospitalization, creatine kinase level had returned to 230 IU/L and the patient was discharged. Telaprevir was considered the probable causative agent of an interaction with simvastatin according to the Drug Interaction Probability Scale. The interaction is due to inhibition of CYP3A4-mediated simvastatin clearance. Simvastatin plasma concentration increased 30 times in this patient and statin induced muscle toxicity is related to the concentration of the statin in blood. In conclusion, with this case we illustrate that telaprevir as well as statins are susceptible to clinical relevant drug-drug interactions.
Adding the protease inhibitor telaprevir to pegylated interferon alfa and ribavirin increases the sustained virological response rates in patients with a chronic hepatitis C virus (HCV) genotype 1 infection significantly.1 Drug-drug interactions with tel-aprevir are common2,3 and may result in serious complications. We report a patient who developed rhabdomyolysis due to concomitant use of telaprevir and simvastatin.
Case ReportA 46-year old man from the Republic of Azerbaijan started treatment for chronic HCV infection with ribavirin, pegylated interferon alfa and telaprevir at standard doses through an extended access program (EAP). A complete overview of his medication seemed to be clear at that time, but turned out to be incomplete possibly through language problems.
One month later, the patient was admitted to the hospital because of nausea without vomiting or abdominal pain and decreased appetite existing for 2 to 3 weeks. Since 10 days he was exhausted quickly and noticed muscle weakness in his legs and hands; when walking his legs were trembling. He also reported pain in his back and shoulders, dyspnea on exertion and a painful miction but no hematuria.
Physical examination showed a not acutely ill man with a blood pressure of 110/76 mm Hg and a pulse rate of 99 beats/minute. His medical history included diabetes mellitus type 2, hypersomnia, liver cirrhosis due to chronic genotype 1b HCV infection and previous alcohol abuse with portal hypertension and esophageal varices. The patient had no ascites. At admission his medications included enalapril 20 mg once-daily (QD), hydrochlorothiazide 12.5 mg QD, metformin 500 mg QD, omeprazole 40 mg QD, lactulose syrup 40 g thrice daily (TID) and simvastatin 80 mg QD, telaprevir 750 mg thrice daily (TID)’, pegylated interferon alfa 180 mcg once week ly and ribavirin 600 mg twice daily. Laboratory evaluation at admission revealed a creatine kinase of 34,010 IU/L, aspartate aminotransferase of 882 IU/ L, alanine aminotransferase of 423 IU/L, gamma glutamyl-transferase of 63 IU/L, alkaline phosphatase of 94 IU/L, lactate dehydrogenase of 1438 IU/L, albumin of 2.9 g/dL, total bilirubin was 2.69 mg/dL, direct bilirubin 0.77 mg/dL, glucose 135 mg/ dL, serum creatinine 0.74 mg/dL and a hemoglobin level of 11.6 g/dL.
The clinical diagnosis was rhabdomyolysis. Simv-astatin, metformin and all antiviral drugs were discontinued because toxicity due to a drug-drug interaction was suspected. The patient was treated with intravenous normal saline. The creatine kinase peaked at 62,246 IU/L on the third day of admission and rapidly declined hereafter. The patient’s renal function remained unaffected. He developed edema, for which he received furosemide. During admission, the patient also developed fever and increased infection parameters, for which he was successfully treated with cefuroxime. The source of the infection was not clear but responded to antibiotics and therefore a bacterial infection is suspected. At discharge (14 days after hospitalization), the patient was in a good disease-related health condition and creatine kinase level had returned to 230 IU/L.
The patient is still very motivated to be treated for HCV and will restart therapy in the near future.
DiscussionTo date, this is the first report showing the pharmacokinetic drug-drug interaction between telaprevir and simvastatin in a chronic HCV infected patient.
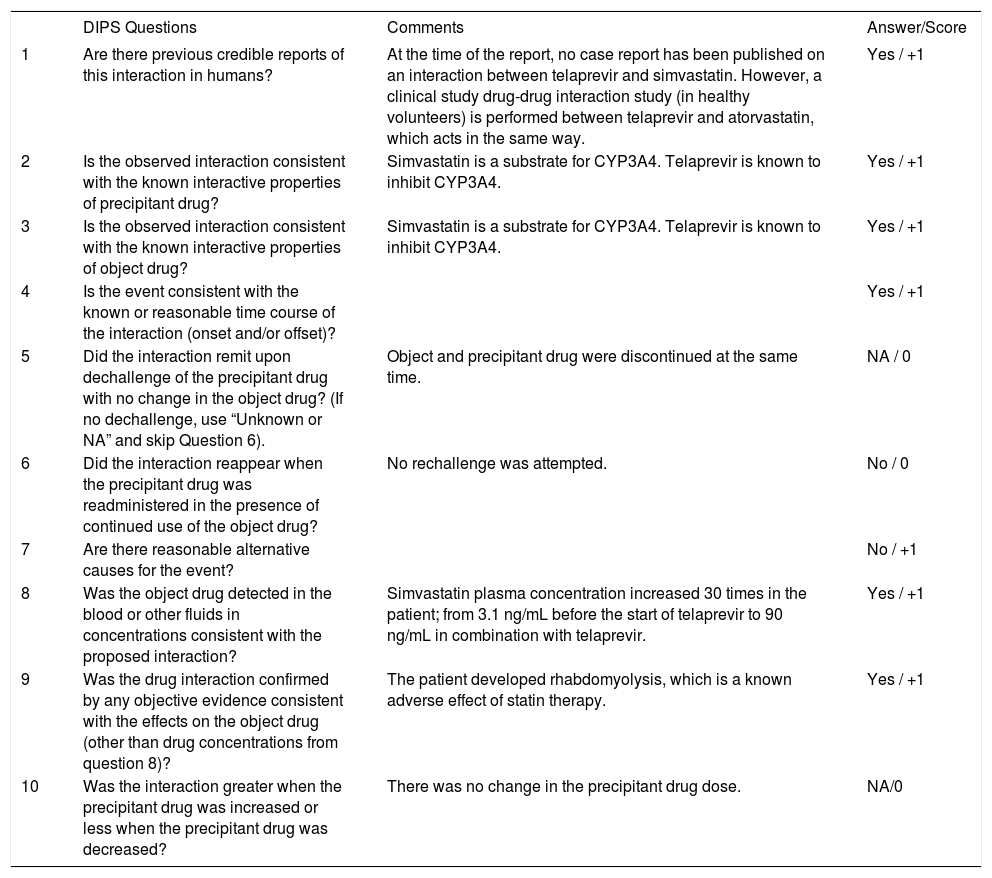
Telaprevir was considered the probable causative agent of an interaction with simvastatin according to the Drug Interaction Probability Scale, see table 1.4
The Drug Interaction Probability Scale (DIPS).
| DIPS Questions | Comments | Answer/Score | |
|---|---|---|---|
| 1 | Are there previous credible reports of this interaction in humans? | At the time of the report, no case report has been published on an interaction between telaprevir and simvastatin. However, a clinical study drug-drug interaction study (in healthy volunteers) is performed between telaprevir and atorvastatin, which acts in the same way. | Yes / +1 |
| 2 | Is the observed interaction consistent with the known interactive properties of precipitant drug? | Simvastatin is a substrate for CYP3A4. Telaprevir is known to inhibit CYP3A4. | Yes / +1 |
| 3 | Is the observed interaction consistent with the known interactive properties of object drug? | Simvastatin is a substrate for CYP3A4. Telaprevir is known to inhibit CYP3A4. | Yes / +1 |
| 4 | Is the event consistent with the known or reasonable time course of the interaction (onset and/or offset)? | Yes / +1 | |
| 5 | Did the interaction remit upon dechallenge of the precipitant drug with no change in the object drug? (If no dechallenge, use “Unknown or NA” and skip Question 6). | Object and precipitant drug were discontinued at the same time. | NA / 0 |
| 6 | Did the interaction reappear when the precipitant drug was readministered in the presence of continued use of the object drug? | No rechallenge was attempted. | No / 0 |
| 7 | Are there reasonable alternative causes for the event? | No / +1 | |
| 8 | Was the object drug detected in the blood or other fluids in concentrations consistent with the proposed interaction? | Simvastatin plasma concentration increased 30 times in the patient; from 3.1 ng/mL before the start of telaprevir to 90 ng/mL in combination with telaprevir. | Yes / +1 |
| 9 | Was the drug interaction confirmed by any objective evidence consistent with the effects on the object drug (other than drug concentrations from question 8)? | The patient developed rhabdomyolysis, which is a known adverse effect of statin therapy. | Yes / +1 |
| 10 | Was the interaction greater when the precipitant drug was increased or less when the precipitant drug was decreased? | There was no change in the precipitant drug dose. | NA/0 |
Total score: 7 (probable)
Telaprevir is a strong inhibitor of Cytochrome P450 (CYP) enzyme 3A4.5 It can therefore reduce the metabolism of drugs using this enzyme and hence lead to markedly increased drug concentrations and concomitant toxicities of co-medications. In this case, the patient used simvastatin which is a pure substrate of CYP3A4. Although a drug-drug interaction study with simvastatin and telaprevir has not been conducted, the combination of atorvastatin, also a CYP3A4 substrate, with telaprevir has been studied, and showed the exposure to atorvastatin to be 7.88 times higher with versus without telaprevir.6 Based on this study, increased plasma concentrations of simvastatin can be expected when co-administered with telaprevir. Indeed, the simvastatin plasma concentration increased 30 times in this patient; from 3.1 ng/mL before the start of telaprevir to 90 ng/mL in combination with telaprevir.
Statins are generally well tolerated, but skeletal muscle-related events are the most common adverse events of statin treatment.7 The spectrum of toxicity ranges from life-threatening rhabdomyolysis, occurring at a rate of 0.44 per 10,000 patient-years, to myalgias, which may trouble 9-20% of statin users in clinical practice.8 It is therefore important to monitor the (laboratory parameters of the) patient for statin induced myotoxicity. Risk factors for developing statin induced muscle toxicity include patient characteristics (demographic characteristics, co-morbidities, genetic factors), drug properties (specific statin molecule, dose, pharmacokinetic properties) and concomitant interacting medication.7
Statin induced muscle toxicity is related to the concentration of the statin in blood.9 The blood concentration in this patient will be largely influenced by the large dose of simvastatin administered (80 mg), and by the drug interaction with telaprevir. These two factors probably both contributed to the development of rhabdomyolysis in this patient. One might speculate about a potential role of increased statin metabolites on a cirrhotic liver, but studies evaluating the pharmacokinetics of various statins in patients with compensated cirrhosis have not shown any significant alteration.10
Our case illustrates that when prescribing telaprevir, it is extremely important to have knowledge of possible drug-drug interactions in order to anticipate them in the best possible way. For instance, some statins are not metabolized by CYP3A4 (i.e. pravastatin, rosuvastatin), however not much is known about possible drug-drug interactions involving drug transporters. Moreover in many patients, treatment with a statin can be temporarily discontinued during the 12 weeks of telaprevir treatment.
Another very important aspect that this case shows, is that it should be clear to the treating physican and pharmacist which medication a patient uses. This should also include self-prescribed medications, such as over-the-counter medication or complementary and alternative medicines. Patients are sometimes unaware that these drugs can also in- teract with their prescribed medications. In this case the full medication list remained unclear. Only with a complete list of medications it is feasible to check for possible drug-drug interactions and perform the adequate actions.2,3,11,12 Finally, a complicating factor in this case was that telaprevir was available through an EAP and (thus) not yet included in the electronic drug-drug interaction databases.
ConclusionsWith this case we illustrate that telaprevir as well as statins are susceptible to clinical relevant drugdrug interactions. To be up to date with all the medication a patient uses and knowledge of interactions with telaprevir is important to provide patients with the best possible care.
Disclosure StatementThe authors have no conflicts of interest to declare.