Hepatocellular carcinoma is a common malignancy affecting approximately one million people around the world every year. The incidence is low in the occidental world and high in locations such as Southeast Asia and sub-Saharan Africa. Hepatocellular carcinoma primarily affects old people, reaching its highest prevalence among those aged 65 to 69 years old. Chronic infection by the hepatitis B virus is the most common cause of this disease. Other important causes are cirrhosis, chronic viral hepatitis (hepatitis C virus, and hepatitis B plus D viruses), alcohol abuse, obesity, hemochromatosis, alfa1-antitripsin deficiency, and toxins similar to aflatoxin. In most cases, hepatocellular carcinoma is asymptomatic and has a low life expectancy. This article presents a review of the most important epidemiological, diagnostic and treatment data about this disease.
Abbreviations:
AFP: alfa-fetoprotein
CC: cryptogenic cirrhosis
CEA: carcinoembrionary antigen
DN: dysplastic nodules
HBV: hepatitis B virus
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
HH: hereditary hemochromatosis
IGF2r: insulin-like growth factor 2 receptor
LCD: large cell-dysplasia
MRI: magnetic resonance imaging
NAFLD: nonalcoholic fatty liver disease
NASH: nonalcoholic steatohepatitis
NK: natural killer cells
PBC: primary biliar cirrhosis
SCD: small cell-dysplasia
TGF-β: transforming growth factor β
IntroductionHepatocellular carcinoma (HCC) is a malignant tumor that arises from hepatocytes, the major cell type in the liver. HCC is the most common primary hepatic tumor and the fifth most common tumor worldwide. It has a high incidence in sub-Saharan Africa and Asia. The HCC 5-year survival rate is less than 5 per cent without treatment. Any chronic inflammatory liver disease has the potential to induce HCC, but the pathophysiological process found in up to 80 per cent of cases of the disease is cirrhosis. Approximately 90 to 95 percent of these tumors are the biologic consequences of persistent hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. Certain diseases other than chronic Hepatitis B or C are associated with increased HCC incidence; iron overload cirrhosis (hemochromatosis), long-standing alcoholic cirrhosis, alpha1-antitrypsin deficiency, and tyrosinemia. The disease is often clinically silent until it is well advanced or tumor diameter exceeds 10 cm. Given the poor prognosis and lack of effective therapies for hepatocellular carcinoma, prevention programs are desperately needed. Surgical resection is the treatment of choice for patients with HCC when the tumor is small and limited to one lobe of the liver. In cases where the tumor is larger or involves more than one lobe of the liver such that it cannot be removed, liver transplantation has also been performed. In either case, the cure rate averages only 20-30 per cent, which has somewhat limited the use of liver transplantation for this problem. Surveillance for HCC in patients with cirrhosis may lead to an improved survival in cohort studies.
The aim of this review is to analyze information regarding epidemiology, risk factors, clinical manifestation, diagnosis and actual treatment of HCC
EpidemiologyHepatocellular carcinoma (HCC) represents approximately 6 per cent of all malignancies. It is the fifth most common malignancy in men and ninth in women, with an estimated 500,000 to 1 million new cases annually around the world.1 Its incidence is low in the occidental world and high in Southeast Asia and sub-Saharan Africa, even though it has risen in the United States, Japan, England, and France.2 HCC is considered a disease of older persons, with a high incidence in people between 65 to 69 years old. However, the prevalence in young people has risen in recent years due to environmental risk factors at birth.3 HCC is the most common primary liver cancer (78 per cent of all primary liver cancer in the United States) with an incidence of 2.4 for every 100,000 persons living in the United States from 1991- 1995. Its incidence rises with age, as well as in highrisk populations including Hispanic groups, Native Americans and Asians. The three most important risk factors for HCC development in the United States are Hepatitis C virus infection (HCV), Hepatitis B virus infection (HBV) and cirrhosis caused by alcoholic liver disease. In people with HCV or HBV chronic liver disease, HCC can develop in approximately 10 to 30 years.3 Some studies have shown that high alcohol consumption (more than 80 g per day) and cirrhosis caused by alcohol consumption are strongly associated with HCC development even in the absence of viral infection.4,5 In one of these studies, high alcohol consumption and viral hepatitis (primarily HCV infection) represented 63 per cent of HCC cases.4 In Brazil, the most common causes of HCC were HCV and HBV infection.6 At this time we have no HCC incidence studies in Mexico. In a study made in a general hospital in Mexico City of 12,556 cases of necropsy, an HCC prevalence of 0.59 percent (n = 73) was found. The age at death among these patients lay between 25 and 90 years, with a median of 65 years.7 In Spain, the most common risk factors for HCC development in Child Pugh A-B cirrhotic patients were age of 54 years or older, low prothrombin activity, low platelet count, and chronic HCV infection.8
Risk factors, predisponent conditions and pathogenesisHCC etiology varies depending on the geographical location. As indicated in Table 1, in countries where HCC is endemic (sub-Saharan Africa, Asia and Alaska), the most common cause is HBV infection, but in low-risk countries the most common HCC cause is cirrhosis caused by chronic viral infection or alcohol consumption.9
CirrhosisIndependent of its cause, cirrhosis is considered a major clinical and histopathological risk factor for HCC development. Five per cent of all cirrhotic patients develop HCC.9 In a Mexican study,10 the main causes of cirrhosis among 1,486 patients were alcohol (587; 39.5 per cent), HCV (544; 36.6 per cent), cryptogenic (154; 10.4 per cent), primary biliar cirrhosis (PBC) (84; 5.7 per cent), HBV (75; 5.0 per cent). Cortes-Espinosa et al. found cirrhosis in 75 per cent of all HCC cases.7 We know that cirrhosis caused by alcohol consumption alone is an important risk factor for HCC development, but in a Japanese study, alcohol consumption was a co-factor to prior exposure to HBV infection in accelerating HCC development.5 According to liver disease prevalence trends in Mexico, nearly 2 million cases of chronic liver disease are expected between 2005 and 2050, with alcohol-related liver disease the most important cause.11
Precursor histological injuriesHepatocarcinogenesis is the development and progression of a HCC chronic liver disease. Hepatocarcinogenesis is a multistep process characterized by the accumulation of poorly understood interacting genetic alterations. HCC coexists with a number of microscopically distinct lesions that are thought to be its precursors.
Regenerative nodules are characteristic lesions of the cirrhotic liver. They exhibit a lack of bile ducts and poorly organized hepatocytes surrounded by fibrosis and proliferating cholangiocytes. These lesions are arbitrarily classified as micro or macronodular (cut point 0.3 cm).
Regenerative nodules may present dysplastic foci, which are smaller than 1 mm and can only be recognized by microscopic studies. There are two types of dysplastic foci in cirrhotic livers, the small cell-dysplasia (SCD) and the large cell-dysplasia (LCD), according to the nucleocytoplasmic ratio of each one (high in SCD and normal in LCD). SCD are thought to be HCC precursor lesions that result from the proliferation of hepatocytes and oval cells. On the other hand, LCD apparently arise from persistent necroinflammation-induced senescent hepatocytes and are therefore not considered to be HCC precursor lesions, although patients with LCD are at an increased risk of HCC.12
Dysplastic nodules (DN) are macroscopically recognizable lesions that show atypical features microscopically, such as increased nucleocytoplasmic ratio, nuclear contour, thickness of hepatocellular plates and compression of adjacent hepatocytes. DN represents parts of a spectrum that is arbitrarily divided for the purposes of clinical utility into low-grade or high-grade DN, according to the presence of cytological or structural atypia or both.13 The risk of HCC in patients with high-grade DN is four-fold higher. By contrast, patients with only low-grade DN are not at a significantly increased risk of HCC.14
Hepatitis B infectionChronic HBV infection is well established as a risk factor for HCC development. In the United States, 25 per cent of patients with HCC are chronic carriers of HBV.2 Sixty to ninety per cent of patients with HBV-related HCC have cirrhosis, but cirrhosis development is not necessary for HCC development.9 HBV chronic infection raises HCC risk because of different factors. Genetic alteration in hepatocytes because of viral DNA; viralinduced chronic inflammation with high cellular proliferation and replication errors with low DNA restoration, producing premalignant cells; and HBVmediated low activity of intrahepatic Natural Killer cells (NK), induce low immunological surveillance. Gender is important in these patients because there is an association between high testosterone levels and HCC in early tumors. Vaccination against HBV has diminished the mobility and mortality of this infection as well as risk for HCC development.15
Hepatitis C virus infectionHCV infection is recognized as a significant risk factor for HCC development, with 6–75 per cent of HCC cases having positive antibodies for HCV.9,16 In the United States, HCV accounts for approximately 50 per cent of HCC cases.15 Some studies identified genotype 1b with a high risk for HCC development.16 A number of studies have demonstrated a direct relationship between HCC incidence and advanced stages of hepatic fibrosis in chronic active hepatitis.15 Because of a HCV-related nonspecific inflammatory process that induces hepatocyte proliferation associated with a rise in alanine-aminotransferase (ALT) levels, patients with high inflammatory and proliferation activity are more prone to the progression to HCC.17 There is evidence of a direct viral effect on carcinogenesis, such as HCV core protein inhibition of apoptosis. High incidence of HCC is seen in people with HCV infection and high alcohol consumption.15
HVC/HBV infectionCo-infection of HBV in people with HCV infection elevates HCC development risk. The mechanisms that cause this high incidence include augmented fibrosis, and inflammation and high cellular re-change.15
AflatoxinsAflatoxin is a toxin produced by Aspergillus flavus and A. parasiticus, which grow in foods like peanuts.9 It causes alterations in the hepatocyte DNA (see genetic alterations). It is related to HCC in countries where infestation of crops and animal feed is common.18 Aflatoxin metabolism produces aflatoxin B1-8,9-epoxide, a toxic product that induces a G to T mutation of the p53 gene at codon 249 up-regulating insulin-like growth factor II that leads to a reduction of apoptosis and HCC formation.19,20
Hereditary hemochromatosisHereditary hemochromatosis (HH) is an autosomic recessive disease in which an alteration in iron absorption, inducing deposition in the liver and other organs occurs.9 HH is a significant risk factor for HCC development. Its presence is associated with a 200 major risk for HCC. A case-control study demonstrated a 1.8 relative risk for HCC development in HH patients compared with non-HH chronic liver disease patients.21 Iron toxicity in the liver is produced by free radical formation, lipid peroxidation of cell organs causing cell death with fibrosis and cirrhosis.9
α-1-antitripsin deficiencyAlpha-1-antitrypsin is the archetypal member of the serine proteinase inhibitor (or serpin) superfamily, playing an important role in the control of proteinases involved in the inflammatory, complement, coagulation and fibrinolytic cascades.22 α-1-antitripsin deficiency is an autosomic recessive disease, with an abnormal accumulation of α-1-antitripsin in the hepatocyte endoplasmic reticulum resulting in hepatic cells dysplasia and cirrhosis.9 Although many [alpha]1-antitrypsin deficiency variants have been described, only two other mutants of [alpha]1-antitrypsin have been associated with plasma deficiency and hepatic inclusions: [alpha]1-antitrypsin Siiyama (53Ser->Phe) and [alpha]1-antitrypsin Mmalton (52Phe deleted).23
Wilson’s diseaseWilson’s disease is a heritable disease with mutations in the gene ATP7B and alteration in plasma copper circulation and its bile excretion. Excessive free copper can provoke cytoplasmic injury, cirrhosis and sometimes HCC.9
Cryptogenic cirrhosisIn 1980, Ludwig et al. gave the name nonalcoholic steatohepatitis (NASH) to an advanced form of fatty liver disease, defining it as a well-recognized clinical-pathologic syndrome primarily occurring in obese female populations with diabetes mellitus, with histological similarities to alcoholic liver disease in the absence of heavy alcohol consumption.24 Nonalcoholic fatty liver disease (NAFLD) affects 10 to 24 per cent of the total population in various countries.25 This prevalence is higher in highrisk groups with a prevalence of 70 to 86 per cent in obese and/or diabetic patients.26 NASH is estimated to occur in 10 per cent of NAFLD patients. NASH has been posited as a possible cause of cryptogenic cirrhosis (CC).27 Mortality trends in Mexico show a significant association between increased prevalence of obesity and increases in mortality caused by chronic liver disease.28
Patients with CC also develop HCC. There is increasing evidence that obesity and NAFLD are risk factors for HCC as the link between CC and nonalcoholic fatty liver disease (NAFLD) in many patients is strengthened.29 Bugianesi et al.30 performed a case-control study in which 23 retrospectively identified patients with CC and HCC were compared to 641 age- and sex-matched patients with alcohol or viral cirrhosis and HCC. The prevalence of obesity and diabetes was higher in the CC patients. In addition, CC patients had higher glucose, cholesterol, and triglyceride plasma levels, and increased insulin resistance. Overweight patients with cryptogenic cirrhosis had a greater risk of developing HCC compared to lean patients with cryptogenic cirrhosis.31
Although NASH may progress to cirrhosis, it is not known if NASH has a role in the development of HCC. These data show that features suggestive of NASH are frequently observed in patients with CC-associated HCC. Some studies have confirmed that HCC may represent a late complication of CC cirrhosis in patients with metabolic syndrome.31,32
Genetic alterationsSome genetic alterations have been associated with HCC development.
- •
p53, localized in chromosome 17p, is mutated in 30 per cent of HCC cases worldwide. This mutation primarily occurs either because of aflatoxins or HCV, HBV chronic infection.33 A protein is produced by p53 that recognizes injured DNA and controls cell replication.34 B1-8,9-epoxid-aflatoxin is a toxic product of aflatoxin metabolism and it is metabolized by the epoxid hydrolase and glutation-S-transferase. If this toxin is not metabolized, it combines with genomic structures to create mutations in p53, producing toxic accumulation.9,34
- •
Insulin-like growth factor 2 receptor (IGF2r) and SMAD4 y SMAD2 genes. The primary function of IGF2r is the activation of the transforming growth factor β (TGF-β) and the SMAD4 and SMAD2 intracellular mediators of the TGF-β, resulting in growth inhibition and apoptosis.33 Mutation and chromosomic deletion of IGF2r occurs in 61 per cent of HCC cases associated with other factors such as viral hepatitis and cirrhosis.34
- •
Table II summarizes the most important genes implicated in HCC.
Table II.Chromosomal localization of potential and candidate suppressor genes for HCC.
Chromosomic region Potential and candidate suppressor genes 1p36 p73 (functionally related to p53) 4q Potential genes include albumin, alcohol dehydrogenase (ADH3), fibrinogen, and UDP-glucoronyl-transferase 6q26-27 Insulin-like growth factor 2 8q21-22 PDGF-receptor beta-like tumor suppressor 13q12-q32 BRCA2 and retinoblastoma gene 17p13.1 p53
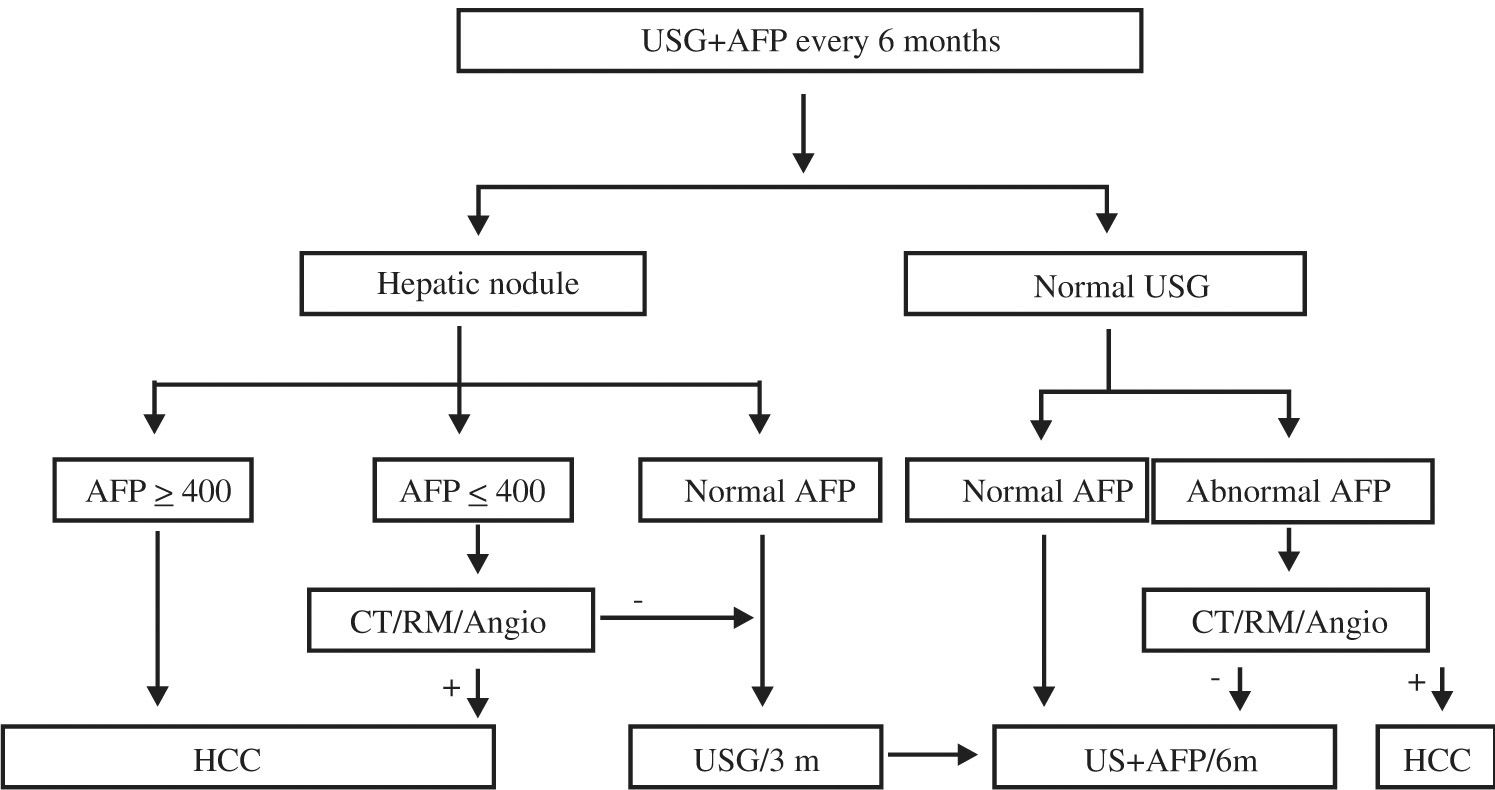
The clinical presentation of HCC differs slightly in low and high incidence areas (Table III). There are three main clinical forms: a) right superior quadrant pain; b) worsening of general conditions in cirrhotic patients; c) asymptomatic (found as a result of screening, see figure 1).35 HCC at the beginning is asymptomatic, and when the disease becomes symptomatic in most cases the disease is advanced and spread. Lungs, adrenal glands and bone are the most common sites of metastasis.36
HCC clinical manifestation in low and high incidence areas.
| Symptoms | Low incidence areas1 | High incidence areas2 |
|---|---|---|
| Abdominal pain | 53%-58% | 62%-95% |
| Weight loss | 19%-73% | 19%-73% |
| Abdominal mass | 33% | < 33% |
| Anorexia | 33% | 47%-60% |
| Hematemesis | 1%-19% | 15% |
| Bone ache | 3%-12% | |
| Signs | ||
| Hepatomegaly | 56%-74% | 86%-98% |
| Ascitis | 55%-61% | 30%-51% |
| Splenomegaly | 15%-48% | 27%-57% |
| Fever | 10% | 38% |
| Jaundice | 44% | 25% |
Hepatic ultrasound is useful in detecting HCC larger than 2 cm, but is a poor tool for detecting lesions smaller than 2 cm. As a result, computed tomography has replaced ultrasound in HCC detection. Contrast tomography in HCC has three phases: before contrast infusion; arterial phase (2 to 40 seconds after infusion) where tumor presence is more evident; portal vein phase (50 to 90 seconds after contrast infusion) where liver parenchyma is more evident. Magnetic resonance imaging (MRI) is useful for HCC image diagnostics because it can differentiate between regenerative nodules and early high fatcontaining HCC through T1-sequencing.37 For an example of MRI imaging in HCC, please see figure 2.
MRI imaging of HCC. Axial images showing three separated nodules in the right lobe, with round borders, satellite lesions, and a hypointense region in the center of the largest nodule, suggesting necrosis. A) T1/SE; B) T1/FAT SAT; C) T2; D) T2/ FAT SAT. Courtesy of Dr. Roberto Corona-Cedillo, Medica Sur, Mexico.
Alfa-fetoprotein (AFP) is the most important proteic component of fetal serum. It is synthesized in the visceral endoderm of the vitelin sac in the first part of fetal development, after which it is synthesized in the liver. AFP levels eventually diminish after birth to virtually undetectable levels, and only elevate under pathological conditions. AFP is a 591 aminoacid-glycoprotein, with a weight of 70Kd.38 It has been known for more than four decades that AFP expression becomes notable in patients with HCC. Other gastrointestinal tumors and benign liver diseases like hepatitis and cirrhosis also elevate AFP levels. Approximately 10 per cent of HCC patients have AFP levels greater than 1,000 ng/mL. The sensitivity and specificity of AFP varies according to its serum levels. AFP levels less than 500 ng/mL in patients with chronic liver disease trigger an obligation to determine whether there is any other type of hepatopathy. When image studies detect a hepatic mass in patients with chronic liver disease and AFP levels > 500 ng/mL, a virtual diagnosis of HCC can be made. In most cases, AFP levels rapidly return to normal when HCC is completely resected. Such low AFP levels do not exclude recurrence because low AFP-producing metastasis can persist.39
Carcinoembrionary antigenCarcinoembrionary antigen (CEA) was first described in 1996. It is a member of the immunoglobulin family and it is important for some biological functions including:
- a)
Cellular adhesion: CEA plays an important role in Ca++ dependent cellular adhesion and in the process of metastasis. These actions are the result of a direct effect (tumoral membrane CEA union with Kupffer cell or hepatic sinusoidal receptors) or because tisular response modulation supporting cellular anklage. CEA and other similar molecules react with their receptors, facilitating cytokine secretion (IL-1, IL-5, α-NTF) that stimulates the expression of adhesion molecules and consequent retention in the liver of tumor cells.
- b)
Tumorigenicity: CEA contributes to tumorigenicity in three ways: 1) Cellular differentiation inhibition; 2) Immunity diminution — CEA diminishes tumoral cells and NK cells’ ratio with less tumoral lysis and a reduced activity of T and B lymphocytes; 3) Interrelations with Lewis blood group antigens, facilitating migration, tissue protection and differentiation of normal tissue, neutrophil migration, tumoral differentiation and neoplasic dissemination.
Other oncogenes relations: CEA relates and cooperates with some oncogenes such as ras, mos, v-myc y bcl-2. Kiras cells have twice the amount of Catepsin B and tumor dissemination protease. CEA blocks cellular differentiation. CEA-positive cells have a higher multidrugresistance gene (mdr1) expression and higher glutationtransferase pi (gst-pi) expression. All of these factors contribute to low cell sensitivity to some drugs.40,41
- c)
Microorganism recognition and protection: Evidence of this phenomenon is first seen in the digestive tract.
Serum PIVKA II (protein induced vitamin K absence) is elevated in one third of HCC cases, including some cases with normal AFP levels.
Diagnostic CriteriaHCC diagnostic criteria adopted by the European Association for the Study of the Liver in Patients with Cirrhosis in 2000 are:
Non-invasive methodsRadiological criteria: focal lesion ≥ 2 cm with arterial hypervascularization demonstrated with two different radiological diagnosis methods: Doppler-ultrasound, helicoidal tomography, magnetic resonance and angiography.
Mixed criteria: AFP > 400 ng/dL + one suggestive HCC image method.
Invasive methodsHistological diagnosis: Fine-needle aspiration biopsy.
Liver biopsyLiver biopsy is an important element in HCC diagnosis, but its utilization is controversial, particularly in patients who can be cured by liver transplant or resection. Liver biopsy can be done with diverse methods: guided and surgical ultrasound or tomography. One of the risks of percutaneous aspiration is tumor extension in the punction zone (1 per cent).37
StagingAfter HCC diagnosis, staging of the carcinoma is important to separate patients into different groups to determine the most adequate treatment modality, and mortality. The TNM system evaluates tumor size, effects on lymphatic nodules and presence of metastasis. Although surgeons often use the TNM system for assessing the success of surgical resection and liver transplantation, it has been criticized for a lack of prognostic value, and has been virtually abandoned.42 The Okuda system includes tumor size parameters and liver disease status. Although easily applicable, the Okuda system is also outdated.2 The CLIP system (Table IV) includes important variables such as biochemical variables, liver ultrasound and physical examination. In 2002, Levy and colleagues compared the CLIP and Okuda classifications in Canadian patients. This group showed the CLIP score more accurately defined HCC patients with good and poor prognoses.43 In a retrospective study comparing the CLIP in a Japanese population, TNM scores confirmed the discriminatory ability and predictive power of the CLIP score.44 The HCC study clinic in Barcelona (BCLC) proposed an HCC classification (Table V) that has 4 principal stages, and divides patients into early (A), intermediate (B), advanced (C), and terminal (D) stages. This system utilizes the clinical significance that HCC has in every patient for normal daily activities. CLIP and BCLC systems have been shown to provide more precise estimates of survival than the Okuda system. The BCLC system also appeared to be more accurate than the CLIP score in identifying cases with better prognosis (small tumors, 3 cm in patients with well compensated liver function and no portal hypertension).45
CLIP staging system for HCC.
| Variables | Points | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Child-Pugh | A | B | C |
| Tumor morphology | Single node Extension area ≤ 50% | Multiple nodules and Extension area ≤ 50% | Massive or Extension area ≥ 50% |
| AFP (ng/mL) | < 400 | ≥ 400 | |
| Portal vein thrombosis | No | Yes | |
Note: For a total, all variables have to be present
BCLC system for HCC staging.
| Stage | PST Tumor | Okuda stage | Hepatic function |
|---|---|---|---|
| Stage A: Early HCC | |||
| A1 | 0 single < 3 cm | I | Without portal hypertension and normal serum bilirubin |
| A2 | 0 single < 3 cm | I | With portal hypertension and normal serum bilirubin |
| A3 | 0 single < 3 cm | I | Portal hypertension and abnormal bilirubin levels |
| A4 | 0 3 tumors < 3 cm | I-II | Child-Pugh A-B |
| Stage B: Intermediate | Large and | ||
| HCC | 0 multinodular | I-II | Child-Pugh A-B |
| Stage C: advanced HCC | 1-2 Vascular invasion or extrahepatic metastasis | I-II | Child-Pugh A-B |
| Stage D: Terminal HCC | 3-4 Any | III | Child-Pugh C |
Stage A y B: Require all criteria
Stage C: At least one criteria PST 1-2 or vascular invasion/extrahepatic metastasis
Stage D: At least one criteria PST 3-4 or Okuda III/Child-Pugh C
Only 10 to 13 per cent of HCC patients can be cured with liver transplant, surgical resection and tumor ablation therapies. Overall, liver transplants have low mortality.2
Non-surgical treatmentArterial liver chemotherapySelective administration of chemotherapy in the hepatic artery is based on the idea that HCC irrigates from this artery so that the drug can be delivered direct to the tumor. The most common drugs used for this procedure are 5-fluorouracil and 5-fluorouracildesoxiribonucleosid. Unfortunately most patients with advanced HCC have associated thrombocytopenia that contraindicates this procedure. This procedure has not been demonstrated to lower HCC mortality.46
ChemoembolizationChemoembolization is the most commonly used treatment for HCC that cannot be submitted to surgery.47 It is based on the objective of tumor devascularization, in which the oxygen and nutrient supply to the tumor is blocked, resulting in tumor necrosis. The most commonly used agents for this treatment are Gelfoam, polivinilic acid, collagen, iodinized oil and angiotensine II.46,47
Ethanol percutaneous injectionIntratumoral injection of ethanol causes dehydratation, intracellular coagulation, necrosis, vascular occlusion and tumor fibrosis. This technique has been used primarily in small tumors, 3-5 cm.46
RadiationRadiotherapy is not commonly used as a single treatment. It can be used on tumors with diameters smaller than 8 cm in patients with Child A and smaller than 5 cm in patients with Child B.47
CryosurgeryCryosurgery has been used in patients with HCC and cirrhosis, with inadequate hepatic reserves and inadequate or multifocal lesions. Survival is approximately 20 per cent in three years.47
ThermotherapyThermotherapy uses changes in temperature for tumor destruction. It is estimated that tumors as large as 9 cm can be cured with this method.46
Systemic chemotherapyThe most common drugs used as palliative therapy are: 5-fluorouracil, doxorrubicin, interferon, cisplatine, tamoxifen, and capecitabine.47 Interferon has been shown to reduce incidence and recurrence of HCC in patients with HCV, even in the absence of virological response.48
Surgical treatmentAfter decades of poorly defined decision-making criteria, surgery is the therapy of choice for HCC in selected cases.49 Surgical options are liver resection and transplantation. For the small group of non-cirrhotic patients and cirrhotic patients with acceptable residual liver function, liver resection is the first choice of treatment. Usually, right hepatectomy induces greater decompensation than left hepatectomy. Indications for resection depend on tumor size, number and extrahepatic involvement according to the Milano criteria.50 Patients with solitary tumors of less than 5 cm, or up to three tumors of less than 3 cm, without extrahepatic involvement, are candidates for resection, with a five-year survival up to 70 per cent in some series.51 Abnormal serum bilirubin and portal hypertension are the main clinical prognostic indicators of survival after liver resection for HCC;52 these factors are associated with a decrease in survival to less than 50 per cent at five years. Portal hypertension is suspected in patients with less than 100,000 platelets/mm3 and splenomegaly in patients with ascites requiring diuretics, and confirmed by measuring hepatic vein pressure gradient or finding esophageal varices in upper endoscopy.53 In patients with high bilirubin levels, low platelets or splenomegaly, transplant is the treatment of choice, although only 5 per cent of patients with HCC and cirrhosis are chosen for this kind of treatment.49 The best histological predictor of recurrence in operated patients is microvascular invasion and additional tumor sites.54
Preoperative chemoembolization has not shown clear benefits.55 Preoperative embolization of the hepatic artery and the portal vein of the affected hepatic lobes have probable benefits by inducing growth of the nonaffected lobes. However, this procedure carries the potential risk of malignant hepatocytes being stimulated by ischemia to induce angiogenesis and tumor growth.56 In some cases, ethanol ablation or thermoablation might be useful as a bridge to surgical resection or transplantation.
Liver transplantation is indicated in patients with advanced liver disease who meet the Milano criteria. MELD scores are currently used to allocate organ distribution, although MELD is considered a poor prognostic tool for HCC. Delays in proceeding could lead to disease progression and a dismal prognosis. Twenty-two additional points are added to the MELD scores of patients with HCC if they meet surgical criteria, and a 10 per cent increase is made for every three months of waiting.57 The chance of five-year survival after liver transplantation is 60-70 per cent. Special postransplant management is a question of debate and frustration. Prevention of graft involvement during viral infection is mandatory, although not satisfactory; especially in Hepatitis C infected patients in whom graft infection is observed in 90-100 per cent of cases. Some reports suggest that postoperative systemic chemotherapy (adriamycin) added to immunosuppressive regimens reduces the risk of recurrence after liver transplantation.58
ConclusionsHCC is a tumor that primarily affects patients of advanced age. Its detection is very difficult because in most cases it has an asymptomatic evolution and when symptoms begin, most cases are already at an advanced stage with a low survival rate. Early detection is important in order to begin treatment as soon as possible and reduce mortality rates.