Currently, there are limited data on the epidemiology and disease characteristics of patients with chronic hepatitis C (CHC) in Latin America. The primary objective of this study was to evaluate demographic and disease characteristics of patients with CHC in Latin America.
Patients and methodsHEPLA was a non-interventional, multicenter study of the epidemiology and disease characteristics of patients with CHC in Argentina, Brazil, Chile, Colombia, and Mexico.
ResultsOf the 817 included patients, the median age was 58 years, 53.9% were female, and 39.3% had cirrhosis. Overall, 41.2% were treatment naive, 49.8% were treatment experienced, and 8.9% were currently undergoing treatment. In patients with available data, genotype 1b accounted for 41.6% of infections, followed by genotype 1a (29.9%) and genotype 3 (11.3%). Probable mode of infection was transfusion in 46.8% of patients. Liver-related comorbidities were present in 26.4% of patients and non-liver-related comorbidities were present in 72.3%. Most patients (71.8%) received concomitant medications, with proton-pump inhibitors (20.8%) being the most commonly reported.
ConclusionsAt the time the HEPLA study was carried out, the data from this cross-section of patients in Latin America showed that the CHC population has variation in disease and viral characteristics, with a minority of patients receiving treatment and many patients having advanced disease. Increased awareness and access to treatment are necessary in Latin America in order to meet the goal of hepatitis C virus elimination by 2030.
The global prevalence of hepatitis C virus (HCV) is approximately 1%, corresponding to a total of 71.1 million infected individuals, of whom approximately 3.8 million are in Latin America [1]. The estimated viremic prevalence of HCV varies markedly between Latin American countries, ranging from 0.3% in Chile up to 0.9% in Brazil [1]. However, as there are no large-scale general population studies on HCV prevalence and available data are based mainly on spontaneous-demand studies, blood donors, and specific small communities in which seroprevalence was suspected to be high, there remain knowledge gaps in the characteristics of the HCV-infected population in Latin America [2–4].
Latin America suffers a high burden of HCV disease, with the majority of HCV-infected individuals unaware of their status [5–8]. It has been suggested that Latin American patients exhibit a faster progression of disease, because studies have shown that higher histologic activity, higher fibrosis progression rates, and higher risk of cirrhosis were attributed to Latino patients compared with white and African-American patients [9–11].
The treatment for HCV has evolved rapidly with interferon (IFN)-free direct-acting antivirals (DAAs) replacing IFN-based therapy in many parts of the world. DAAs have dramatically altered the landscape of treatment for HCV by demonstrating high rates of sustained virologic response (SVR), a well-tolerated safety profile, and considerably shorter treatment durations [12].
Due to limited data on the disease characteristics of patients with chronic hepatitis C (CHC) in Latin America, more real-life data on patient and disease characteristics are needed to help medical communities and government agencies manage disease burden and develop optimal strategies for the elimination of HCV. HEPLA (A Multicenter, Observational Cohort Study on Demographic and Disease Characteristics of Patients Seeking Care for Chronic Hepatitis C in Latin America) was initiated to further evaluate demographic and disease characteristics of patients with CHC in Latin America.
2Materials and methodsHEPLA is a non-interventional study of the epidemiology and characteristics of patients with CHC using patient chart data. The study was conducted at 30 sites in Argentina, Brazil, Chile, Colombia, and Mexico. The research sites were medical centers experienced in the treatment of CHC. The first patient visit was on August 20, 2014 and last patient visit was on December 29, 2015.
2.1PatientsPatients eligible for the study were at least 18 years of age with a current diagnosis of CHC. Both HCV treatment-naive and HCV treatment-experienced patients as well as patients currently on anti-HCV treatment were eligible to participate in the study. Treatment decisions were at the discretion of the physician. Patients were excluded if they were participating in any concurrent interventional clinical trial. Patients were identified consecutively and invited to participate. Those who were eligible and provided written consent were enrolled into the study until the target sample size was reached. Enrollment caps were applied at individual sites to ensure a real-life patient representation. For each patient, the study consisted of one visit only. The study was designed and conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki and applicable local regulations. All authors had access to study data and participated in analysis of the results.
A target study size of 1000 patients was established in order to generate two-sided 95% confidence intervals not greater than 6.2% for a proportion of 50% (e.g., the proportion of females). The study had 817 patients with evaluable data, providing sufficient power to generate two-sided 95% confidence intervals not greater than 6.8% for a 50% proportion.
2.2Reported dataThe following data were collected from medical records for each patient at the study sites, whenever available:
- •
Demographics: age, sex, race/ethnic origin, height, body weight, and IL28B single nucleotide polymorphisms genotype (rs12979860)
- •
HCV disease characteristics: estimated year and mode of infection, HCV genotype and subtype, liver fibrosis stage, most recent biopsy results, most recent FibroScan results, Child–Pugh score, and the presence or absence of esophageal varices
- •
Comorbidities: co-infection with HIV or hepatitis B virus, hepatitis A virus, liver-related comorbidities, and other extrahepatic conditions
- •
Concomitant medications: any medication at time of inclusion into study
- •
CHC treatment status: HCV treatment naive, HCV treatment experienced, or currently on anti-HCV treatment
- •
Clinical status: laboratory assessments (e.g. HCV RNA viral load, clinical chemistry, and hematology evaluations)
For the patients that were on treatment at the time of the single study visit, the frequency of each antiviral medication and combination regimen was documented. Dual therapy was defined as taking pegylated IFN- (pegIFN-) or IFN-alpha and ribavirin (RBV), whereas triple therapy was defined as taking pegIFN-or IFN-alpha and RBV plus one DAA. All other therapies were summarized as “other therapy.”
The presence and degree of hepatic fibrosis were assessed by the following methods: clinical/best guess only (in the clinical opinion of the physician), non-invasive only, biopsy only, or a combination of two or more of those methods.
2.3Statistical analysesThe HEPLA study was exploratory in nature; no hypotheses or primary endpoints were pre-specified. Descriptive methods were used to summarize the data collected from source documents. No imputation methods were used to replace missing values. All statistical analyses were carried out using SAS software (SAS Institute; Version 9.4).
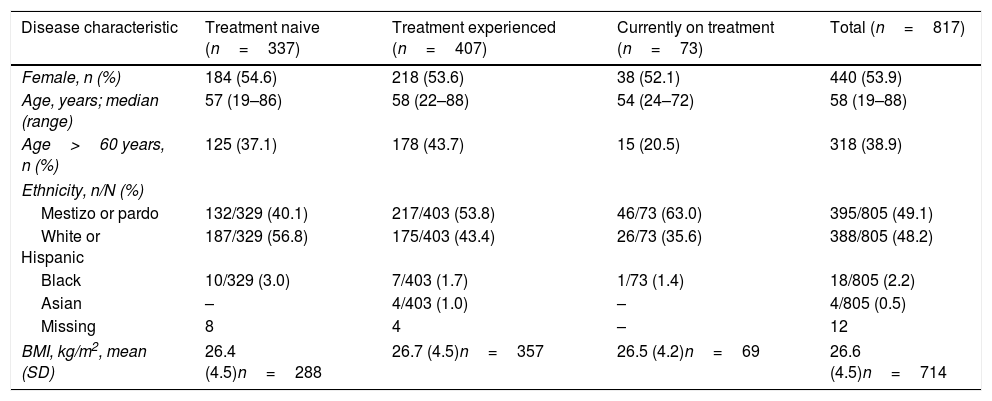
3ResultsA total of 850 patients were enrolled, and 817 were available for analysis. A total of 33 patients were excluded because they did not fulfill the selection criteria: 32 patients did not have proof to support a diagnosis of CHC and 1 patient was participating in a concurrent interventional therapeutic trial. Patient demographic data are shown in Table 1 and HCV disease characteristics are shown in Table 2. Of the 817 evaluable patients, 337 were HCV treatment naive (41.2%), 407 were HCV treatment experienced (49.8%), and 73 were currently undergoing anti-HCV treatment (8.9%) at the time of the single study visit (Table 1). Nearly all patients described themselves as either mestizo/pardo or white/Hispanic, with a small number of patients listed as black, Asian, or having missing data (Table 1).
Demographic data by current treatment status.
| Disease characteristic | Treatment naive (n=337) | Treatment experienced (n=407) | Currently on treatment (n=73) | Total (n=817) |
|---|---|---|---|---|
| Female, n (%) | 184 (54.6) | 218 (53.6) | 38 (52.1) | 440 (53.9) |
| Age, years; median (range) | 57 (19–86) | 58 (22–88) | 54 (24–72) | 58 (19–88) |
| Age>60 years, n (%) | 125 (37.1) | 178 (43.7) | 15 (20.5) | 318 (38.9) |
| Ethnicity, n/N (%) | ||||
| Mestizo or pardo | 132/329 (40.1) | 217/403 (53.8) | 46/73 (63.0) | 395/805 (49.1) |
| White or Hispanic | 187/329 (56.8) | 175/403 (43.4) | 26/73 (35.6) | 388/805 (48.2) |
| Black | 10/329 (3.0) | 7/403 (1.7) | 1/73 (1.4) | 18/805 (2.2) |
| Asian | – | 4/403 (1.0) | – | 4/805 (0.5) |
| Missing | 8 | 4 | – | 12 |
| BMI, kg/m2, mean (SD) | 26.4 (4.5)n=288 | 26.7 (4.5)n=357 | 26.5 (4.2)n=69 | 26.6 (4.5)n=714 |
BMI, body mass index; SD, standard deviation.
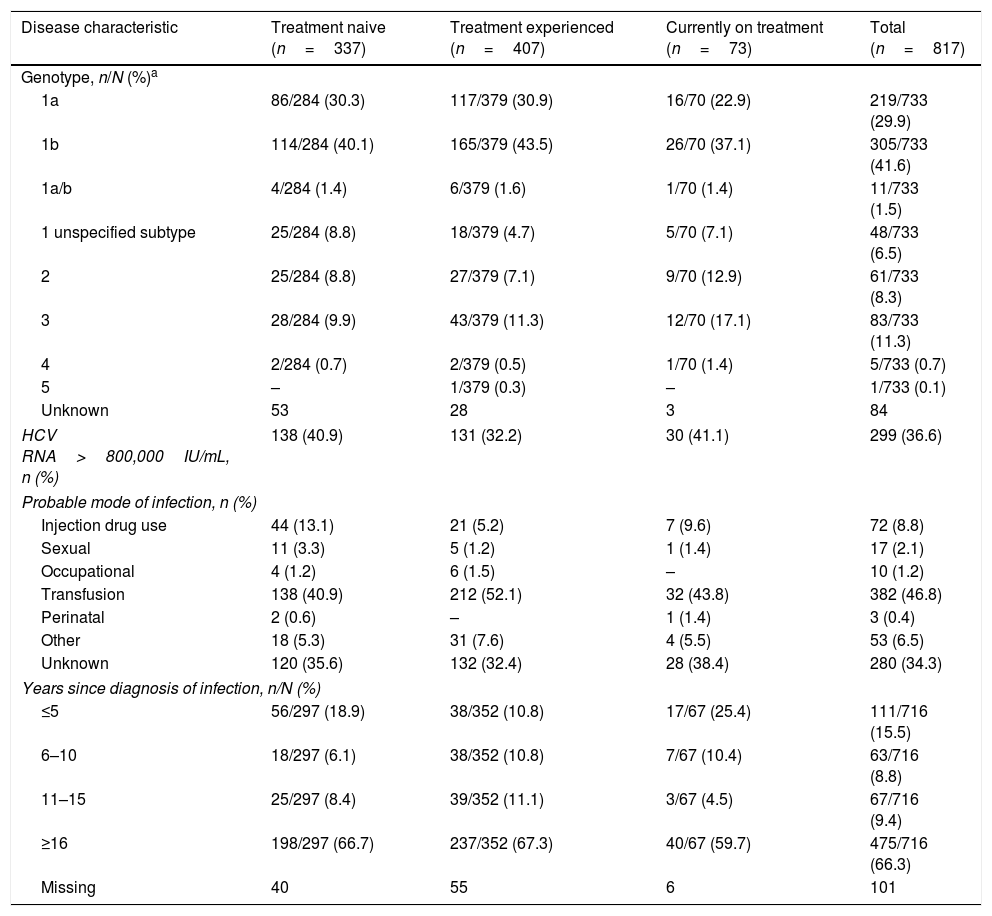
HCV disease characteristics by current treatment status.
| Disease characteristic | Treatment naive (n=337) | Treatment experienced (n=407) | Currently on treatment (n=73) | Total (n=817) |
|---|---|---|---|---|
| Genotype, n/N (%)a | ||||
| 1a | 86/284 (30.3) | 117/379 (30.9) | 16/70 (22.9) | 219/733 (29.9) |
| 1b | 114/284 (40.1) | 165/379 (43.5) | 26/70 (37.1) | 305/733 (41.6) |
| 1a/b | 4/284 (1.4) | 6/379 (1.6) | 1/70 (1.4) | 11/733 (1.5) |
| 1 unspecified subtype | 25/284 (8.8) | 18/379 (4.7) | 5/70 (7.1) | 48/733 (6.5) |
| 2 | 25/284 (8.8) | 27/379 (7.1) | 9/70 (12.9) | 61/733 (8.3) |
| 3 | 28/284 (9.9) | 43/379 (11.3) | 12/70 (17.1) | 83/733 (11.3) |
| 4 | 2/284 (0.7) | 2/379 (0.5) | 1/70 (1.4) | 5/733 (0.7) |
| 5 | – | 1/379 (0.3) | – | 1/733 (0.1) |
| Unknown | 53 | 28 | 3 | 84 |
| HCV RNA>800,000IU/mL, n (%) | 138 (40.9) | 131 (32.2) | 30 (41.1) | 299 (36.6) |
| Probable mode of infection, n (%) | ||||
| Injection drug use | 44 (13.1) | 21 (5.2) | 7 (9.6) | 72 (8.8) |
| Sexual | 11 (3.3) | 5 (1.2) | 1 (1.4) | 17 (2.1) |
| Occupational | 4 (1.2) | 6 (1.5) | – | 10 (1.2) |
| Transfusion | 138 (40.9) | 212 (52.1) | 32 (43.8) | 382 (46.8) |
| Perinatal | 2 (0.6) | – | 1 (1.4) | 3 (0.4) |
| Other | 18 (5.3) | 31 (7.6) | 4 (5.5) | 53 (6.5) |
| Unknown | 120 (35.6) | 132 (32.4) | 28 (38.4) | 280 (34.3) |
| Years since diagnosis of infection, n/N (%) | ||||
| ≤5 | 56/297 (18.9) | 38/352 (10.8) | 17/67 (25.4) | 111/716 (15.5) |
| 6–10 | 18/297 (6.1) | 38/352 (10.8) | 7/67 (10.4) | 63/716 (8.8) |
| 11–15 | 25/297 (8.4) | 39/352 (11.1) | 3/67 (4.5) | 67/716 (9.4) |
| ≥16 | 198/297 (66.7) | 237/352 (67.3) | 40/67 (59.7) | 475/716 (66.3) |
| Missing | 40 | 55 | 6 | 101 |
Of the 733 patients with known HCV genotype data, 583 (79.5%) were infected with HCV GT1 (Table 2), including 219 patients (29.9%) infected with GT1a, 305 (41.6%) infected with GT1b, 11 (1.5%) infected with mixed GT1a/b, and 48 (6.5%) infected with unspecified GT1. Overall, GT3 accounted for 11.3% (n=83) of HCV infections, with a further 8.2% (n=67) documented to be infected with GT2, GT4, or GT5. Patients currently on treatment were numerically more likely to be infected with GT3 infection compared with treatment-naïve or treatment-experienced patients (Table 2).
Among patients with a documented probable mode of infection, transfusion accounted for 46.8% (n=382) of patients and injection drug use accounted for 8.8% (n=72) of patients (Table 2). An exploratory analysis looking at differences between treatment-status groups showed that the mode of infection differed between the treatment groups (Kruskal–Wallis test p=0.0006, excluding patients with unknown mode of infection), with injection drug use more frequently cited as cause of infection among treatment-naïve patients.
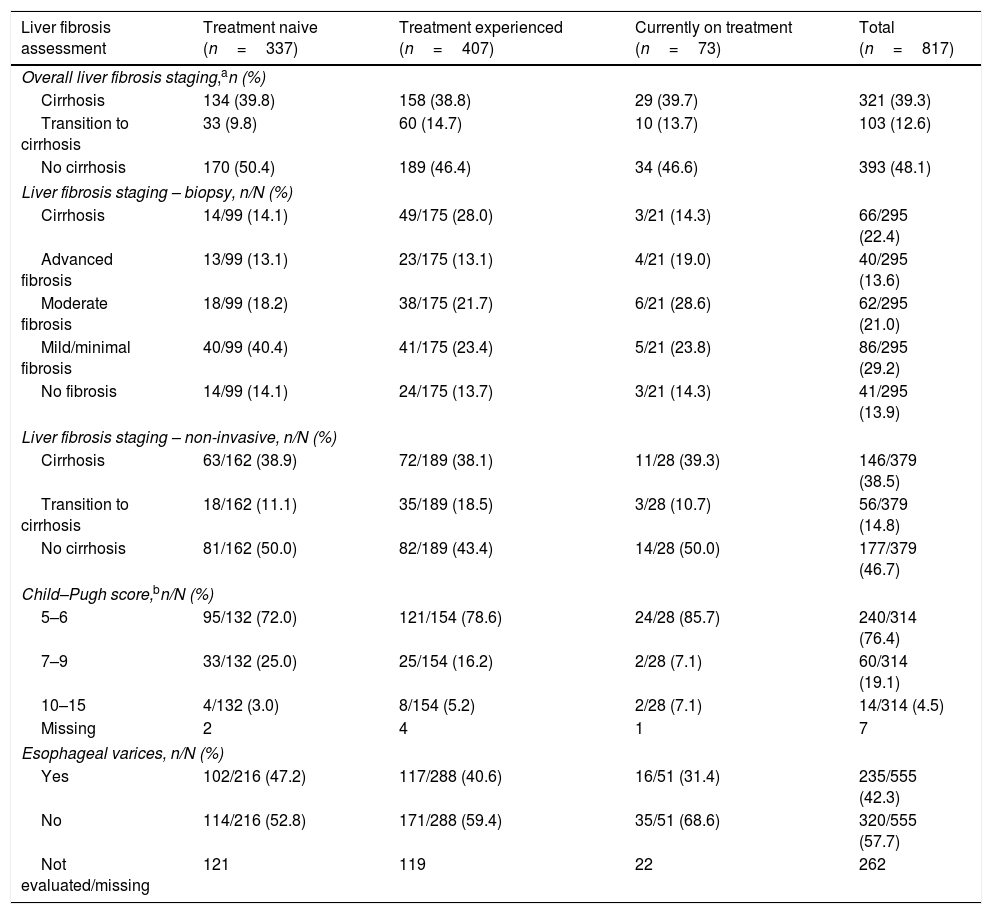
In the overall liver fibrosis assessment, the percentage of patients without cirrhosis, with transition to cirrhosis, and with cirrhosis was 48.1%, 12.6%, and 39.3%, respectively (Table 3). Among patients with cirrhosis with a reported Child–Pugh score (n=314), 240 (76.4%) were classified as being Child–Pugh A, 60 (19.1%) were classified as being Child–Pugh B, and 14 (4.5%) were classified as being Child–Pugh C (Table 3).
Liver fibrosis assessments by current treatment status.
| Liver fibrosis assessment | Treatment naive (n=337) | Treatment experienced (n=407) | Currently on treatment (n=73) | Total (n=817) |
|---|---|---|---|---|
| Overall liver fibrosis staging,an (%) | ||||
| Cirrhosis | 134 (39.8) | 158 (38.8) | 29 (39.7) | 321 (39.3) |
| Transition to cirrhosis | 33 (9.8) | 60 (14.7) | 10 (13.7) | 103 (12.6) |
| No cirrhosis | 170 (50.4) | 189 (46.4) | 34 (46.6) | 393 (48.1) |
| Liver fibrosis staging – biopsy, n/N (%) | ||||
| Cirrhosis | 14/99 (14.1) | 49/175 (28.0) | 3/21 (14.3) | 66/295 (22.4) |
| Advanced fibrosis | 13/99 (13.1) | 23/175 (13.1) | 4/21 (19.0) | 40/295 (13.6) |
| Moderate fibrosis | 18/99 (18.2) | 38/175 (21.7) | 6/21 (28.6) | 62/295 (21.0) |
| Mild/minimal fibrosis | 40/99 (40.4) | 41/175 (23.4) | 5/21 (23.8) | 86/295 (29.2) |
| No fibrosis | 14/99 (14.1) | 24/175 (13.7) | 3/21 (14.3) | 41/295 (13.9) |
| Liver fibrosis staging – non-invasive, n/N (%) | ||||
| Cirrhosis | 63/162 (38.9) | 72/189 (38.1) | 11/28 (39.3) | 146/379 (38.5) |
| Transition to cirrhosis | 18/162 (11.1) | 35/189 (18.5) | 3/28 (10.7) | 56/379 (14.8) |
| No cirrhosis | 81/162 (50.0) | 82/189 (43.4) | 14/28 (50.0) | 177/379 (46.7) |
| Child–Pugh score,bn/N (%) | ||||
| 5–6 | 95/132 (72.0) | 121/154 (78.6) | 24/28 (85.7) | 240/314 (76.4) |
| 7–9 | 33/132 (25.0) | 25/154 (16.2) | 2/28 (7.1) | 60/314 (19.1) |
| 10–15 | 4/132 (3.0) | 8/154 (5.2) | 2/28 (7.1) | 14/314 (4.5) |
| Missing | 2 | 4 | 1 | 7 |
| Esophageal varices, n/N (%) | ||||
| Yes | 102/216 (47.2) | 117/288 (40.6) | 16/51 (31.4) | 235/555 (42.3) |
| No | 114/216 (52.8) | 171/288 (59.4) | 35/51 (68.6) | 320/555 (57.7) |
| Not evaluated/missing | 121 | 119 | 22 | 262 |
Overall, liver- and/or CHC-related comorbidities were present in 26.4% (n=216) of patients, with a numerically smaller proportion occurring in those currently on treatment. Non-liver comorbidities were common, with 32.7% (n=267) of patients having a diagnosis of cardiovascular disease, 16.0% (n=131) with diabetes mellitus, and 10.3% (n=84) with psychiatric disorders. In addition, 5.6% (n=46) of patients suffered from chronic kidney disease, of whom 10 were on dialysis.
The majority of patients (71.8% [n=587]) received concomitant medication other than for CHC. The concomitant medications most frequently taken were proton-pump inhibitors (PPIs) (20.9%), followed by propranolol (15.5%). Overall, there was no difference between the use of concomitant medications in patients not receiving CHC therapy versus patients receiving CHC therapy. However, some concomitant medications were taken at a lower frequency among patients currently on treatment compared with treatment-naive patients such as omeprazole, propranolol, spironolactone, losartan, levothyroxine, metformin, and furosemide.
4DiscussionThe HEPLA study was a large, Latin American, multicenter, non-interventional, epidemiologic, cross-sectional study and was designed to describe the demographic and disease characteristics of patients with CHC receiving medical care, predominantly in specialized centers in Latin America.
Among patients with known HCV genotype data, almost 80% were infected with HCV GT1, primarily GT1b, with fewer patients infected with other genotypes. These data are broadly consistent and comparable with previous findings, with GT1 accounting for approximately 60% of cases or greater [1,13,14].
Nearly half of the patients in this study had transfusion as the probable mode of infection, which is consistent with historical data in Latin America [3]. A systematic review of the prevalence of HCV in high-risk populations in Latin America and the Caribbean estimated the pooled regional prevalence of anti-HCV antibodies to be 49% in injection drug users, with significant regional variation [15]. In this study, the probable mode of HCV infection was injection drug use in 9% of participants, though for a large proportion of patients (34%), the mode of infection was unknown.
There are limited data on comorbidities and concomitant drug use in Latin America. In this study, liver-related comorbidities were present in 26.4% of patients while 72.3% of patients suffered from comorbidities not related to the liver. Cardiovascular disease was the most common class of non-liver comorbidity. Nearly three-quarters of the patients in this study were receiving concomitant medications other than for treatment of CHC. The most frequently taken concomitant medication was omeprazole and, overall, PPIs were taken by over 20% of patients. Consistent with the high rate of cardiovascular disease in the population, propranolol was the next most common concomitant drug whereas other beta-blockers such as atenolol, carvedilol, and metoprolol were taken, but at a lower rate.
According to the overall liver fibrosis assessment, approximately half of the patients in this study had cirrhosis or advanced fibrosis (stage F3), and approximately 25% of those with cirrhosis were classified as Child–Pugh B or C. Rates of cirrhosis of >50% have been reported in other Latin American cohorts, including up to 91% [13,14,16]. In this study, nearly 60% of patients were on treatment or treatment-experienced to HCV therapy, though less than 10% overall were currently on treatment. Other real-world Latin American cohorts have reported populations to be between 40% and 50% treatment experienced [13,14,16], suggesting an increase in treatment-naïve patients seeking treatment.
These data represent patients treated in the years 2014 and 2015, and HCV treatment in Latin America has evolved since then. In Mexico, IFN-free therapy has been recommended for all patients since treatment guidelines were updated in July of 2018, although only patients with high risk of morbidity and mortality have an expedited or priority treatment recommendation [17]. In Brazil, although government treatment guidelines recommending IFN-free therapy were implemented earlier, patients without advanced fibrosis/cirrhosis were included in the recommendations only recently [18]. Argentina also has treatment guidelines recommending the use of IFN-free DAAs [19] and a national healthcare program providing HCV care; however, as in other countries in Latin America, a low number of patients have been treated. Therefore, despite these few advances in Latin America, many challenges remain in improving access to treatment, including incomplete epidemiologic data, inadequate awareness and screening, political barriers, limitations of healthcare providers, and lack of knowledge of region-specific risk factors [20,21].
Although HEPLA provides valuable insights, there are some limitations to this study. As it was conducted over five different Latin American countries, there may be variability in how data were reported. For example, ethnicity was self-reported and certain ethnic terms may have different meanings in different countries or regions. Furthermore, as all the centers were in urban areas, patient, viral, and disease characteristics reported here may differ from those of rural areas. A further limitation is that these data represent a “snapshot” of each patient at a single visit with no follow-up. Finally, as participants had to have current CHC, those who were previously successfully treated for HCV were not eligible for the study and thus a description of those cured of HCV is not available.
The HEPLA study showed that the Latin American CHC population is heterogeneous in terms of disease and viral characteristics, with a high rate of advanced liver disease and a low rate of treatment, which must be improved to meet the goal of HCV elimination by 2030.AbbreviationsHCV hepatitis C virus genotype interferon direct-acting antiviral sustained virologic response chronic hepatitis C A Multicenter, Observational Cohort Study on Demographic and Disease Characteristics of Patients Seeking Care for Chronic Hepatitis C in Latin America human immunodeficiency virus pegylated interferon ribavirin proton-pump inhibitor
AbbVie provided funding for this study and participated in design, research, data collection, interpretation of data, and writing, reviewing, and approving the publication.
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Conflict of interestThe authors have no conflicts of interest to declare.
Medical writing assistance was provided by Anna Bacon and Scott Battle of Medical Expressions, funded by AbbVie.