The development of cirrhosis and portal hypertension in the natural history of chronic liver disease is associated with many complications. A transjugular intrahepatic portosystemic stent shunt (TIPS) is a metal prosthesis that has been shown to be very effective in lowering sinusoidal portal pressure, and therefore is effective in the management of complications of cirrhosis, especially those related to portal hypertensive bleeding and sodium and water retention. In patients with acute variceal bleeding not responding to pharmacologic and endoscopic treatments, a reduction of the hepatic venous pressure gradient to < 12 mmHg or by > 20% with TIPS has been shown to be effective in controlling the acute bleed and in preventing rebleeding. For stable patients whose acute variceal bleed is controlled, TIPS is equal to combined beta-blocker and band ligation in the prevention of recurrent variceal bleed. TIPS is also more effective than large volume paracentesis in the control of refractory ascites, and may confer a survival advantage over repeated large volume paracentesis. TIPS has also been used in the management of other complications related to portal hypertension including ectopic varices, hepatic hydrothorax, and hepatorenal syndrome with some success, but experience is still rather limited. Miscellaneous uses include treatment of Budd Chiari Syndrome, portal hypertensive gastropathy and hepatopulmonary syndrome. Careful patient selection is vital to a successful outcome, as patients with severe liver dysfunction tend to die post-TIPS despite a functioning shunt. All patients who require a TIPS for treatment of complications of cirrhosis should be referred for consideration of liver transplant.
The development of cirrhosis and portal hypertension in the natural history of chronic liver disease is associated with many complications. Over the last few decades, there have been significant advances in the management of portal hypertension. Surgical reduction of portal hypertension, by means of portosystemic shunting, was in vogue more than 20 years ago. The procedure, although effective, was associated with many adverse events, and significant mortality, especially since many of these patients had advanced liver cirrhosis.1 This was followed by the development of simple and effective pharmacological and endoscopic treatments for bleeding varices, one of the major complications of portal hypertension,2,3 thereby significantly improving the prognosis of these patients. Despite this, there is still a proportion of patients whose bleeding varices cannot be controlled by both pharmacological and endoscopic means.4 In addition, there are other complications of cirrhosis and portal hypertension such as ascites and hepatorenal syndrome, which have not fared as well with pharmacotherapy. Therefore, the need for other methods of reducing portal hypertension has led to the development of the transjugular intrahepatic portosystemic shunt.
A transjugular intrahepatic portosystemic shunt effectively functions as a side-to-side portal caval shunt and therefore is effective in lowering portal hypertension. The very first transjugular intrahepatic portosystemic shunt was a venous tissue shunt in the liver parenchyma between the portal and hepatic veins,5 initially devised to decompress the portal system as a treatment for esophageal varices. It was soon realized that the major complication with the tissue shunt was shunt occlusion.5 The introduction of an expandable flexible metal shunt prosthesis, originally designed for strictures of the biliary tract (Figure 1), has proven to be successful in maintaining shunt patency. Since then, the metal prosthesis, or TIPS, has enjoyed much popularity in the management of complications of portal hypertension.
1) TIPS insertionPreparationThe insertion of TIPS is a radiological procedure. Therefore, it is usually performed by an interventional radiologist. TIPS insertion can be done either as an emergency procedure as in the case of refractory bleeding varices, or as an elective procedure as in the case of treatment for refractory ascites. Since the insertion of TIPS significantly alters the splanchnic, pulmonary and systemic hemodynamics,6 it is important that the patient undergoes appropriate investigations of these various circulations prior to the procedure. As the insertion of TIPS returns a significant blood volume from the splanchnic circulation to the systemic circulation, the cardiac function has to be adequate, and pulmonary hypertension should not be present in order to receive the extra blood volume. In the immediately post-TIPS period, as blood is being rapidly shunted through the TIPS, the remainder of the liver can become relatively ischemic. Therefore, TIPS may precipitate liver failure in patients with poor liver reserve as indicated by a high Child-Pugh score. Patients who are receiving TIPS as a treatment for refractory ascites should have organic renal diseases excluded, as these patients required relatively normal renal function in order to eliminate their ascites. Therefore, these patients should have a normal urinalysis, and 24-hour urinary protein excretion of < 500 mg, and normal kidney size on abdominal ultrasound. In addition, both the portal and hepatic veins should be patent on doppler ultrasound for TIPS insertion to be technically feasible. Finally, because the TIPS directly lines a vascular tract, any infection of the TIPS will become a constant source of septicemia, it is imperative that all sources of infection, including dental infections are eradicated prior to TIPS insertion. Table I lists the recommended investigations prior to TIPS insertion. In the case of an emergency TIPS insertion for bleeding varices, the minimal investigation that is required is a doppler ultrasound of the abdomen to ensure that the procedure is at least technically feasible.
Recommended investigations prior to TIPS insertion.
| •Normal electrocardiograph |
| •Normal chest X-ray |
| • 2 dimensional echocardiogram showing ejection fraction of ≥ 55%, normal estimated right sided cardiac pressures and normal diastolic function |
| •Normal radionuclide angiography |
| •Patent portal and hepatic veins on Doppler ultrasound |
| •Child-Pugh score ≤ 12, or INR ≤ 2 and bilirubin ≤ 85 pmol/L |
| •Clearance of all sepsis including dental sepsis |
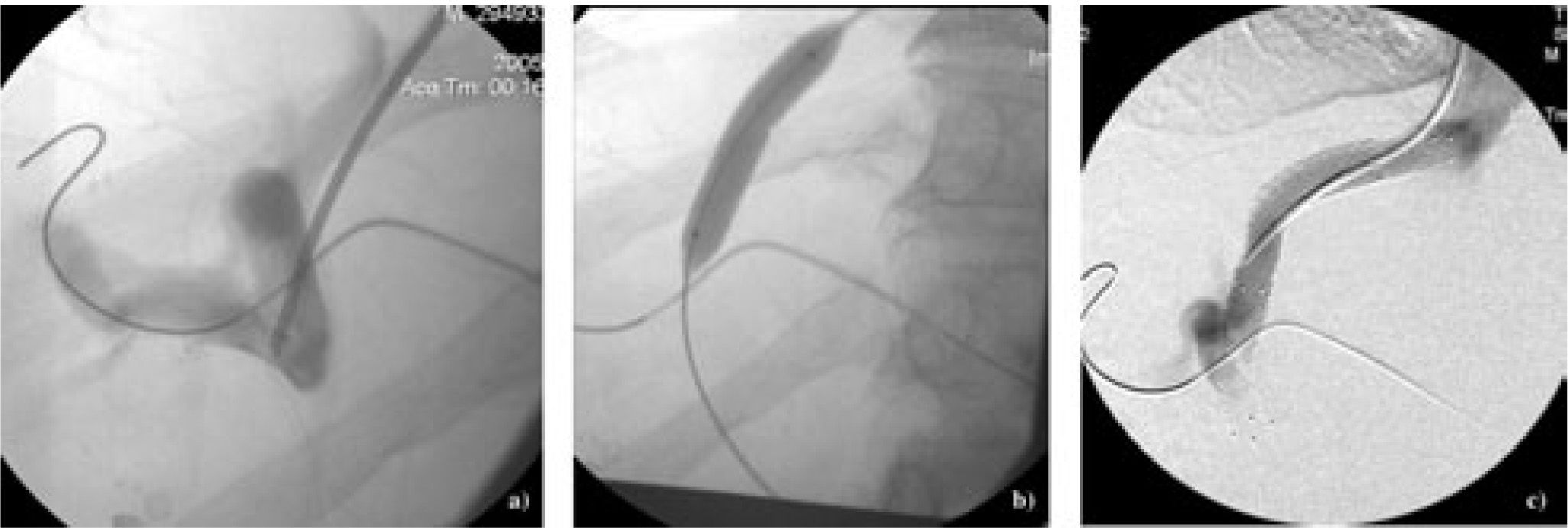
Under conscious sedation, or under general anaesthesia, one of hepatic vein branches, usually the right hepatic vein is cannulated via the transjugular approach. Under fluoroscopic guidance, a needle is then passed through the cannula to puncture the liver, aiming at the intrahepatic portion of a main branch of the portal vein, which has been identified by ultrasound and marked by an radio-opaque marker. Once the needle is in the portal vein, the cannula is advanced over the needle (Figure 2a). A portal venogram confirms that the portal vein has been entered. The cannula is then replaced with an angioplasty balloon. The intrahepatic tract is dilated (Figure 2b). The angioplasty catheter is then replaced with the stent that is then deployed in the intrahepatic tract. A repeat venogram confirms the patency of the shunt (Figure 2c). Embolization of varices may also be performed at the time of TIPS insertion in patients with bleeding varices to reduce the risk of rebleeding in the immediate postoperative period.7 In patients whose intrahepatic tissue tract is too long to be covered by one TIPS stent, a second stent can be inserted in series to cover the entire intrahepatic tract, ensuring that the final appropriate portosystemic gradient is reached. In centers where TIPS insertion is routinely performed, the technical success rate is over 90%. As these cirrhotic patients usually have some degree of coagulopathy, and the use of anti-coagulation has not been shown to reduce the risk of TIPS thrombosis in the post-operative period,8 anticoagulation is generally not recommended with TIPS insertion. Antibiotic prophylaxis is also not universal, as the infection rate with TIPS insertion as reported in the literature is not significant.
TIPS insertion-a) Approaching from the hepatic venous end, a cannula is advanced over a needle after the needle has punctured the intrahepatictributary of a portal vein branch; b) dilatation of the intrahepatic tract using an angioplasty balloon; c) after deployment of the TIPS stent, avenogram confirms shunt patency.
On the day after the TIPS procedure, all patients should undergo an abdominal ultrasound with doppler interrogation of the TIPS shunt. A shunt flow velocity of > 100 cm/ sec at the portal venous, mid shunt, and hepatic venous ends suggests adequate shunt patency. All patients with shunt flow velocity of < 100 cm/sec should undergo a TIPS venogram, as the TIPS shunt may retract after the initial deployment, creating an acute angle between the TIPS shunt and the tissue tract that it lies in. Any angulation at either the portal venous or hepatic venous ends can be straightened with the insertion of a second TIPS stent. Thrombosis within the TIPS shunt occasionally occurs, and tends to be an early event.9 This requires immediate attention in order to re-establish shunt patency. Doppler ultrasound is once again required after thrombolysis in order to confirm shunt patency. If shunt patency cannot be re-established with thrombolysis, then a new TIPS will need to be inserted using a different portal and hepatic veins.
The routine use of lactulose as a prophylaxis against the development of hepatic encephalopathy is controversial. A recent randomized controlled trial did not show any benefit of prophylactic treatment in reducing the incidence of hepatic encephalopathy, especially in the first month post-TIPS.10 However, in patients who are prone to develop hepatic encephalopathy, such as patients who are older than 60 years of age, with a past history of hepatic encephalopathy and a low final portosystemic gradient post-TIPS10,11(Table II), the prophylactic use of lactulose is advisable. As the clinical presentation of hepatic encephalopathy can be frightening to those who have never witnessed it, both the patient and his family should be instructed about its possible occurrence and to adjust the lactulose dose to produce 2 loose bowel movements per day as well as a normal mental state.
Maintenance of adequate TIPS function requires regular assessment of TIPS patency. Doppler ultrasound assessments of TIPS function is recommended at month 1, and thereafter at 3 monthly intervals for at least a year, as most cases of TIPS stenosis occur in the first year post TIPS.12 In patients who have received a TIPS as a treatment of refractory ascites, TIPS surveillance should be continued at 3 to 6 monthly intervals until elimination of ascites. Any suggestion of TIPS stenosis should be assessed with a TIPS venogram. Stenosis can be treated with either TIPS dilatation or insertion of a new TIPS. Doppler ultrasound should be repeated after every TIPS manipulation to ensure a shunt flow velocity of > 100 cm/sec, and also at any time if ascites recurs after it has been eliminated.
2) ComplicationsThe insertion of TIPS is associated with many complications, some are procedure related, while others are related to the presence of shunting.
Puncture and fistulaePuncture of the liver capsule during TIPS insertion is not uncommon. This is usually recognized during the procedure and the puncture sealed with gelfoam. Occasionally, fistulae can occur between the hepatic artery and the portal vein. The flow within the TIPS shunt becomes pulsatile. This can be managed by embolization.13 A fistula between the portal vein and the biliary tract will lead to hemobilia and melena. The presence of blood in the biliary tract can also cause biliary obstruction and predisposes the patient to biliary sepsis. The use of a polytetrafluoroethylene (PTFE)-covered TIPS stent as a graft revision has been shown to be successful in closing the fistula and maintaining shunt patency.14
TIPS thrombosisThrombosis of the TIPS usually occurs in the immediate post-procedure period in approximately 10-15% of patients. Leakage of bile into the TIPS predisposes to the development of thrombosis of the TIPS.15 This can be detected by the use of doppler ultrasound which shows reduced shunt flow velocity. The thrombus can be macerated and shunt patency re-established. Occasionally, this may cause a shower of pulmonary emboli. It has been suggested that the use of low molecular weight heparin can reduce the risk of pulmonary emboli and re-stenosis.16 Successful thrombectomy within a TIPS using a mechanical device such as an Amplatz Thrombectomy Device has also been reported.17 A rapidly spinning encapsulated impeller creates an aspirating vortex that agitates and pulls the thrombus toward its distal tip. The thrombus is then broken down to small particles, of which more than 99% are less than 13 μm, which will then disintegrate within the circulation.
TIPS stenosisTIPS stenosis occurring more than 30 days after insertion is common. It is estimated that up to 70% of patients with a TIPS will experience some degree of TIPS stenosis within the first year.18 This is due to overgrowth of the endothelium within the TIPS, narrowing the lumen. TIPS stenosis can be suspected if doppler ultrasound examination detects reduced shunt flow velocity or if the patient presents with recurrence of complications of portal hypertension. A normal doppler ultrasound examination does not exclude the presence of TIPS stenosis if the patient presents with clinical evidence of portal hypertension. TIPS angiography is required in all cases of suspected TIPS stenosis. TIPS stenosis can be treated by balloon dilatation of the TIPS, and if this is not successful, a new TIPS can be inserted. The recent advent of PTFE covered stents has reduced the incidence of TIPS stenosis significantly, as they have the advantage of avoiding bile leakage into the shunt lumen due to decreased abluminal porosity, and also a smoother internal lining, thereby permitting a more uniform endothelial growth. Several studies have confirmed the superiority of PTFE stents over the bare stent in maintaining shunt patency.19-21 However, its high cost may be the prohibiting factor in its wide application.
Hepatic encephalopathyBecause the TIPS is a large-caliber portosystemic shunt, some degree of hepatic encephalopathy is to be expected. The incidence of new or worsening hepatic encephalopathy is of the order of 20-30%.22,23 In a metaanalysis of randomized controlled trials comparing the efficacy of TIPS versus large volume paracentesis as a treatment for refractory ascites. The patients who received TIPS had an odds ratio of 2.26 for the development of hepatic encephalopathy after TIPS.24 Fortunately, most patients will respond to medical therapy of lactulose.25 There is still much debate as to whether lactulose should be started prophylactically immediately after TIPS, or only given when hepatic encephalopathy occurs. A recent randomized controlled trial did not support the benefit of prophylactic treatment of hepatic encephalopathy with lactulose.10 With the introduction of newer agents such as non-absorbable antibiotics for the management of hepatic encephalopathy, there may not be so much reluctance to start prophylactic treatment,26 as non-absorbable antibiotics tend not to cause unpleasant diarrhea. In a small proportion of patients, the encephalopathy is refractory to medical management, and occlusion of the shunt is then required to control the hepatic encephalopathy.
TIPS induced hemolysisLike any foreign body placed in the circulation, TIPS can induce intravascular hemolysis. This is particularly evident with uncovered stents, occurring in approximately 10% of patients.27 Patients will present with unconjugated hyperbilirubinemia, associated with a fall in the hemoglobin, a rise in the reticulocyte count. The hemolysis can be quite severe in the occasional patients. However, in most cases, it is self-limiting and decreases as the TIPS stent becomes endothelialized in 12 to 15 weeks. Patients with severe hemolysis may require transfusion for support. With the advent of PTFE covered stents, the incidence of intravascular hemolysis should decrease.
TIPS infectionPatients with decompensated cirrhosis are at an increased risk for infections, especially bacterial infections. Despite this, infection of the TIPS is extremely uncommon, even in the absence of widespread use of prophylactic antibiotics for TIPS insertion. Infection of TIPS, or endotipsitis, has been estimated to occur in 1.3% of all procedures.28 The most common presentation includes fever and primary bacteraemia. Most cases are caused by grampositive bacteria, followed by gram-negative bacteria and occasionally fungi. Diagnosis may be difficult to establish. Endotipsitis has to be considered in patients with a TIPS and bloodstream infection that is not clearly attributable to another source. Doppler ultrasound of the TIPS may show vegetations on the TIPS.29 Prolonged courses of antibiotics are required as endotipsitis is similar to any infection in a vascular endoprosthesis. In most cases, the infection can be resolved, although deaths from endotipsitis have been reported.28,29 Liver transplantation will be difficult unless the infection is resolved.
Other complicationsIn a recent meta-analysis of TIPS versus large volume paracentesis for the treatment of refractory ascites, other uncommon complications were also described.24 These include the development of cardiac failure (2.5%), liver failure (1.9%) and hepatorenal syndrome (4.3%). The development of cardiac failure after TIPS may be related to the presence of undiagnosed cirrhotic cardiomyopathy, which is a newly recognized condition present in most patients with decompensated cirrhosis.30 The presence of diastolic and systolic dysfunction, features of cirrhotic cardiomyopathy, may contribute to the inability of the cirrhotic heart to handle the large volume of splanchnic blood volume being returned to the systemic circulation with TIPS insertion, leading to cardiac failure, The development of renal failure post TIPS may be related to the worsening of systemic arterial vasodilatation, not adequately compensated by refilling of the systemic circulation, thereby leading to continued activation of the various vasoconstrictor systems with consequent renal vasoconstriction.31 The development of liver failure tends to occur in patients with advanced liver dysfunction pre-TIPS (Child Pugh Class C),32 and this may be related to the relative ischemia of the liver in the immediate post-TIPS period when most of the portal blood volume is directly shunted through the TIPS to the systemic circulation without passing through the rest of the liver. Finally, there are recent reports of increased incidence of hepatocellular carcinomas during long-term follow-up of TIPS patients.33,34 Although still controversial, it appears that the use of uncovered stents in cirrhotic patients with refractory ascites does increase the risk of post-TIPS hepatomas.
3) Patient selectionGiven the fact that TIPS insertion is associated with so many potential complications, it is imperative that careful patient selection is applied with every case that is being considered for TIPS. Table III provides some guidelines for patient selection for TIPS insertion. Elderly patients with a past history of hepatic encephalopathy should not receive a TIPS. Likewise, patients with known cardiac dysfunction, pulmonary hypertension, or renal impairment should be carefully assessed before being accepted for TIPS. This is despite the fact that TIPS has been used as a treatment for Type 1 hepatorenal syndrome with improvement of renal function post-TIPS, there are also incidences of worsening of renal function post-TIPS. Patients with systemic diseases such as diabetes complicated by renal impairment may have organic renal disease. This will reduce the efficacy of TIPS as a treatment for refractory ascites, and therefore these should also not receive a TIPS unless their organic renal disease is mild. All patients receiving a TIPS should be free of infections, including dental infections. The presence of malignancy should also preclude TIPS insertion.12 For TIPS to be technically feasible, the patient should have a patent portal vein, and there should not be any structural abnormalities such as multiple hepatic cysts.
4) indications for TIPS insertiona) Related to portal hypertensive bleedingAcute variceal bleedThe management of acute variceal bleeding involves a combination of pharmacologic and endoscopic treatments which is usually successful in over 90% of cases. In the few occasions when the combination of pharmacotherapy and band ligation (3%) fail to control the acute variceal bleed, TIPS can be used as a rescue treatment for these patients. Because the definition of treatment failure varies widely between institutions, patients have been referred to TIPS insertion at various time points after presentation. In general, a clinically significant bleed recurring within 48 hours after presentation should prompt the treating physician to consider using a TIPS as a rescue treatment. Likewise, the need for balloon tamponade should also be considered as a treatment failure of pharmacologic and endoscopic therapies.35 Given the fact a hepatic venous pressure gradient (HVPG) of < 12 mmHg has been shown to significant reduce the risk of variceal bleeding,36 the goal is to reduce the HVPG to < 12 mmHg with TIPS insertion. Further lowering of the HVPG to much more than 12 mmHg will significantly increase the risk of hepatic encephalopathy.22 There is recent data to suggest that a reduction of > 20% of the HVPG would be adequate,37,38 and this will certainly reduce the incidence of post-TIPS hepatic encephalopathy.
It is prudent to initiate TIPS insertion early before the patient becomes too ill with superimposed infection or multi-organ failure, otherwise the mortality remains high despite a functioning TIPS and control of variceal bleeding. This is particularly true in patients whose HVPG is > 20 mmHg.39 To date, there are 15 studies reporting the results of over 500 patients who had received a TIPS as a rescue treatment for refractory variceal bleeding.40 TIPS is very effective in controlling the acute bleeding with a success rate of 90-100%, and a rebleeding rate of 7-27%.40 In most series, the mortality remained high (15-75%), mostly likely related to the severity of the underlying liver disease.16,41 This has led some to advocate reserving TIPS for the not so ill patients, that is, those patients who are not ventilated, not septic and not receiving blood pressure support.42 Patch et al have found that moderate/severe ascites, requirement for ventilation, white blood cell count, platelet count, partial thromboplastin time with kaolin (PTTK) and creatinine, when fitted into a prognostic index equation (prognostic index score =1.54 X (ascites) + 1.27 X (need for ventilation) + 1.38 Ln (WBC) + 2.48 Ln (PTTK) + 1.55 Ln (Creatinine) - 1.05 Ln (platelet), where moderate/severe ascites, or need for ventilation scores 1), predicted a 100% mortality when the prognostic index was ≥ 18.52. They therefore advocated assessing each patient prospectively before making a final decision on using TIPS as a rescue treatment for refractory variceal bleed.43
Prevention of recurrent variceal bleedThe control of an acute variceal bleed by endoscopic means is associated with a very high rebleeding rate of up to 80% at 2 years.44 Therefore, various treatment strategies have been devised to prevent the recurrence of variceal bleeding after the initial episode. The combination of non-selective beta-blocker and endoscopic band ligation has been shown to be superior than either therapy alone in the prevention of variceal rebleeding45 and has been accepted as the standard of care for patients who have experienced an episode of variceal bleed. The advent of TIPS has brought much enthusiasm in the use of TIPS as a treatment for the prevention of recurrent variceal bleed. This is because it does not carry the surgical risks of a surgical portosystemic shunt, which has also been proven to be very effective in preventing recurrent variceal bleed.46 Meta-analyses of studies comprising of more than 1,000 patients have proven TIPS to be equivalent to a surgical shunt, but superior than endoscopic therapy in the prevention of recurrent variceal bleed,47,48 but at a cost of significant more hepatic encephalopathy and without any improvement in terms of survival. However, the recurrent bleeding of 18.9% with TIPS is no better than that of 14% with combined beta-blocker and endoscopic band ligation.45 Therefore, TIPS cannot be recommended as the treatment of choice for the prevention of variceal rebleeding. TIPS may have a role in those patients who have failed the combination of beta-blocker and endoscopic band ligation, but such patients will require frequent TIPS surveillance to ensure shunt patency in order to maximize TIPS effectiveness.
Ectopic varicesEctopic varices account for 1 to 5% of all episodes of variceal bleeding.49 They can be difficult to diagnose because of their ectopic locations. They are also difficult to treat because often they are situated at a site along the gastrointestinal tract that is not accessible to the endoscope. When they are not amendable to endoscopic treatment, the use of embolization therapy using radiological techniques can often be successful.50 Both endoscopic treatment (band ligation or sclerotherapy) and embolization therapy are associated with a high rebleeding rate.51,52 Decompression of the portal circulation using either a surgical shunt or TIPS can be considered in patients who continue to bleed despite either endoscopic or embolization therapy.49 In the 2 largest series of cirrhotic patients with ectopic varices treated with TIPS, the technical success rate was at least 90% with control of bleeding.53,54 However, rebleeding also occurred frequently, sometimes despite a patent and functioning shunt with a HVPG of < 12 mmHg.53,54 Embolization at the time of TIPS insertion can reduce the incidence of rebleeding, and therefore should be considered as an adjunct therapy to TIPS insertion for these patients.
Portal hypertensive gastropathyPortal hypertensive gastropathy (PHG) is an entity related to congestive changes in the gastric mucosa due to increased portal pressure. It is characterized by a mosaic or snake-skin like gastric mucosa with hemorrhagic spots. Portal hypertensive gastropathy causes both acute and chronic blood loss from the gastrointestinal tract. Although it is fairly prevalent in patients with portal hypertension, estimated to be present in 50 to 80% of patients with cirrhosis, only 15% to 20% of subjects experience symptomatic gastrointestinal blood loss. The diagnosis is frequently, but not invariably, made by upper endoscopy. Treatment is usually conservative with beta-blockers. In patients who either cannot tolerate or are unresponsive to beta-blockers, a TIPS can be considered. In a small series of 16 patients, TIPS improved PHG in all patients with severe PHG, but was only partially effective in those with mild PHG. This was achieved by reducing the total blood flow, in both the mucosa and the sub-mucosa.55 Others have also reported similar improvement in endoscopic appearance as well as a reduction in transfusion requirement.56,57 Because of its many complications, TIPS should be reserved as a rescue treatment for those patients who are transfusion dependent because of their PHG despite beta-blockers.
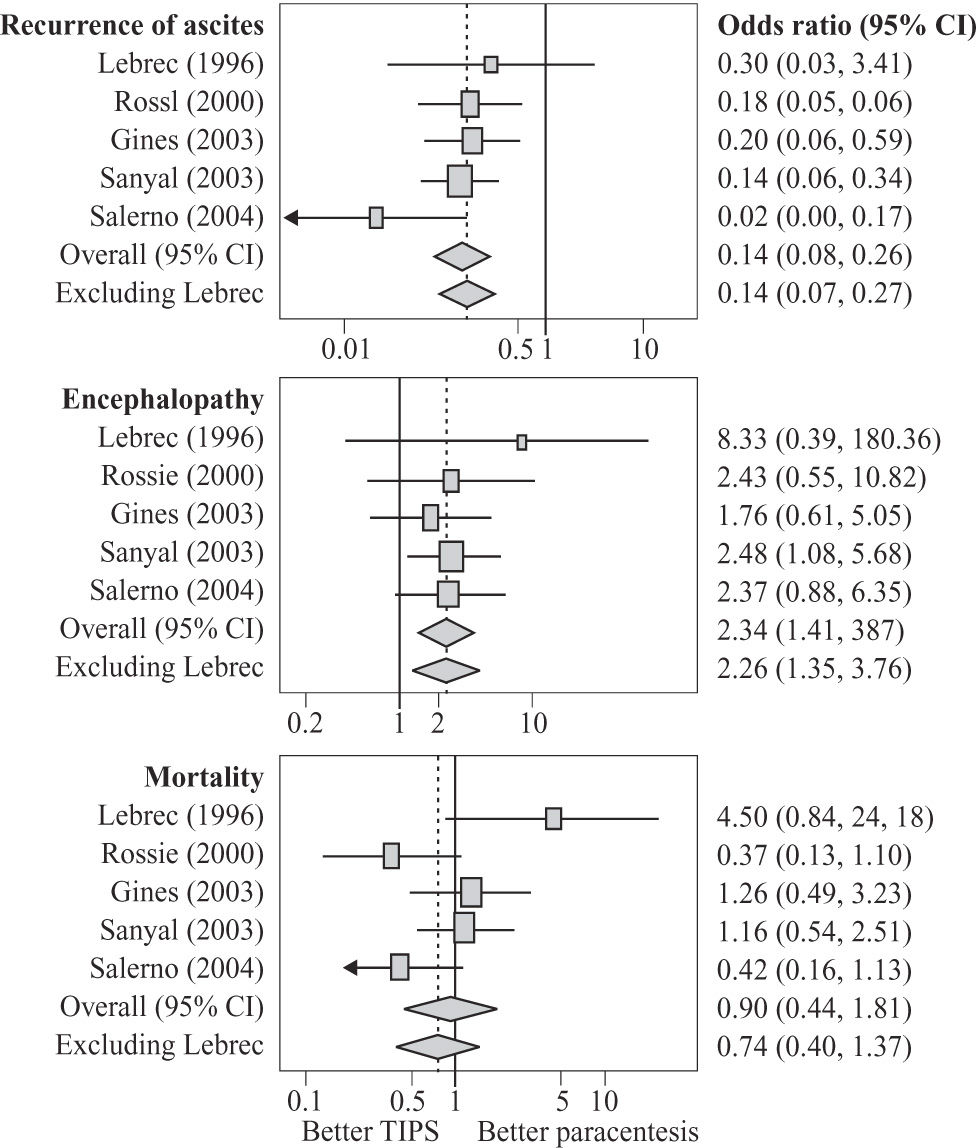
b) Related to sodium and water retention of cirrhosisRefractory ascitesRefractory ascites or resistant ascites is defined as a prolonged history of ascites unresponsive to 400 mg of spironolactone or 30 mg of amiloride plus up to 160 mg of furosemide daily for 2 weeks while on a sodium restricted diet of 50 mmol or less per day. Patients who cannot tolerate diuretics because of side effects are also regarded as diuretic resistant.58 Some 5% to 10% of ascitic patients admitted to hospital for treatment of ascites have refractory ascites. These patients can be treated with repeat large volume paracentesis, which have been shown to be safe and effective.59 However, repeat large volume paracenteses are time consuming for both the physician and the patient, as ascites invariably recurs after each paracentesis. Furthermore, the presence of ascites predisposes the patient to the development of spontaneous bacterial peritonitis, various hernias and hepatorenal syndrome. Therefore, more permanent treatments for the elimination of ascites have been sought. Since one of the pathogenetic factors for the development of sodium and water retention in cirrhosis is sinusoidal portal hypertension, it stands to reason that reduction of portal hypertension should result in increased sodium retention and eventual elimination of ascites. To date, there are 5 published randomized controlled trials comparing the effects of TIPS versus large volume paracentesis as a treatment for refractory ascites.25, 60-63 Of the 330 patients enrolled, 162 patients received TIPS, while 168 patients underwent repeat large volume paracentesis as a treatment for their refractory ascites. TIPS was effective in about two-thirds of patients in eliminating ascites, and this was significantly better than repeated large volume paracenteses which controlled ascites in about 23% of patients. A recent meta-analysis has confirmed that TIPS is better than large volume paracentesis in the control of ascites. However, the insertion of TIPS worsens hepatic encephalopathy, but is associated with a trend towards improved survival (Figure 3).24 With long-term follow up, successful TIPS placement with elimination of ascites results in improved renal function,64 better nutritional status and positive nitrogen balance.65 However, any improvement in quality of life is offset by the increased incidence of hepatic encephalopathy.66
Meta-analysis of all randomized controlled trials of TIPS versus large volume paracentesis as a treatment for refractory ascites, better control of ascites, worsening of encephalopathy and a trend towards better survival (reprinted from D’Amic et al, Gastroenterology 2005; 129: 1282-1293 with permission).
Hepatic hydrothorax is ascites that has tracked up into the pleural cavity through a defect in the diaphragm. It occurs in 5-12% of all cirrhotic patients with ascites.67 In some patients, ascites disappears as the pleural effusion develops. Thoracentesis is the standard treatment as diuretics are seldom effective since the pleural fluid is held under negative pressure. Patients are usually very symptomatic because of the limited space in the pleural cavity. Therefore, thoracenteses are required more often than paracentesis for any given amount of fluid. Since the pathogenesis of hepatic hydrothorax is the same as that of cirrhotic ascites, TIPS should be effective in eliminating hepatic hydrothorax. To date, there is only limited experience in the published literature on the use of TIPS as a treatment for hepatic hydrothorax. These are limited to case reports or case series, totally less than 100 patients.67-70 The overall response is favorable with elimination of the hydrothorax in approximately two-thirds of patients. In the remainder of the patients, the requirement for thoracenteses is reduced. The response of hepatic hydrothorax alone to TIPS is slower than that of ascites alone. This is because the pleural fluid is held under negative pressure in the pleural cavity. In patients with hydrothorax and concomitant ascites, the ascites usually disappears before the hydrothorax. The same patient selection criteria, follow- up and complications should apply as patients with refractory ascites alone.
Hepatorenal syndromeHepatorenal syndrome is defined as the development of renal failure in patients with advanced liver failure (acute or chronic) in the absence of any identifiable causes of renal pathology.71 The Internal Ascites Club further divides hepatorenal syndrome into Type 1 and Type 2.58 Type 1 hepatorenal syndrome is characterized by a rapid decline in renal function defined as a doubling of serum creatinine to a level greater than 220 μmol/L or a halving of the creatinine clearance to less than 20 mL/min within two weeks. The clinical presentation is that of acute renal failure. In Type 2 hepatorenal syndrome, renal function deteriorates more slowly with the serum creatinine increasing to greater than 133 μmol/L or a creatinine clearance decreasing to less than 40 mL/min over the course of weeks to months. The clinical presentation is that of gradual renal failure in a patient with cirrhosis and refractory ascites.
Since sinusoidal portal hypertension plays a pivotal role in the control of renal hemodynamics,72 it is therefore not surprising that the insertion of TIPS, especially in cirrhotic patients with refractory ascites and some degree of renal dysfunction, is associated with improvement in both glomerular filtration rate and renal blood flow.64 In addition, TIPS returns a significant portion of the splanchnic volume into the systemic circulation, leading to suppression of various vasoactive neurohormones, resulting in better renal perfusion.
To date, a total of 7 studies have reported the use of TIPS for hepatorenal syndrome.73-79 Most studies have focused on subjects with type I hepatorenal syndrome while one study studied type II hepatorenal syndrome alone76 and two studies assessed a mixed population of types I and II hepatorenal syndrome.73,74 The majority of these studies were retrospective and either did not have a control group or did not have predefined methods for assigning subjects to TIPS versus control therapy. Therefore, one has to be cautious in interpreting the results. Despite this, all studies showed that TIPS improved renal function, although not to normal levels. The improvement was observed to extend up to six months (the longest duration of follow up) in one study.73 The mechanisms for the failure of renal function to normalize after TIPS are unclear. However, when TIPS was placed after a period of vasoconstrictor therapy,79 renal function returned to near normal levels. The improvement in renal function is usually associated with reduction in ascites in both type I and type II hepatorenal syndrome patients. Long-term improvement in renal function is associated with improved survival over conventional medical therapy. In the largest series,73 20% of the patients with type 1 hepatorenal syndrome were alive 1 year after TIPS creation, whereas with type 2 hepatorenal syndrome, approximately 45% were alive after 1 year. The pre-TIPS bilirubin has been shown to be the best predictor for survival after TIPS in patients with hepatorenal syndrome.73
c) Miscellaneous indicationsBudd-Chiari syndromeBudd-Chiari syndrome is a heterogeneous group of disorders characterized by obstruction of the hepatic outflow tract at the level of the hepatic venules, the large hepatic veins, the inferior vena cava, or the right atrium.80 Liver injury results from hepatic congestion. Depending on the rapidity of the hepatic outflow tract obstruction, the patient could have a fulminant, acute, subacute or chronic course, which will in turn determine the patient’s prognosis. A model based on the presence of ascites, hepatic encephalopathy, prothrombin time and bilirubin has been developed to asses the prognosis81 and patients are then classified accordingly into good, intermediate and poor prognostic categories. TIPS has been used as a treatment for Budd-Chiari syndrome in acute and subacute cases, as well as chronic cases that are not controlled by medical therapy,82 and is slowly replacing surgical shunting as the preferred treatment of hepatic decompression. In the last 2 years, there were 6 case series totaling 194 patients in the English literature reporting on the successful use of TIPS as a treatment for Budd-Chiari syndrome.83-88 The average follow-up was between 20 to 36 months. The majority of patients did well, with relief of symptoms and improvement in liver function, and an average 1 year survival of over 90% and an average 5 year survival of approximately 75%. Because most cases of Budd-Chiari syndrome are related to the presence of a hypercoagulable state, most patients will need to remain on anti-coagulants. The major problem with TIPS was TIPS dysfunction.89 The recent introduction of PTFA stents (covered stents) has significantly reduced the TIPS stenosis rate,85 although some patients with shunt stenosis may not have recurrence of symptoms because of the development of collaterals. Consultation with a surgeon who performs liver transplantation prior to TIPS insertion is an advantage, as the TIPS protruding into the suprahepatic inferior vena case may pose a technical problem should the patient require a liver transplant later.
Hepatopulmonary syndromeHepatopulmonary syndrome is an uncommon complication of liver cirrhosis. It is characterized by intra-pulmonary vasodilatation and arterial hypoxemia.90 Portal hypertension seems to play a crucial role in the pathogenesis of the syndrome, probably by enhancing nitric oxide production. Therefore, TIPS, by reducing portal hypertension, could potentially reverse the syndrome. To date, there are only a handful of case reports on the use of TIPS for hepatopulmonary syndrome. Five of the 6 patients treated showed an improvement in oxygenation, some but not all showed a reduction in intrapulmonary shunting.91-94 Therefore, the use of TIPS in hepatopulmonary syndrome can only be regarded as experimental.
5) MortalityThe mortality rate following TIPS insertion is somewhat dependent on the indication for the TIPS insertion. The median survival for patients who receive a TIPS for variceal bleeding is better than that for patients who receive a TIPS for refractory ascites.43,95 The average 30-day survival for patients who receive a TIPS for variceal bleed is approximately 85-90%, while the 1 year survival for the same indication is approximately 70%.95,96 Various factors have been used to predict survival after TIPS, and the model of end-stage liver disease (MELD) score was developed for predicting survival after TIPS96 for all indications. This score is derived from 3 variables relating to liver and renal function using the following formula: 3.8 X loge (bilirubin [g/dL]) + 11.2 X loge (international normalized ratio) + 9.6 X loge (creatinine [mg/ dL]). The survival rate in patients with MELD scores of 18 or more is higher in comparison to those with MELD scores of 17 or less.96 That is, the more severe the liver dysfunction, the more likely the patients will not survive despite a functioning TIPS. Many other studies have also confirmed that patients with poor pre-TIPS liver function such as high Child-Pugh scores32 or high bilirubin and pre-TIPS encephalopathy unrelated to bleeding do not survive after TIPS.97 Renal impairment pre-TIPS also has been shown independent of the MELD score to have a negative prognostic value.98 In addition, patients with variceal hemorrhage requiring emergent TIPS placement have a 37.5 times relative risk for 30-day mortality.97 These latter variables, together with alanine aminotransferases level (> 100 IU/L) have been put into another model to predict low, median and high risk for mortality for TIPS insertion (Table IV).97 These survival estimates can be used to advise patients when discussing risks for the TIPS procedure or alternatively used to decide which patient will be better served by having a liver transplant without receiving a TIPS.
6) ConclusionsSince TIPS was introduced more than 15 years ago as a treatment for complications of portal hypertension, much has evolved to refine the indications, the techniques for its insertion, and the predictors of outcome. TIPS is now a recognized treatment for refractory variceal bleeding, and is emerging as the preferred treatment for refractory ascites, especially since the latest meta-analysis suggests that TIPS can confer a survival advantage in these patients. The use of TIPS for other complications of portal hypertension are not yet well defined, although entities such as ectopic varices, portal hypertensive gastropathy and hepatic hydrothorax are becoming accepted indications for TIPS insertion. Other conditions such as hepatorenal syndrome and Budd Chiari syndrome are still defining their place in the use of TIPS. TIPS insertion is not for the occasional physician, rather, the successful use of TIPS involves a multi-disciplinary approach including the hepatology, interventional radiology, doppler ultrasound teams as well as the transplant surgeons. Despite its many successes in the management of complications of portal hypertension, TIPS also has its limitations and is associated with many complications. Better patient selection and the use of PTFE covered stents will minimize these TIPS complications. Patients who have had their complications of portal hypertension successfully treated by TIPS will still need to be followed regularly, as the TIPS has not removed the cirrhotic liver and many of these patients are still at risk for the development of complications of cirrhosis such as hepatocellular carcinoma. As the indications for TIPS are now being sorted out, further studies on TIPS must assess its cost effectiveness and its impact on quality of life.