Microtubule-associated protein light chain 3-II (LC3-II), and Sequestosome-1 (SQSTM1) are proteins that can be used as markers for autophagic pathway. Bcl-2 protein is reported to be inversely correlated with apoptosis. We aimed to investigate the effects of curcumin on liver inflammation and fibrosis up to the first dysplastic stage of Hepatocellular carcinoma (HCC) induced by Thioacetamide (TAA) in rats and to clarify the effects of curcumin on LC3-II, SQSTM1, and Bcl-2. Male Sprague-Dawley rats were randomized into four groups: Control group, TAA group, Curcumin low-dose group, and Curcumin highdose group. The last three groups received TAA 200 mg/kg i.p. twice weekly for 18 weeks. Oxidative stress markers as hepatic malondialdehyde (MDA) concentration and superoxide dismutase (SOD) activity were measured by colorimetric methods. Hepatic SQSTM1 concentration was measured by ELISA, and gene expression levels of Bcl-2, and LC3-II were measured by RT-PCR.We also investigated the in vitro effect of curcumin on HepG2 cells viability through MTT assay, and the involvement of autophagy in this effect.
ResultsCurcumin increased the survival percent in rats, decreased -fetoprotein (AFP) concentration, and serum aspartate aminotransferase (AST) activity, and increased serum albumin concentration. Curcumin also significantly reduced oxidative stress in liver, inhibited apoptosis, and induced autophagy. In vitro, curcumin (50 μM) decreased HepG2 cells viabilityand the concentration of SQSTM1.
ConclusionsCurcumin leads to protection against TAA induced HCC up to the first dysplastic stage through activating autophagic pathway and inhibiting apoptosis. Also, the antioxidant activity of curcumin almost prevents liver fibrosis.
Hepatocellular carcinoma (HCC) comes in the third place as the most leading cause of cancer mortality all over the world and in the sixth place as the most frequently diagnosed cancer. HCC usually progresses from chronic hepatitis, to cirrhosis, to dysplastic nodules (low- and high-grade), to malignant tumors.1,2
Curcumin derived from the plant Curcuma longa, is a gold-colored spice commonly used in the Indian subcontinent. Curcumin has been shown to exhibit antioxidant, anti-inflammatory, antiviral, antibacterial, antifungal, and anticancer activities and thus has a potential protective role against various malignant diseases, diabetes, allergies, arthritis, Alzheimer's disease, and other chronic illnesses.
It has also been shown to induce cell death in apoptosis resistant cancer cells.3,4
Autophagy is an evolutionarily conserved self-digestive mechanism, which involves many steps.5 These steps include: aggregation of double-membrane, formation of autophagosome that engulfs the targeted region, its fusion with lysosomes and digestion in autolysosomes. Under starvation or other stresses, autophagy is required for cell survival to eliminate the damaged cellular components and to maintain nutrition and energy homeostasis. Therefore, autophagy has a close relationship with many biological or pathological phenomena including carcinogenesis. Many recent studies have indicated that autophagy has a tumor-suppressive function.2,6 Besides tumor-suppressive function; autophagy has been reported to promote tumor development. Many recent studies have revealed that autophagy was essential for oncogene-mediated tumorigenesis,7 which seemingly raises a paradox. However, autophagy probably has different roles in different stages of tumor development, which is a multistep and complicated process.2,8,9
Microtubule-associated protein light chain 3 is a protein involved in the autophagy process, and exists in either of two forms, a cytosolic form (18 kDa, LC3-I) and membrane-bound form (16 kDa, LC3-II) which results from proteolysis and lipid modification of LC3-I. Since LC3-I is processed into LC3-II which in turn is incorporated into autophagosomal membranes during autophagy, the accumulation of LC3-II is considered as one of the markers of autophagy induction.10
In addition to LC3, SQSTM1 is another autophagy specific substrate, since it has been reported to directly bind to LC3, incorporate into autophagosome, and finally degrade by autophagy. For example, in autophagy-deficient cells, autophagy-inducing stimulus such as starvation failed to reduce SQSTM1 expression. Therefore, SQSTM1 expression level is inversely correlated with autophagic activity.11
Bcl-2 protein is one of the Bcl-2 proteins family that includes both pro- and anti-apoptic proteins. Bcl-2 belongs to the anti-apoptic group that down regulates autophagy through inhibiting other pro-apoptic members of the same family. Besides inactivation of pro-apoptotic Bcl-2 family proteins, the presence of elevated levels of anti-apoptotic family members is an obvious mechanism of apoptosis dysregulation in cancer.12,13
Material and MethodsA in vitro StudyCell LineThe HepG2 cell line was purchased from The Holding Company for Biological Products & Vaccines (Vacsera, Cairo, Egypt), where the HepG2 cells were examined for identity and absence of bacteria, mycoplasma and viruses.
HepG2 cells were grown in Dulbecco's Modified Eagle's medium supplemented with 10% fetal bovine serum and 1% streptomycin and penicillin, and incubated for 24 h at 37 °C in a 5% CO2 incubator to allow the cells to grow.
MTT Assay1 x 104 cells were plated in each well of 96-well plate, and were placed in the humidified 5% CO2 incubator at 37 °C to allow them to grow for 24 h period. Cells were transferred to serum-free medium and exposed to different concentrations of curcumin (5, 10, 20, 40, and 50 μM) and another two combinations were made between curcumin 50 μM and N-Acetyl Cysteine (NAC) (Sigma-Aldrich, St. Louis, MO, USA), and between curcumin 50 μM with hydrogen peroxide (El-Gomhouria Co., Cairo, Egypt) and placed in the humidified 5% CO2 incubator for 24 h for evaluation.
In vivo StudyAnimals and their treatment outlinesThe animal protocol was approved by ethical committee in Faculty of Pharmacy, Mansoura University (proto-col code No. 2012-32) Mansoura, Egypt, in accordance with “Principles of Laboratory Animal Care” (NIH publi-cation No. 85-23, revised 1985). 66 Male Sprague Dawely rats weighing 180-200 g were used. All animals in the study were maintained under standard conditions of temperature 25 ± 2 °C, with regular 12 h light/12 h dark cycle and allowed free access to food and water. Rats were divided into 4 groups:
- •
Control group (10 rats). Rats received corn oil through oral gavage and served as a negative control group throughout the study.
- •
TAA group (24 rats). Rats were injected with thioacetamide (TAA) (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 200 mg/kg, intraperitoneal (i.p.) twice per week for 18 weeks.14
- •
Curcumin low-dose (Cur low-dose) group and Curcumin high-dose (Cur high-dose) group (16 rats each).
The two groups received curcumin 100 mg/kg,15 and 200 mg/kg16 daily, respectively, by oral gavage throughout the whole study and were injected with TAA (200 mg/kg, i.p.) twice per week for 18 weeks starting from the second week after administration of curcumin.
Collection of samplesAfter the experimental period, rats were fasted overnight then blood samples were taken from the heart under diethyl ether anesthesia. The clotted blood samples were centrifuged at 5,000 rpm for 5 min for serum separation. Serum was divided into aliquots for biochemical analysis. Rats were sacrificed by decapitation; the whole liver was dissected, and immersed in ice-cold saline. A small part from right lobe of liver was cut and fixed in 10% phosphate-buffered formalin (pH 7.2) for histopathological examination. The remaining liver was kept in liquid nitrogen for subsequent analysis. It was then homogenized in 10-fold volume of ice-cold sodium potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl. The homogenates were centrifuged at 600 x g for 10 min at 4 °C, and the supernatant was stored at -80 °C.
Histopathological analysis of hepatic tissuePhosphate buffered formalin-fixed liver tissues were embedded in paraffin, cut into 5 μm thick sections and stained with hematoxylin and eosin (H&E) and Masson's trichrome for histopathological examination.
Hepatic specimens were anonymously coded and examined in a masked manner.
Liver function testsSerum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities and albumin and AFP concentrations were measured by the standard methodologies using commercially available kits provided by Bio-diagnostic Company (Dokki, Giza, Egypt).
Assessment of oxidative stressIt was estimated through determination of hepatic malondialdhyde (MDA) concentration, and hepatic superoxide dismutase (SOD) activity using commercially available kits provided by Biodiagnostic Company (Dokki, Giza, Egypt).
Determination of SQSTM1 concentrationThe concentration of biochemical parameter SQSTM1 in the liver, and in HepG2 cells were measured by ELISA using a commercially available SQSTM1 (Enzo life sciences, NY, USA) in accordance with the manufacturer's instructions.
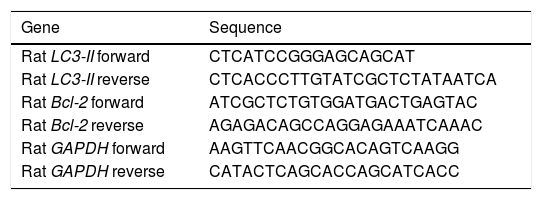
Gene expression of LC3-II, and Bcl-2 by quantitative real-time polymerase chain reaction (RT-PCR)Total RNA for LC3-II, Bcl-2, and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was isolated from euthanized rat liver using GF-1 total RNA extraction kit (Vivantis, Malaysia). One microgram of total RNA was reverse transcribed into single-stranded complementary DNA by using FastQuant RT Kit (with gDNase) (Tain-gen, China). Bcl-2 and LC3-II mRNA levels in different rat liver tissues were determined using Maxima SYBR Green/Fluorescein qPCR Master Mix by Rotor-Gene Q (Qiagen, USA). In the meanwhile, rat GAPDH was used as a housekeeping gene and an internal reference control. Gene-specific PCR primers (Table 1) were obtained using Primer Express 3.0 (Applied Biosystems, USA), and GAP-DH gene sequences were obtained from previous studies.17
Primer sequences used for the RT-PCR step.
| Gene | Sequence |
|---|---|
| Rat LC3-II forward | CTCATCCGGGAGCAGCAT |
| Rat LC3-II reverse | CTCACCCTTGTATCGCTCTATAATCA |
| Rat Bcl-2 forward | ATCGCTCTGTGGATGACTGAGTAC |
| Rat Bcl-2 reverse | AGAGACAGCCAGGAGAAATCAAAC |
| Rat GAPDH forward | AAGTTCAACGGCACAGTCAAGG |
| Rat GAPDH reverse | CATACTCAGCACCAGCATCACC |
For descriptive statistics of quantitative variables, the mean ± standard error was used. One-way analysis of variance (ANOVA) was used to compare means between groups. Rats’ survival was estimated using the Kaplan-Meier method. Statistical computations were done on a personal computer using the computer software Prism 6. Statistical significance was predefined as P < 0.05.
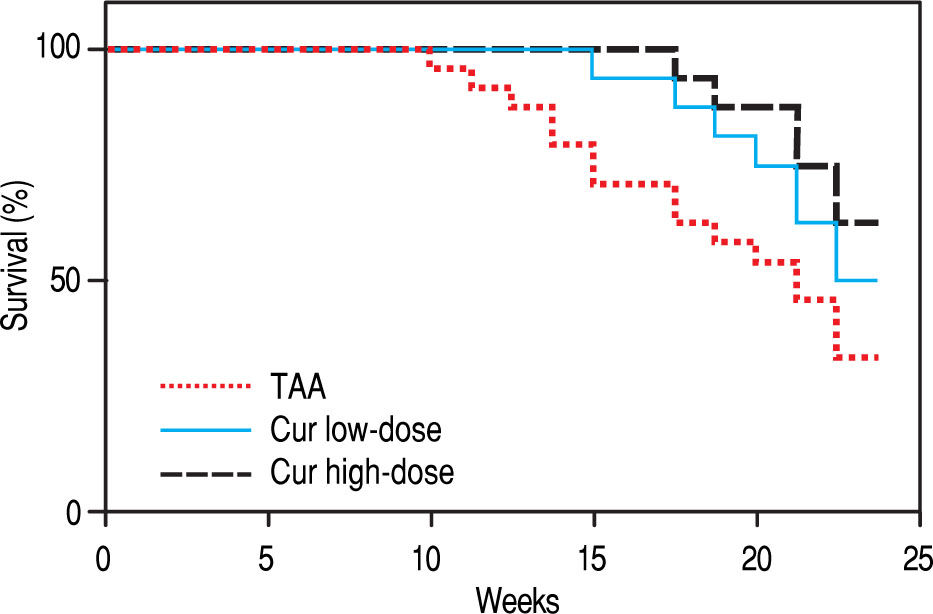
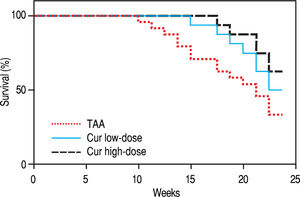
RESULTSEffect of Curcumin low-dose and Curcumin high-dose on survival percentThe survival percent of rats was 33% in TAA group (Figure 1). Cur low-dose showed an increase in animal survival percent to 50%, while Cur high-dose showed an increase in animal survival percent to 62.5%.
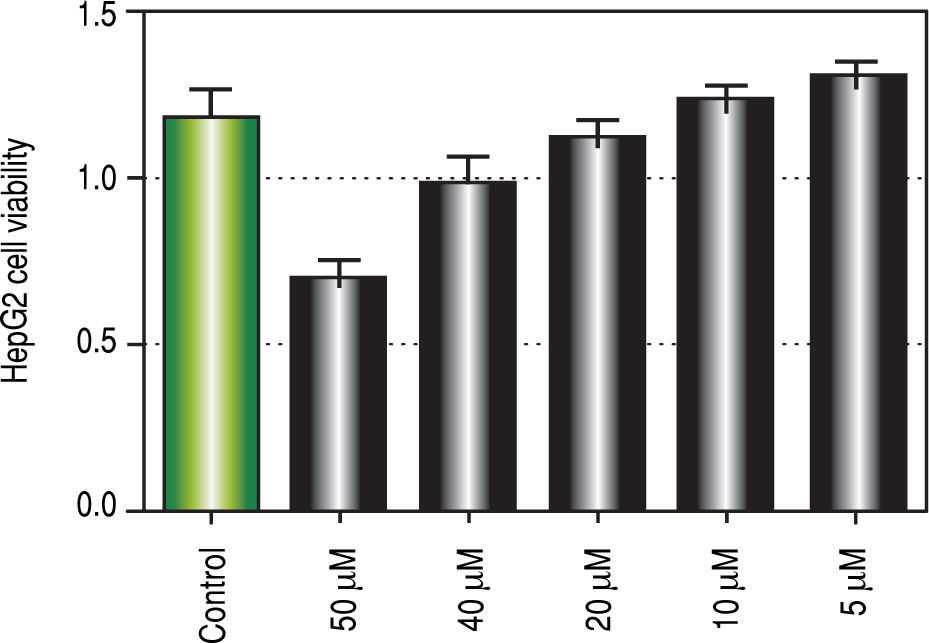
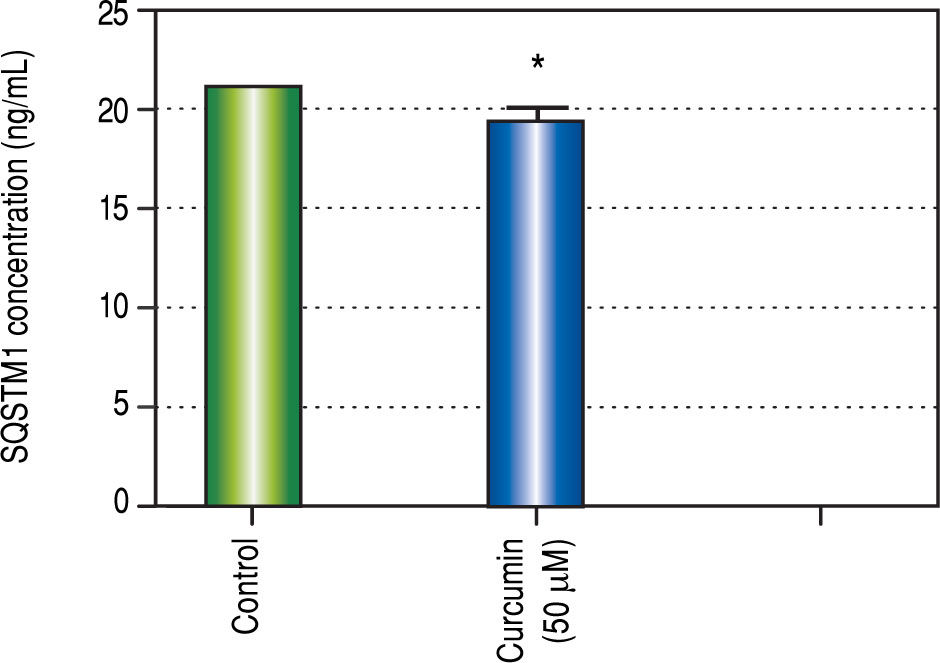
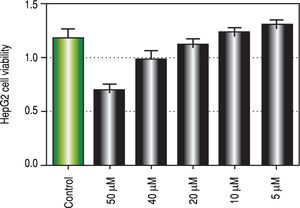
Regarding in vitro studies, treatment with Curcumin showed a significant reduction in the viability of HepG2 cells only with 50 μM dose (Figure 2).
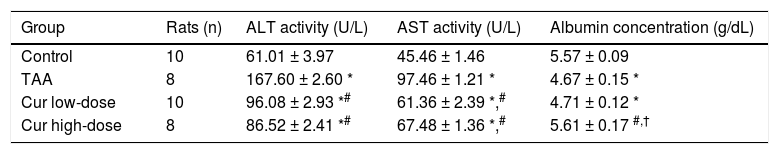
Hepatoprotective effect of Curcumin low-dose and Curcumin high-dose in HCC induced by TAA in ratsNext, we examined the hepatoprotective effect of curcumin against HCC induced by TAA in vivo. TAA rats showed 2.7- and 2.1-fold significant increase in serum ALT, and AST activities, respectively, while Cur low-dose and Cur high-dose groups showed a significant decrease in ALT, and AST activities from TAA group (Table 2). TAA group also showed a 17% significant reduction in serum albumin level in TAA group as compared with the control group, with only Cur high-dose group showing a significant increase in serum albumin level from TAA group (Table 2).
Effect of Cur low-dose (100 mg/kg) and Cur high-dose (200 mg/kg) on serum Alanine aminotransferase activity (ALT), Aspartate aminotransferase activity (AST), and albumin concentration in Thioacetamide (200 mg/kg) induced HCC.
| Group | Rats (n) | ALT activity (U/L) | AST activity (U/L) | Albumin concentration (g/dL) |
|---|---|---|---|---|
| Control | 10 | 61.01 ± 3.97 | 45.46 ± 1.46 | 5.57 ± 0.09 |
| TAA | 8 | 167.60 ± 2.60 * | 97.46 ± 1.21 * | 4.67 ± 0.15 * |
| Cur low-dose | 10 | 96.08 ± 2.93 *# | 61.36 ± 2.39 *,# | 4.71 ± 0.12 * |
| Cur high-dose | 8 | 86.52 ± 2.41 *# | 67.48 ± 1.36 *,# | 5.61 ± 0.17 #,† |
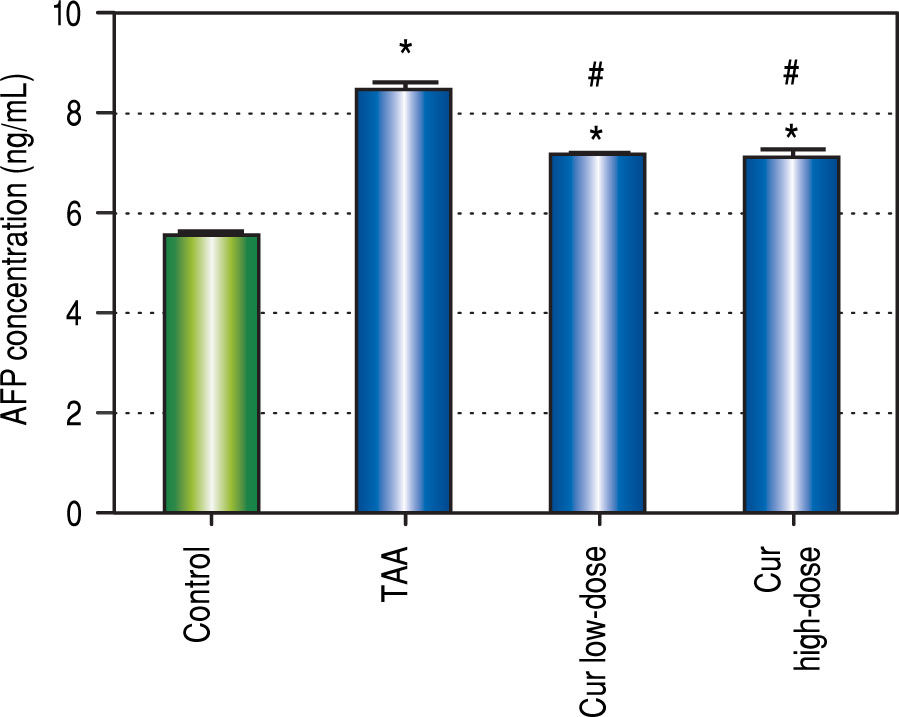
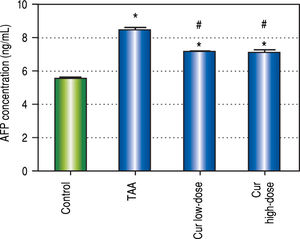
We also found 1.5-fold increase in serum AFP in TAA group as compared to control group (Figure 3). Both curcumin groups blocked the increase in serum AFP level in TAA group.
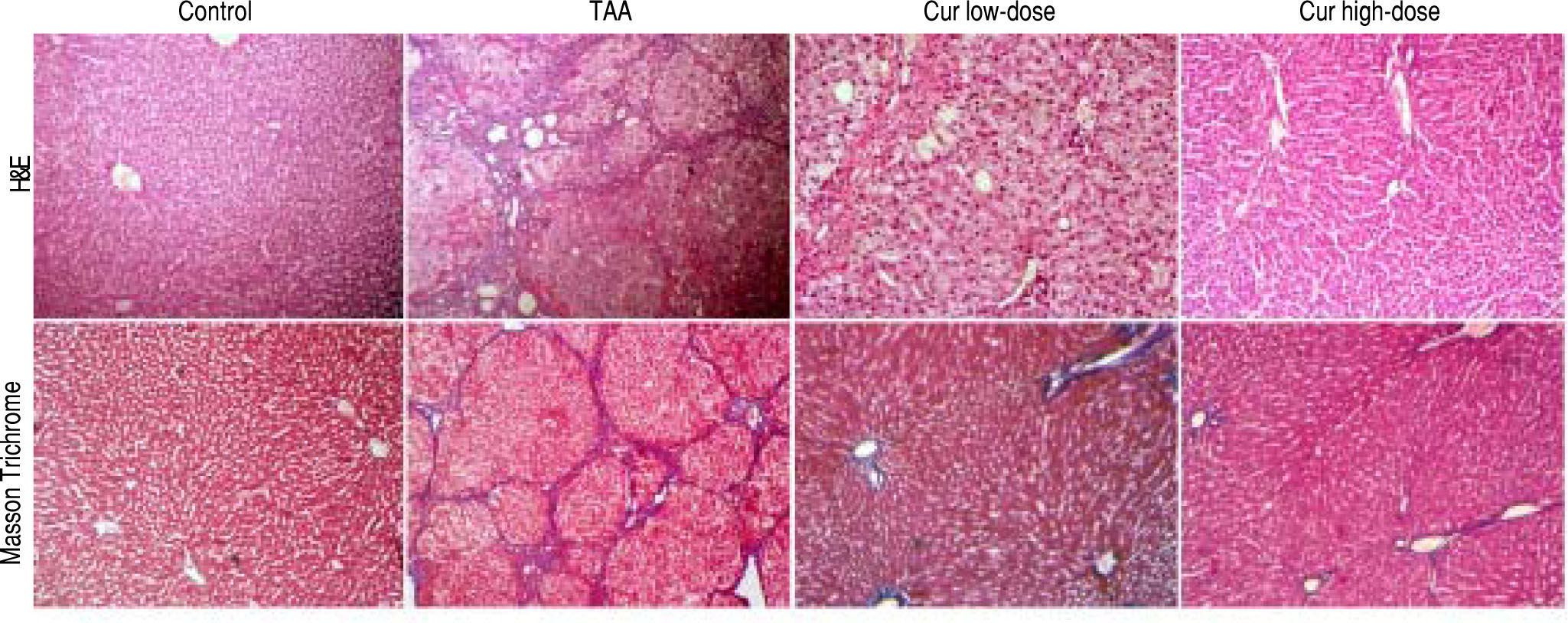
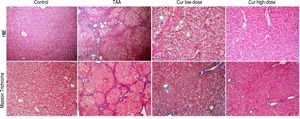
In addition, liver sections stained with H/E showed marked necroinflammatory changes with dysplastic nodules. However, sections from curcumin groups showed nearly normal appearance of hepatic lobule, with liver sections from Cur high-dose group (200 mg/kg daily) showing more improvement than Cur low-dose (100 mg/ kg daily), while liver sections stained with Masson Trichrome showed marked collagen deposition with complete cirrhosis in TAA group with less collagen deposition in Cur high-dose group than Cur low-dose group (Figure 4).
Liver sections stained with H/E, and Masson Trichrome of Control, TAA (thioacetamide 200 mg/kg twice weekly), Cur low-dose (100 mg/kg daily), and Cur high-dose (200 mg/kg daily) groups. Upper panel shows more improvement in Cur high-dose group than Cur low-dose group compared to marked necroinflammatory changes with dysplastic nodules in the TAA group. Lower panel showed marked collagen deposition with complete cirrhosis in TAA group with reduction of collagen deposition in Cur high-dose group than Cur low-dose group.
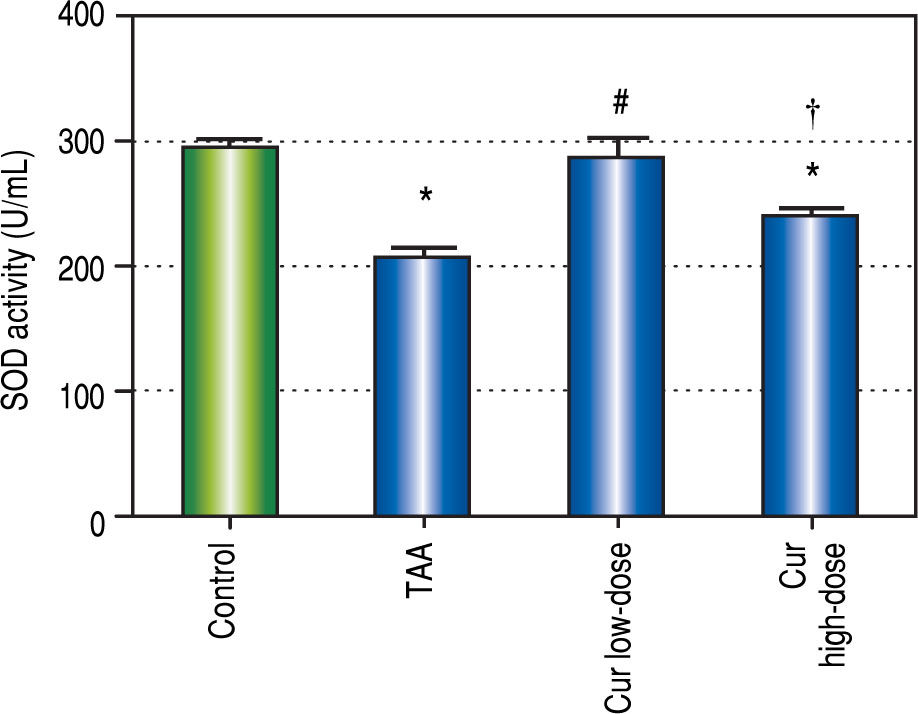
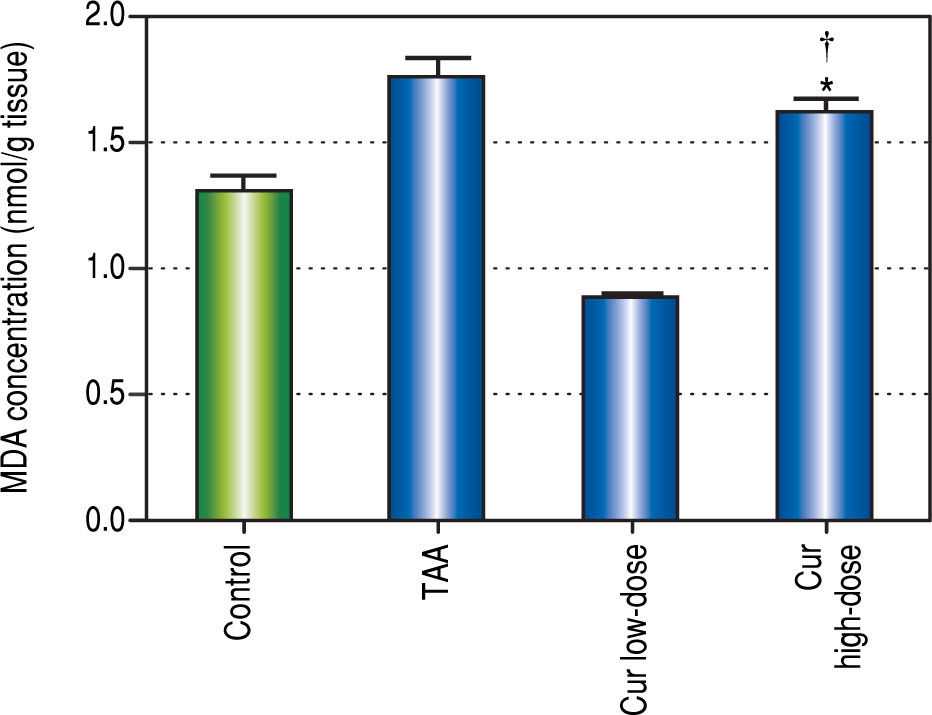
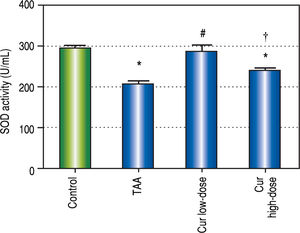
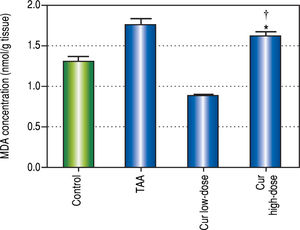
It is known that the oxidative stress status has a key role in HCC development and progression. We found 0.7-fold significant decrease in hepatic SOD activity (Figure 5), and 1.3-fold significant increase in hepatic MDA levels (Figure 6) in TAA group as compared with the control group.
Effect of Cur low-dose (100 mg/kg) and Cur high-dose (200 mg/ kg) on hepatic superoxide dismutase activity (SOD) (mean ± SE) in Thioacetamide (200 mg/kg) induced HCC. * Significant against control group at P < 0.05. # Significant against TAA group at P < 0.05. † Significant against Cur low-dose group at P < 0.05.
Effect of Cur low-dose (100 mg/kg) and Cur high-dose (200 mg/ kg) on hepatic malondialdehyde (MDA) concetration (mean ± SE) in Thioacetamide (200 mg/kg) induced HCC. * Significant against control group at P < 0.05. # Significant against TAA group at P < 0.05. † Significant against Cur low-dose group at P < 0.05.
Rats in Cur low-dose group showed significant reduction to the elevated MDA levels in TAA group, and significant increase to the reduced SOD activity in TAA group. Rats in Cur high-dose group did not show a significant change from TAA group in both oxidative stress markers.
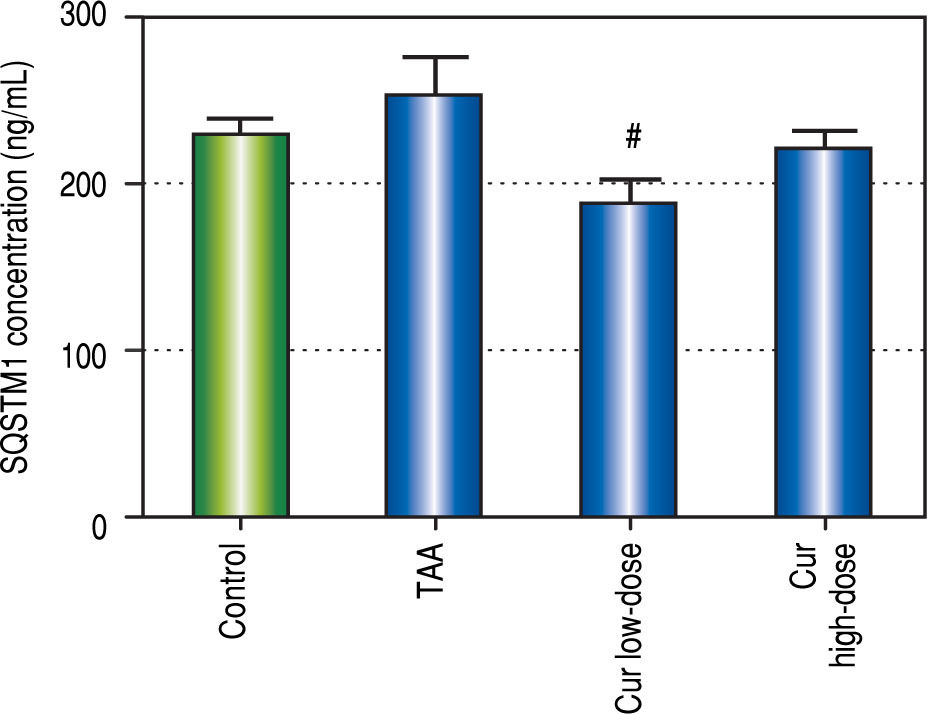
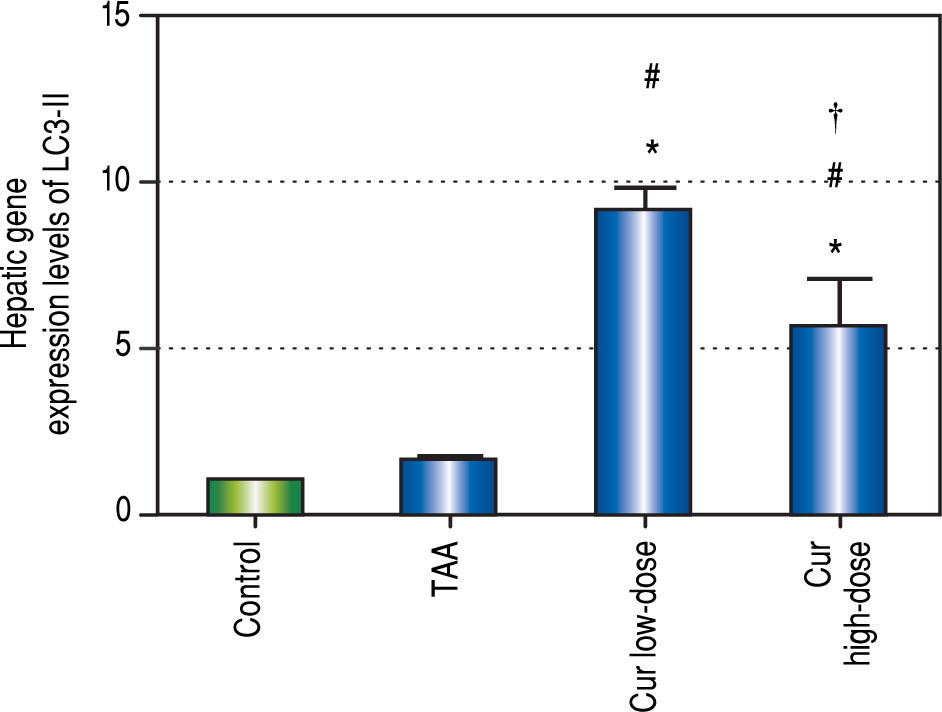
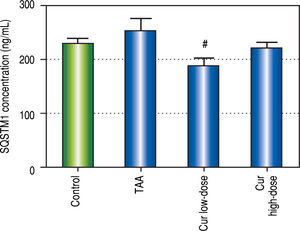
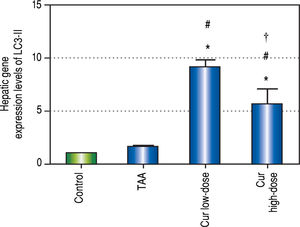
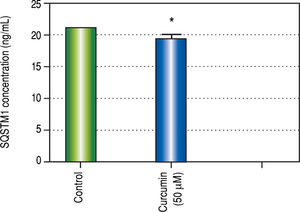
Effect of Curcumin low-dose and Curcumin high-dose on autophagic and apoptic pathwayAs shown in figure 7, TAA group showed non-significant increase in SQSTM1 concentration when compared with control group. Cur low-dose group showed significant decrease in SQSTM1 concentration compared with control group, and showed significant increase in LC3-II hepatic gene expression levels compared with control group. Cur high-dose group showed non-significant decrease in SQSTM1 concentration and significant increase in LC3-II gene expression levels from control group as shown in figure 8. In addition, treatment of HepG2 cells with curcumin caused a significant decrease in SQSTM1 (Figure 9).
Effect of Cur low-dose (100 mg/kg) and Cur high-dose (200 mg/ kg) on microtubule-associated protein light chain 3 II (LC3-II) gene expression levels (mean ± SE) in Thioacetamide (200 mg/kg) induced HCC. * Significant against control group at P < 0.05. #Significant against TAA group at P < 0.05. † Significant against Cur low-dose group at P < 0.05.
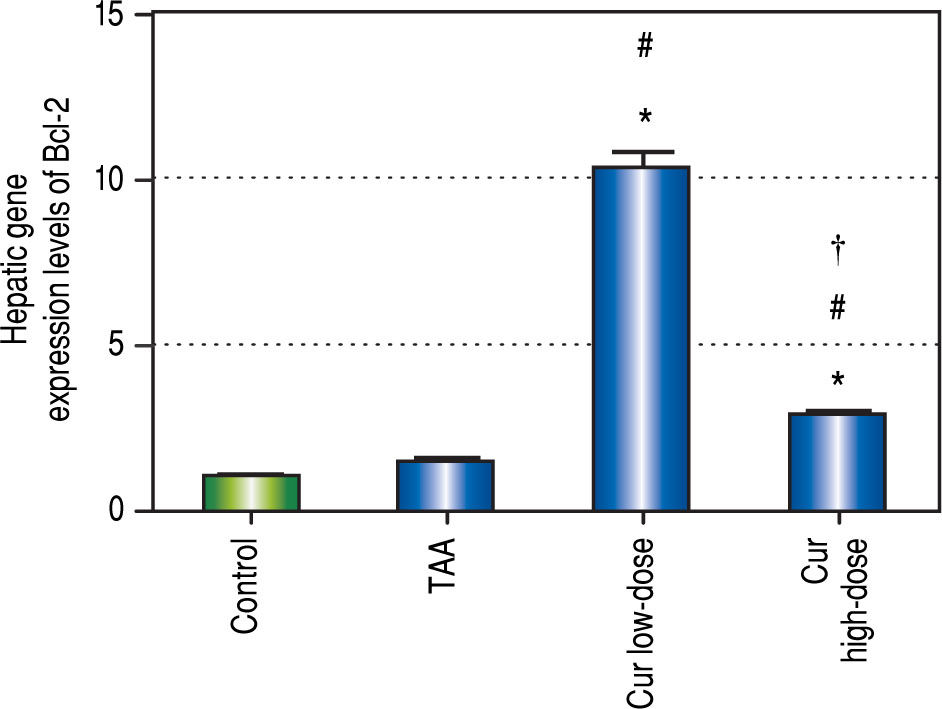
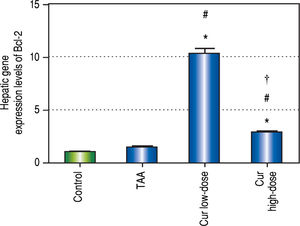
Curcumin also showed significant increase in Bcl-2 gene expression levels in both concentrations (Figure 10), a gene that is known to inhibit apoptosis, which means that curcumin had an inhibitory effect on apoptosis in this study. Moreover the effect of curcumin in induction of autophagy in both doses was highly correlated with the inhibitory effect on apoptosis for each dose.
Effect of Cur low-dose (100 mg/kg) and Cur high-dose (200 mg/kg) on Bcl-2 gene expression levels (mean ± SE) in Thioacetamide (200 mg/kg) induced HCC. * Significant against control group at P < 0.05. # Significant against TAA group at P < 0.05. † Significant against Cur low-dose group at P < 0.05.
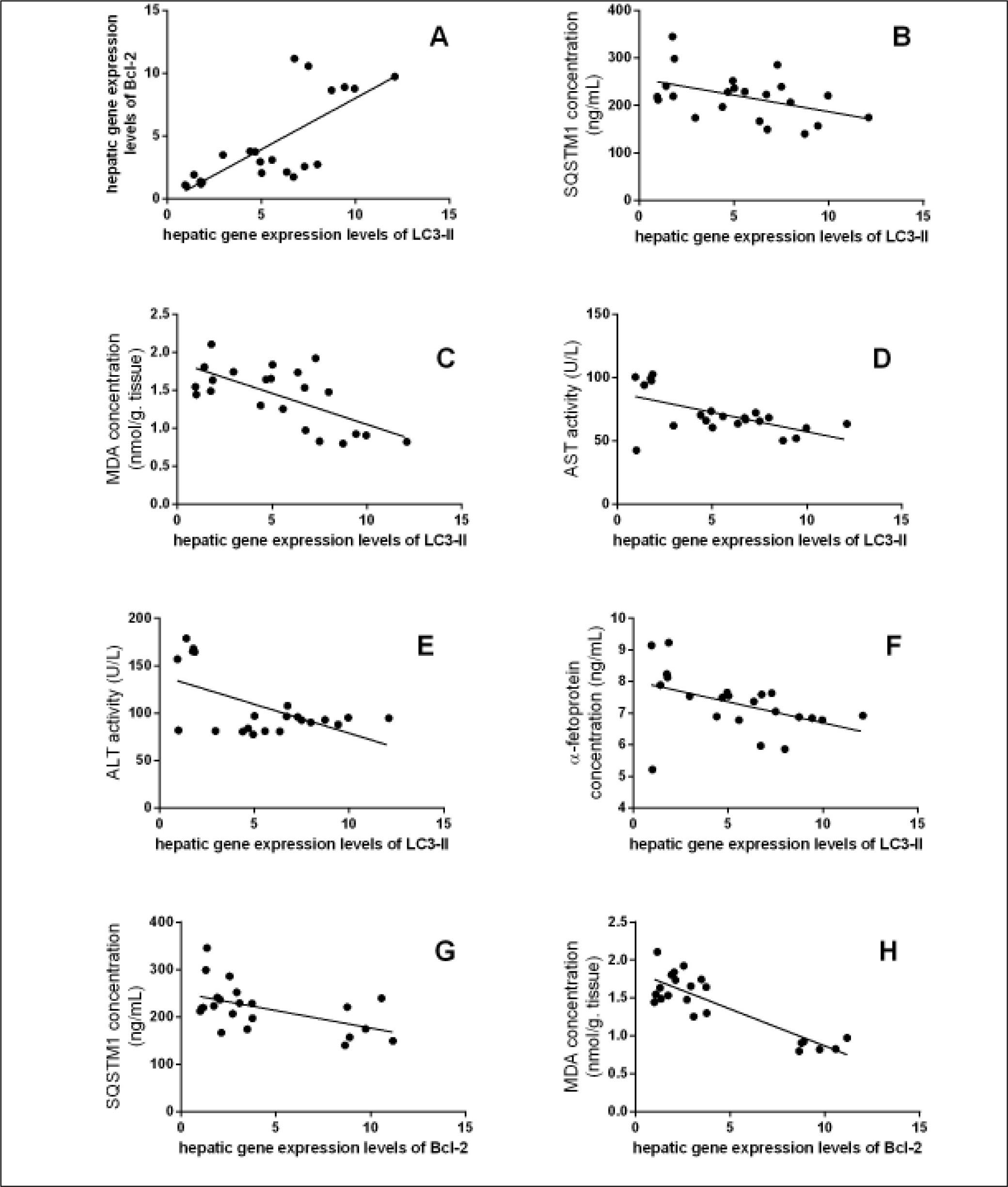
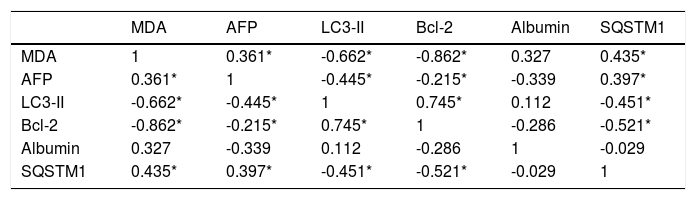
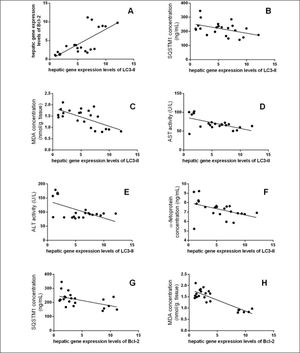
The data in table 3, and figure 11 show the correlation analysis of the studied parameters. LC3-II gene expression level is positively correlated with Bcl-2 gene expression level (0.745, P < 0.05), and negatively correlated with each of liver AFP level (-0.445, P < 0.05), serum MDA level (-0.662, P < 0.05), and hepatic SQSTM1 concentration (-0.451, P < 0.05). Bcl-2 gene expression level is negatively correlated with SQSTM1 concentration (-0.521, P < 0.05), and serum MDA level (-0.862, P < 0.05).
Correlation analysis of the studied parameters.
| MDA | AFP | LC3-II | Bcl-2 | Albumin | SQSTM1 | |
|---|---|---|---|---|---|---|
| MDA | 1 | 0.361* | -0.662* | -0.862* | 0.327 | 0.435* |
| AFP | 0.361* | 1 | -0.445* | -0.215* | -0.339 | 0.397* |
| LC3-II | -0.662* | -0.445* | 1 | 0.745* | 0.112 | -0.451* |
| Bcl-2 | -0.862* | -0.215* | 0.745* | 1 | -0.286 | -0.521* |
| Albumin | 0.327 | -0.339 | 0.112 | -0.286 | 1 | -0.029 |
| SQSTM1 | 0.435* | 0.397* | -0.451* | -0.521* | -0.029 | 1 |
Significant correlation between hepatic gene expression levels of LC3-II with (A) hepatic gene expression levels of Bcl-2 (r = 0.745, P < 0.0001), (B) SQSTM1 concentration (r = -0.451, P < 0.05), (C) MDA concentration (r = -0.662, P < 0.05), (D) Serum AST activity (r = -0.579, P < 0.05), (E) Serum ALT activity (r = -0.571, P < 0.05), and (F) AFP concentration (r = -0.445, P < 0.05), and between hepatic gene expression levels of Bcl-2 with (G) SQSTM1 concentration (r = -0.521, P < 0.05), and (H) MDA concentration (r = -0.862, P < 0.0001).
The role of autophagy induced by curcumin in protection and treatment of HCC was assayed in this study. Autophagy and apoptosis are both considered two different mechanisms for programmed cell death. In this study, we examined how curcumin affects both autophagy and apoptosis, and how they affect HCC prognosis. We are the first to investigate the effect of curcumin on both autophagy and apoptosis in TAA induced HCC, and to relate these effects to the concentration of ROS.
The experimental model was designed to reach only the first dysplastic stage of HCC. This is because autophagy has been proved to have different and even contradicting effects on HCC prognosis in both the dysplastic and tumor-forming stages of HCC.2 Cells in the late tumor stages are characterized from cells in the early stages in having higher metabolic activity, which leads to higher levels of ROS.18,19 Based on that difference between the two stages, it was found that inhibiting autophagy leads to excessive ROS accumulation which leads to cell death by apoptosis, while in the dysplastic stage inhibiting autophagy was shown to lead to cell survival and cancer proliferation.2,20 In our work, we aimed to investigate the hepatoprotective effect of activating autophagy in the dysplastic stage instead of inhibiting it in the late tumor-forming stage.
To investigate the involvement of autophagic pathway in the mechanism of curcumin's effect, LC3-II gene expression, and SQSTM1 concentration were measured, and the results showed significant increase in LC3-II gene expression in Cur low-dose, and Cur high-dose groups. The results also showed significant decrease in SQSTM1 concentration with both curcumin doses. Our results also indicated that curcumin lead to significantly decreasing HepG2 cells viability in a dose of 50 μM. This dose also showed a significant decrease in SQSTM1 concentration. These results are in agreement with other studies that suggested that curcumin among other natural products leads to autophagic cell death.21-23
The results of measuring the anti-apoptic Bcl-2 gene expression showed that curcumin significantly inhibited apoptosis in both concentrations. A correlation study between curcumin's effect on both Bcl-2 gene expression levels, and LC3-II gene expression levels showed significant high positive correlation (r = 0.745, P < 0.0001). Thus, our study shows that curcumin activates autophagy and simultaneously deactivates apoptosis. Previous studies showed that autophagy and apoptosis can happen together,3 independently of each other,24 or activating one can actually lead to inhibiting the other.2 Studies have also suggested that curcumin may lead to cell death through apoptosis,25,26 or through autophagy without affecting apoptosis at all.24 Other studies also showed that apoptosis can be activated to treat different types of cancer,12 but in some cases cells are resistant to apoptosis,24 which makes an option like autophagic cell death much more valuable in treating of or protecting against cancer.
Results of this study showed that curcumin in both doses significantly blocked the elevation of AFP concentration found in TAA group. Curcumin groups also significantly blocked the elevation in ALT and AST activities in TAA group. Cur high-dose group significantly blocked serum albumin concentration reduction found in TAA group. Histopathological examination of liver sections showed marked improvement in both curcumin groups than TAA group, with more improvement in Cur highdose group. These results show that curcumin in both doses help in protection against HCC induced by TAA in rats. Correlation studies also showed significant correlations between LC3-II gene expression levels as a marker of autophagy and each of AFP concentration (r = - 0.445, P < 0.05), ALT activity (r = - 0.571, P <0.05), and AST activity (r = - 0.579, P < 0.05).
The induction or inhibition of autophagy to treat and protect against different types of cancer, including HCC, has been thoroughly studied recently. Recent studies found that activating autophagic cell death can lead to a better cancer prognosis in many cancer types including cervical cancer, nasopharyngeal carcinoma, pancreas adenocarcinoma, and HCC as it leads to cancer cell death.27-29 Other recent studies found that inhibition of autophagy may enhance the effect of some drugs like bevacizumab in treatment of HCC because autophagy in this case is used as a survival mechanism by cancer cells.25,30 A recent study suggested an explanation of this paradoxical role of autophagy in HCC by clarifying that the induction of autophagy treats cancer in the first dysplastic stage of HCC, while in the later tumor-forming stages, induction of autophagy leads to worse cancer prognosis.2
This study also aimed to understand the relationship between activating autophagy and increasing ROS levels. ROS play a vital role in the proliferation and survival of cancer cells. However, excessive ROS generation has been reported to cause cancer cell death through autophagic or apoptic pathways.9,31 TAA is known as a hepatocarcinogen that increases the hepatic concentration of ROS that leads to liver fibrosis, cirrhosis, and finally HCC.32,33 It also affects liver functions significantly, and interferes with the liver's ability to synthesize proteins like albumin.34 In this study, we measured SOD activity and found increased activity in curcumin groups compared with TAA group, while MDA concentration showed significant decrease only in Cur low-dose group compared with TAA group. This comes in agreement with other studies that state that curcumin has an antioxidant activity.35 Correlation studies showed significant negative correlation between MDA concentration as a marker of ROS level and each of LC3-II gene expression levels (r = - 0.662, P < 0.05), Bcl-2 gene expression levels (r = - 0.862, P < 0.0001).
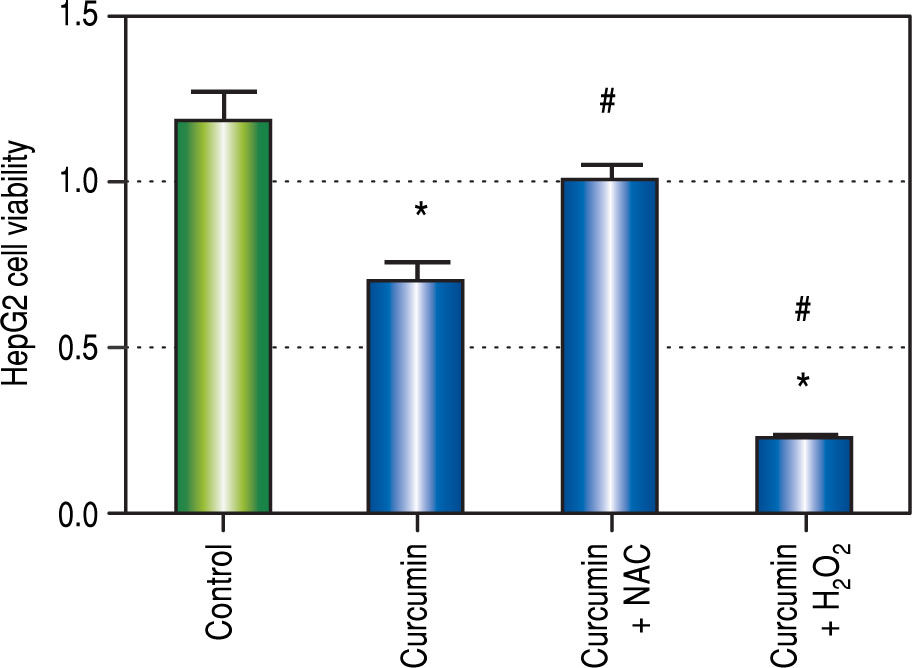
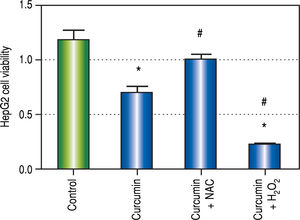
In both SOD and MDA, Cur low-dose group showed significantly better antioxidant activity when compared with Cur high-dose group. It was observed that Cur highdose had less distinct effect on decreasing oxidative stress that Cur low-dose. Also, in the in vitro experiment, treating HepG2 cells with curcumin alone (50 μM), and in combinations with NAC, or with hydrogen peroxide, showed that NAC decreased the effect of curcumin on HepG2 cells viability, while hydrogen peroxide increased that effect (Figure 12). The applied method of adding NAC and hydrogen peroxide separately to a certain drug to study and prove the involvement of ROS in its mechanism has been used to confirm that ROS can either increase or inhibit a certain effect of the drug.36-38 Results from our in vitro experiment come in agreement with a recent research article that demonstrated the involvement of ROS in the activation of autophagy using the same technique of adding a ROS initiator like hydrogen peroxide, and a ROS scavenger like NAC but in a different model.39 These two results suggest that curcumin may initially lead to increasing ROS as a part of its mechanism to induce autophagy, and activation of autophagy may then lead to decreasing ROS.
This comes in agreement with recent studies which showed that in order for curcumin to induce autophagy, it leads first to increasing ROS which in turn activates autophagy.22,40,41 Recent studies have further explained the relationship between ROS and autophagy, showing that ROS could initiate autophagosome formation, and autophagic degradation acting as cellular signaling molecules.42 Autophagy, in contrast, leads to reducing oxidative damage and ROS levels by removing protein aggregates and damaged cellular organelles such as mitochondria.43
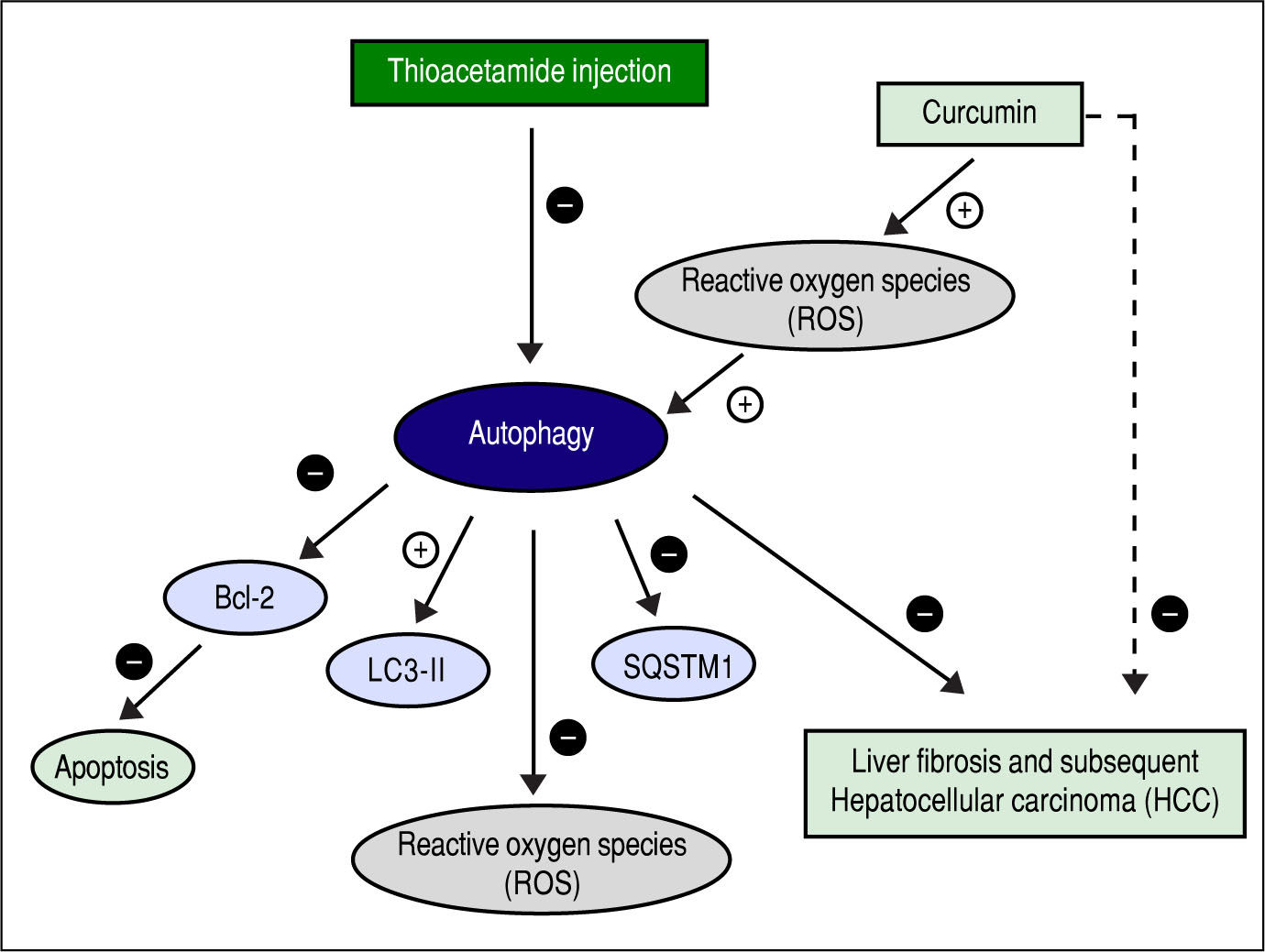
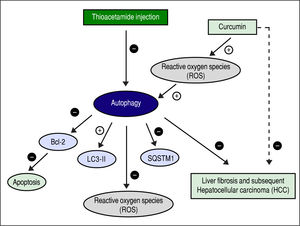
Figure 13 shows the suggested mechanism for curcumin's effect in protection and treatment of HCC. The figure shows that TAA inhibits autophagy, and that for curcumin to induce autophagy again it needs to increase the concentration of ROS first. Once ROS concentration is increased and the process of autophagy is induced, the concentration of SQSTM-1 decreases, and the gene expression of LC3-II increases as shown in the results. According to our findings, when autophagy is induced in our model, it also leads to inhibition of apoptosis which was evident by the gene expression levels of Bcl-2. Finally, when autophagy is activated, it leads to decreasing the levels of ROS which is responsible for the hepatoprotective effect of curcumin in this early stage of HCC.
Another interesting finding was that changing between different concentrations of curcumin can change the extent to which it inhibits apoptosis and activates autophagy. Curcumin is even reported to induce apoptosis in some cases as discussed before. In this study, Cur high-dose showed a slightly better liver protection than Cur low-dose despite activating autophagy to a lesser extent. This could be due to the fact that Cur high-dose group inhibited apoptosis less than Cur low-dose. This suggests that a complete inhibition of apoptosis might not be beneficial, and striking a certain balance between the two mechanisms of cell death can lead to achieving better results in HCC prevention and treatment.
ConclusionFinally, curcumin protects against fibrosis and subsequent HCCup to the first dysplastic stage of HCC by up-regulating autophagy (increasing LC3-II gene expression, and decreasing SQSTM1 concentration) and inhibiting apoptosis (increasing Bcl-2 gene expression). Cur highdose protected more against liver fibrosis by significantly blocking the increase in ALT, AST, and AFP concentrations, and significantly blocking the reduction in albumin concentration caused by TAA. Cur low-dose showed better antioxidant activities by blocking the increase in MDA concentration, and decreasing the activity of SOD. In vitro, Curcumin50 μM showed a significant decrease in HepG2 viability and in SQSTM1 concentration. These findings suggest that curcumin and other autophagy activators can be used in protection against the early stages of HCC. Studying how to manipulate autophagy levels in both early and late stages of HCC, and a better understanding of the complicated relationship between autophagy and apoptosis can lead to better cancer treatment options.
Abbreviations- •
AFP: α-fetoprotein.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
Cur High-dose: curcumin high-dose.
- •
Cur low-dose: curcumin low-dose.
- •
GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
- •
H&E: hematoxylin and eosin.
- •
HCC: hepatocellular carcinoma.
- •
LC3-II: microtubule-associated protein light chain 3-II.
- •
MDA: malondialdhyde.
- •
NAC: N-Acetyl Cysteine.
- •
PCR: real-time polymerase chain reaction.
- •
SOD: superoxide dismutase.
- •
SQSTM1: sequestosome-1.
- •
TAA: thioacetamide.