Liver cirrhosis is a major public health issue associated with high morbidity and mortality. The ANSWER trial showed that long-term human albumin (LTA) infusions led to significant reduction of complications and mortality in patients with uncomplicated ascites. The present study aimed to assess the incremental cost of cirrhosis patients treated with LTA plus standard medical treatment (SMT) versus those treated with SMT from the perspective of the Mexican Social Security Institute (IMSS).
Material and methodsCost of illness for patients with cirrhosis and grade 2-3 ascites treated with SMT or with SMT and LTA (following the treatment regimen from ANSWER) over a one-year period was estimated according to the IMSS perspective. Rates of treatments, complications and hospitalizations were based on results from the ANSWER trial. Unit costs from IMSS were gathered from public sources and transformed to 2020 Mexican $ (Mex$).
ResultsThe use of LTA is estimated to require additional annual expenditure derived from the pharmacological cost of human albumin and by the follow up visits required for LTA administration (Mex$28,128). However, this cost may potentially be counterbalanced by the reduction in paracentesis, cirrhosis-related complications and hospitalizations which would lead to cost savings of Mex$33,417 per patient/year.
ConclusionsBased on the ANSWER trial results, our study suggests that LTA may result in improved clinical outcomes and reduced costs for the IMSS when administered to cirrhosis patients with uncomplicated ascites.
Liver cirrhosis is a major public health issue associated with high morbidity and mortality, that can lead to a significant burden for patients and healthcare systems [1–3]. In 2017, cirrhosis was estimated to cause 40,509 deaths in Mexico, with hepatitis C virus (HCV) infection and alcoholic liver disease being the most common causes [1,4,5].
As cirrhosis progresses, one of the first signs of liver decompensation is the development of ascites, which is an indicator of subsequent serious complications and carries a poor prognosis [6]. The therapeutic strategy for decompensated cirrhosis is generally driven by the management of each individual complication [6–8]. For instance, first-line treatment for grade 2 ascites consists of dietary sodium restrictions and diuretics, whereas grade 3 ascites requires large-volume paracentesis (LVP) in combination with human albumin. The early recurrence after initial paracentesis, despite sodium dietary restrictions and diuretics, is indicative of refractory ascites, which is generally associated with a much worse prognosis [6,9].
Albumin is the most abundant protein in the blood plasma and plays a crucial role in oncotic pressure maintenance, exogen and endogen substance transport and disposal, inflammation, oxidative stress, and endothelial stabilization. The intravenous administration of human albumin in cirrhosis patients seems to entail benefits that go far beyond its volume expansion capacity.10,11 Its acute use in LVP, spontaneous bacterial peritonitis (SBP), and acute kidney injury hepatorenal syndrome (AKI-HRS) is well established and proven to reduce the incidence of complications and mortality [7,8,10-14]. However, its long-term chronic use in this population has been debated for years [7,8,15].
The ANSWER trial was a multi-center Italian study that included 431 patients with cirrhosis and grade 2-3 ascites who were randomized to receive standard medical treatment (SMT) and/or 40 g of albumin twice weekly for 2 weeks, and 40 g weekly thereafter in addition to the SMT for 18 months [16]. Patients treated with long-term albumin (LTA) presented with significantly higher 18-month survival and significantly lower rates of complications, such as SBP, AKI-HRS, hepatic encephalopathy and renal dysfunction, among others [16].
In the context of the ANSWER trial a one-year cost-effectiveness analysis was performed from the perspective of the Italian Health System. This economic evaluation assessed the incremental cost of patients treated with LTA and SMT vs. those treated with SMT only and compared it to the quality-adjusted life years (QALY) gained. The incremental cost per QALY was estimated at 21,265 € which is well below the cost-effectiveness threshold of 35,000 € per QALY considered for the Italian Health System [16]. More recent work, adapted from the ANSWER results to the perspective of the Spanish Health System showed potential cost savings when comparing the incremental cost of chronic LTA infusions added to SMT vs SMT only [17].
Literature evaluating the economic impact of LTA in cirrhosis patients is scarce and its cost-effectiveness is still under debate; and needs to be assessed on a country-per-country basis [7,15]. The present study aims to utilize the clinical results from the ANSWER trial to assess the potential incremental cost of illness of cirrhosis patients treated with LTA and SMT versus those treated with SMT only from the perspective of the Mexican Social Security Institute (IMSS, as per its abbreviation in Spanish).
2Material and methodsCost of illness for patients with cirrhosis and grade 2-3 ascites treated with SMT or with SMT and LTA (following the treatment regimen from ANSWER) over a one-year period was estimated according to the IMSS perspective. The overall cost per patient considered the rates of complications, healthcare resource utilization (HCRU) and treatments for patients under SMT only or SMT and LTA reported in the ANSWER trial (Table 1).
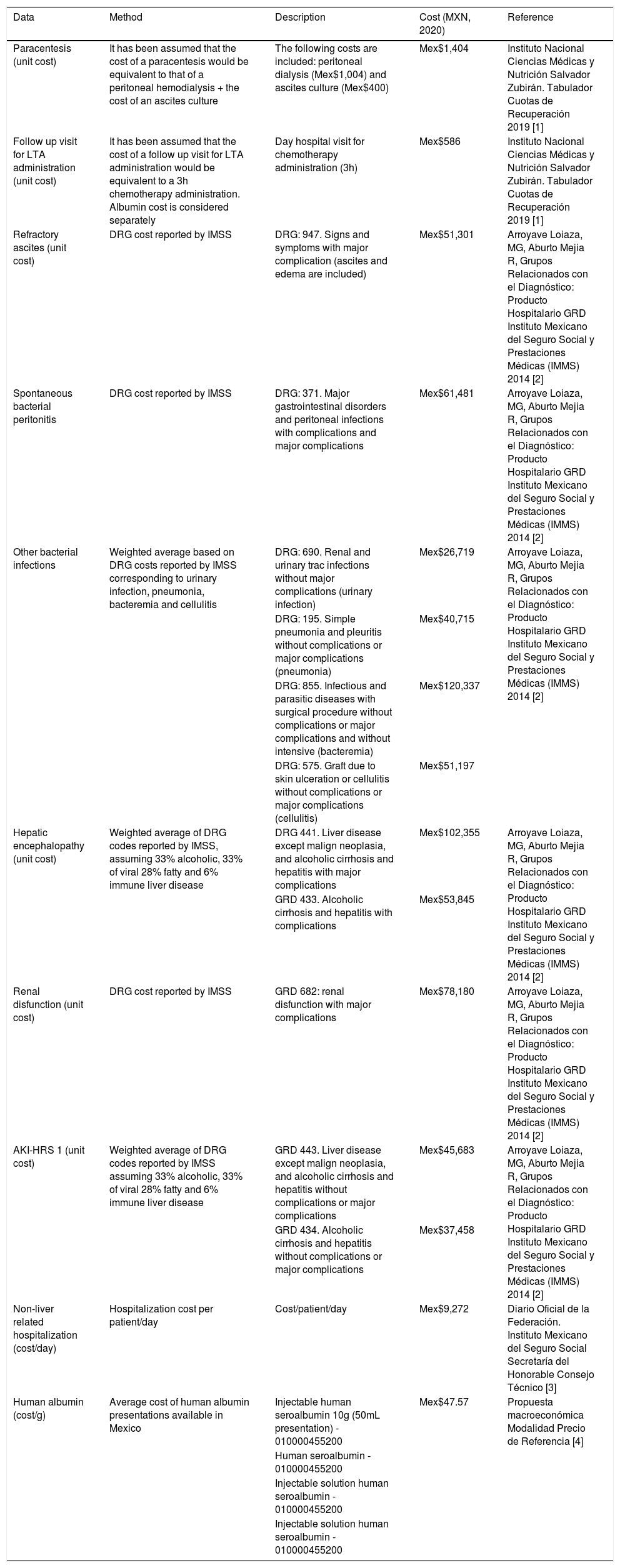
Unit costs from IMSS were gathered from public sources and transformed to 2020 Mexican $ (Mex$) and are detailed in Table 2[18–21]. The pharmacological cost of human albumin for LTA administration considered the average listing cost/g of all brands marketed in Mexico (Mex$47.57/g) and the ANSWER regimen (48 infusions of 40 g per patient/year). The pharmacological cost of SMT considered the cost of the 100 mg presentation of spironolactone (Mex$0.121/mg) and a regimen of 200 mg/day and the 40 mg presentation of furosemide (Mex$0.007/mg) and a posology of 25 mg/day.
The incremental cost per patient treated with LTA was calculated as the difference in cost/patient/year treated with LTA and SMT versus SMT only. No discount rate was applied. A detailed list of assumptions is included in Table A1 of the Appendix. All costs and assumptions were validated by an expert panel that consisted of three physicians experienced in managing cirrhosis patients in Mexico.
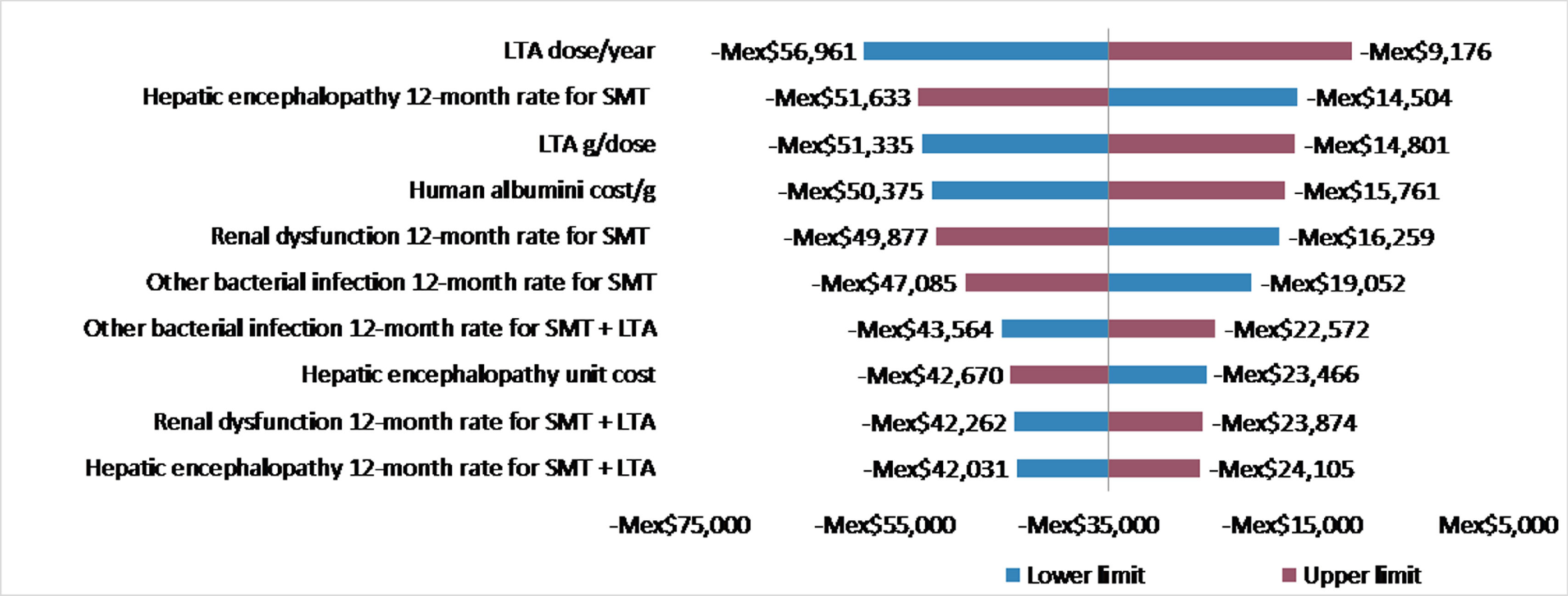
In order to validate the robustness of our findings, a univariate sensitivity analysis of the incremental cost/patient/year treated with LTA and SMT vs SMT only was performed by applying a 20% increase and decrease to all variables considered in the economic evaluation. Results of the sensitivity analyses for the 10 variables with the highest impact on the incremental cost per patient were displayed in a tornado diagram.
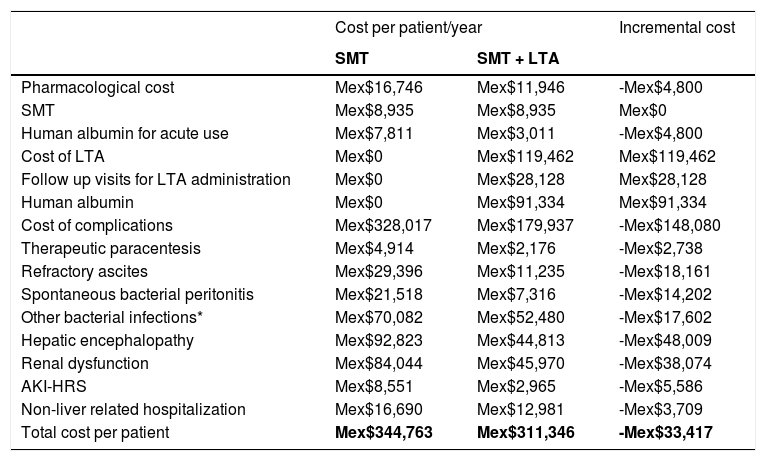
3ResultsThe overall cost/patient/year treated with SMT or SMT and LTA is displayed in Table 3. The use of LTA is estimated to require additional annual expenditure derived from the pharmacological cost of human albumin (Mex$91,334) and by the follow up visits required for LTA administration (Mex$28,128).
list of assumptions for the calculation of the cost of illness from the perspective of the Mexican Healthcare System.
| Data | Method | Description | Cost (MXN, 2020) | Reference |
|---|---|---|---|---|
| Paracentesis (unit cost) | It has been assumed that the cost of a paracentesis would be equivalent to that of a peritoneal hemodialysis + the cost of an ascites culture | The following costs are included: peritoneal dialysis (Mex$1,004) and ascites culture (Mex$400) | Mex$1,404 | Instituto Nacional Ciencias Médicas y Nutrición Salvador Zubirán. Tabulador Cuotas de Recuperación 2019 [1] |
| Follow up visit for LTA administration (unit cost) | It has been assumed that the cost of a follow up visit for LTA administration would be equivalent to a 3h chemotherapy administration. Albumin cost is considered separately | Day hospital visit for chemotherapy administration (3h) | Mex$586 | Instituto Nacional Ciencias Médicas y Nutrición Salvador Zubirán. Tabulador Cuotas de Recuperación 2019 [1] |
| Refractory ascites (unit cost) | DRG cost reported by IMSS | DRG: 947. Signs and symptoms with major complication (ascites and edema are included) | Mex$51,301 | Arroyave Loiaza, MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMMS) 2014 [2] |
| Spontaneous bacterial peritonitis | DRG cost reported by IMSS | DRG: 371. Major gastrointestinal disorders and peritoneal infections with complications and major complications | Mex$61,481 | Arroyave Loiaza, MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMMS) 2014 [2] |
| Other bacterial infections | Weighted average based on DRG costs reported by IMSS corresponding to urinary infection, pneumonia, bacteremia and cellulitis | DRG: 690. Renal and urinary trac infections without major complications (urinary infection) | Mex$26,719 | Arroyave Loiaza, MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMMS) 2014 [2] |
| DRG: 195. Simple pneumonia and pleuritis without complications or major complications (pneumonia) | Mex$40,715 | |||
| DRG: 855. Infectious and parasitic diseases with surgical procedure without complications or major complications and without intensive (bacteremia) | Mex$120,337 | |||
| DRG: 575. Graft due to skin ulceration or cellulitis without complications or major complications (cellulitis) | Mex$51,197 | |||
| Hepatic encephalopathy (unit cost) | Weighted average of DRG codes reported by IMSS, assuming 33% alcoholic, 33% of viral 28% fatty and 6% immune liver disease | DRG 441. Liver disease except malign neoplasia, and alcoholic cirrhosis and hepatitis with major complications | Mex$102,355 | Arroyave Loiaza, MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMMS) 2014 [2] |
| GRD 433. Alcoholic cirrhosis and hepatitis with complications | Mex$53,845 | |||
| Renal disfunction (unit cost) | DRG cost reported by IMSS | GRD 682: renal disfunction with major complications | Mex$78,180 | Arroyave Loiaza, MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMMS) 2014 [2] |
| AKI-HRS 1 (unit cost) | Weighted average of DRG codes reported by IMSS assuming 33% alcoholic, 33% of viral 28% fatty and 6% immune liver disease | GRD 443. Liver disease except malign neoplasia, and alcoholic cirrhosis and hepatitis without complications or major complications | Mex$45,683 | Arroyave Loiaza, MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMMS) 2014 [2] |
| GRD 434. Alcoholic cirrhosis and hepatitis without complications or major complications | Mex$37,458 | |||
| Non-liver related hospitalization (cost/day) | Hospitalization cost per patient/day | Cost/patient/day | Mex$9,272 | Diario Oficial de la Federación. Instituto Mexicano del Seguro Social Secretaría del Honorable Consejo Técnico [3] |
| Human albumin (cost/g) | Average cost of human albumin presentations available in Mexico | Injectable human seroalbumin 10g (50mL presentation) - 010000455200 | Mex$47.57 | Propuesta macroeconómica Modalidad Precio de Referencia [4] |
| Human seroalbumin - 010000455200 | ||||
| Injectable solution human seroalbumin - 010000455200 | ||||
| Injectable solution human seroalbumin - 010000455200 |
AKI-HRS: Acute Kidney Injury Hepatorenal Syndrome; DRG: Diagnosis related group; IMSS: Mexican Social Security Institute; LTA: Long-term albumin
Instituto Nacional Ciencias Médicas y Nutrición Salvador Zubirán. Tabulador Cuotas de Recuperación. http://www.incmnsz.mx/opencms/contenido/transparencia/Tabulador.html; 2019 [accessed 16 March 2021]
Arroyave Loiaza MG, Aburto Mejia R, Grupos Relacionados con el Diagnóstico: Producto Hospitalario GRD Instituto Mexicano del Seguro Social y Prestaciones Médicas (IMSS). https://www.imss.gob.mx/; 2014 [accessed 14 March 2021].
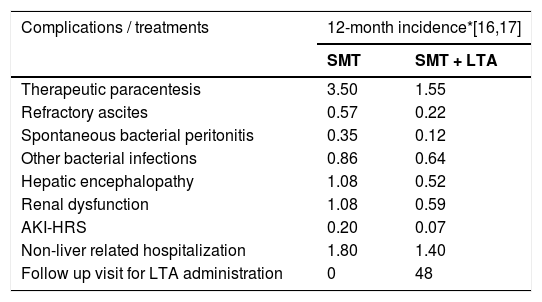
Twelve-month incidences of complications and treatments per patient following SMT or SMT and LTA based on the ANSWER trial.
| Complications / treatments | 12-month incidence*[16,17] | |
|---|---|---|
| SMT | SMT + LTA | |
| Therapeutic paracentesis | 3.50 | 1.55 |
| Refractory ascites | 0.57 | 0.22 |
| Spontaneous bacterial peritonitis | 0.35 | 0.12 |
| Other bacterial infections | 0.86 | 0.64 |
| Hepatic encephalopathy | 1.08 | 0.52 |
| Renal dysfunction | 1.08 | 0.59 |
| AKI-HRS | 0.20 | 0.07 |
| Non-liver related hospitalization | 1.80 | 1.40 |
| Follow up visit for LTA administration | 0 | 48 |
AKI-HRS: Acute Kidney Injury Hepatorenal Syndrome; LTA: Long-term albumin; SMT: standard medical treatment
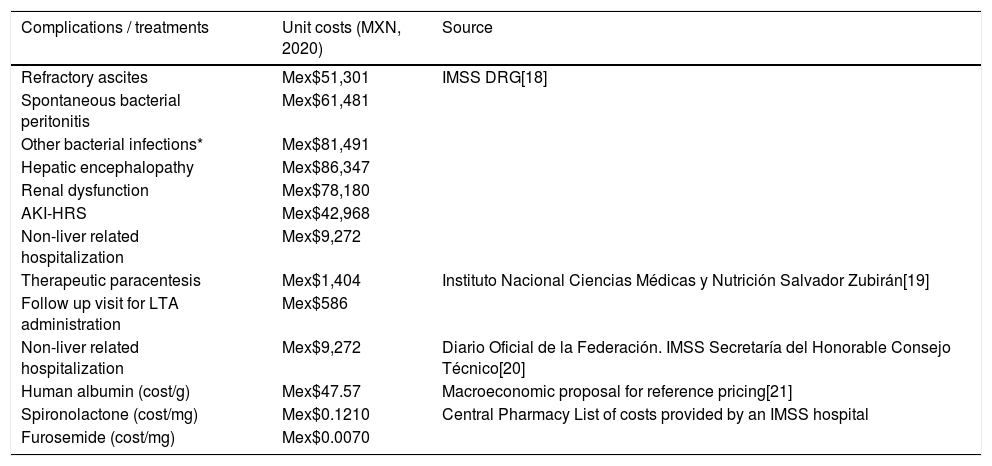
Unit costs and source.
| Complications / treatments | Unit costs (MXN, 2020) | Source |
|---|---|---|
| Refractory ascites | Mex$51,301 | IMSS DRG[18] |
| Spontaneous bacterial peritonitis | Mex$61,481 | |
| Other bacterial infections* | Mex$81,491 | |
| Hepatic encephalopathy | Mex$86,347 | |
| Renal dysfunction | Mex$78,180 | |
| AKI-HRS | Mex$42,968 | |
| Non-liver related hospitalization | Mex$9,272 | |
| Therapeutic paracentesis | Mex$1,404 | Instituto Nacional Ciencias Médicas y Nutrición Salvador Zubirán[19] |
| Follow up visit for LTA administration | Mex$586 | |
| Non-liver related hospitalization | Mex$9,272 | Diario Oficial de la Federación. IMSS Secretaría del Honorable Consejo Técnico[20] |
| Human albumin (cost/g) | Mex$47.57 | Macroeconomic proposal for reference pricing[21] |
| Spironolactone (cost/mg) | Mex$0.1210 | Central Pharmacy List of costs provided by an IMSS hospital |
| Furosemide (cost/mg) | Mex$0.0070 |
AKI-HRS: Acute Kidney Injury Hepatorenal Syndrome; DRG: Diagnosis Related Groups; IMSS: Mexican Social Security Institute; LTA: Long-term albumin
Cost per patient/year treated with SMT and with SMT +LTA.
AKI-HRS: Acute Kidney Injury Hepatorenal Syndrome; LTA: Long-term albumin; SMT: standard medical treatment.
The main drivers of cost-savings associated with the use of LTA would be the reduction of hepatic encephalopathy (−Mex$48,008), followed by the reduction in renal dysfunction (-Mex$38,073) and refractory ascites (−Mex$18,160). Overall the incremental cost per patient treated with SMT and LTA vs SMT only could lead to savings of Mex$33,417 per patient/year for the Mexican Healthcare System (Mex$311,346 patient/year for patients treated with SMT and LTA vs. Mex$344,763 patient/year for patients treated with SMT only).
Fig. 1 displays the results from the univariate sensitivity analysis. All scenarios assessed led to negative incremental costs (indicating cost savings) in all scenarios. Variables with the highest impact on the incremental cost per patient were the number of LTA administrations per year, the 12-month rate of hepatic encephalopathy for patients treated with SMT, and the dose (g/administration) of LTA (Fig. 1).
4DiscussionThe use of LTA in cirrhosis patients has been under debate for years. One of the reasons is the perception that LTA would entail increased resources and expenditure [7,15]. In order to further inform decision making, herein, we have estimated the incremental cost of treating cirrhosis patients with uncomplicated ascites with SMT and LTA following the regimen evaluated in the ANSWER trial from the IMSS perspective. Despite the perception that LTA would result in a significant cost burden when added to SMT, the results from the ANSWER trial suggest that these additional costs could be counterbalanced by the reduction in complications, hospitalizations, and acute albumin use, which could potentially lead to cost savings of Mex$33,417 per patient/year.
There is limited evidence on the cost of liver disease in Mexico. Quiroz et al. estimated the cost of liver cirrhosis based on Child Pugh stage from the IMSS perspective (all costs were reported in 2009 US$) [3]. Cost of illness was estimated using two different approaches: a) through micro-costing based on HCRU gathered from a retrospective cart review, and b) through opinion from an expert panel. The micro-costing approach considered medical and emergency room visits, hospitalizations, pharmacological treatment and diagnostic tests. Cost per patient ranged from US$1,634 to US$19,660 per patient based on the micro-costing approach and from US$4,269 to US$30,249 based on the expert panel [3]. Although comparison between our results and those reported by Quiroz et al is challenging due to the different methodologies applied, both studies seem to indicate that liver cirrhosis entails a relevant burden for the IMSS. Assessment of new therapeutic strategies should therefore consider both clinical and economic impacts.
Fernandez et al. had previously assessed the economic impact of LTA based on the ANSWER trial results for the Spanish Healthcare System (costs reported in 2019 €). Our results seem to be aligned with those reported by Fernandez et al. who estimated potential costs savings of €1,377 per patient/year for the Spanish Health System (approx. Mex$32,442) [17].
As main strengths, our study has assessed the cost of illness of patients with cirrhosis and uncomplicated ascites treated with SMT or SMT and LTA and the incremental cost of LTA based on IMSS costs gathered from public sources. All assumptions and costs have been validated by an expert panel experienced in the management of cirrhosis patients in Mexico.
Our study does entail some limitations, for instance, the fact that the rates of complications, hospitalizations and treatments were gathered from the ANSWER trial which was conducted in Italy, patient characteristics and usual clinical practice may not be fully representative of the IMSS. Also, since the economic evaluation has been performed from the IMSS perspective, no direct costs for the patient or indirect costs have been considered.
Although further work is needed to confirm how the efficacy of LTA from the ANSWER trial would translate to real-world effectiveness for Mexican patients, our study suggests that LTA may result in cost savings for the IMSS when administered to cirrhosis patients with uncomplicated ascites based on the ANSWER trial results.
Funding sourceThis study was funded by Grifols, a manufacturer of plasma-derived albumin products.
Author's contributionCarlos Moctezuma, Graciela Castro and Aldo Torre contributed to the literature review, data validation and interpretation of results, Pablo Simó and Elisabet Viayna contributed to the study conceptualization, data analysis and interpretation of results. Susana Aceituno and Maria Soler contributed to the study conceptualization and data analysis. The manuscript was written by Elisabet Viayna. All authors critically reviewed the manuscript and approved the final version and its submission to Annals of Hepatology. Aldo Torre is responsible for the integrity of the work as a whole.
Dr. Paolo Caraceni and the authors of the ANSWER trial for providing 12-month rates of treatments, complications and hospitalizations. Francisco Mota and Cristina Fuster from Grifols S.A. for their support in the literature and manuscript review. Jordi Bozzo from Grifols S.A. for reviewing the manuscript and for his editorial support. Dannya Calles and Sprim Américas Mexico for their support in gathering local unit costs for cirrhosis management in Mexico.
All authors critically reviewed the manuscript and approved the final version and its submission to Annals of Hepatology. Aldo Torre is responsible for the integrity of the work as a whole.