Hepatitis C virus (HCV) and human T-cell lymphotropic virus type 1 (HTLV-1) infections have chronic courses. HCV is primarily transmitted via the hematogenous route, whereas HTLV-1 is primarily transmitted sexually, although it can also be transmitted by blood. Individuals chronically infected with either HTLV-1 or HCV can differ in terms of behavioral characteristics and personality traits. This study compared the occurrence of risk behaviors and impulsivity aspects between HCV and HTLV-1 carriers.
Materials and methodsObservational, comparative and cross-sectional study that involved a sample of outpatients who had HCV or HLTV-1, by way of a sociodemographic and behavioral questionnaire and the Barratt Impulsiveness Scale – BIS-11. 143 individuals with HCV and 113 individuals with HTLV-1 were evaluated.
ResultsThere was a difference with regards to gender among patients, with mostly males affected in the HCV group. Risk behaviors commonly mediated by impulsiveness were significantly more frequent in the HCV group. Similarly, overall impulsiveness and domain nonplanning were higher in the HCV group. Multivariate analysis showed that increased age, male gender, higher nonplanning scores and HCV infection were independent factors for the occurrence of risk behaviors. Both groups presented high rates of other sexually transmitted diseases and a low rate of condom use in sexual relations.
ConclusionsThis study confirms the higher rate of risk behaviors and the levels of impulsiveness commonly observed in patients with HCV, along with comparisons to patients with HTLV-1.
The total worldwide prevalence of anti-HCV antibodies is estimated to be approximately 1.6% (1.3–2.1%), which corresponds to 115 (92–149) million infected individuals. The majority of these infections, approximately 104 (87–124) million, occur in individuals older than 15 years [1]. Approximately 400 thousand people die annually from hepatitis C virus (HCV), mainly due to cirrhosis and hepatocellular carcinoma; many other people require liver transplantation [2]. In Brazil, the prevalence of chronic infection in people aged 10 to 69 years is 1.38% [3]; the majority of infected individuals are men, with a gender ratio (M:F) of approximately 1.3:1 [4].
HCV transmission mainly occurs via the hematogenous route. People at the greatest risk of infection are those with a history of multiple blood exposures, such as intravenous drug users (IVDUs) or even users of inhaled drugs [5]. HCV infection is commonly associated with risk behaviors, such as the use of injection or inhaled drugs, having multiple sexual partners, having unprotected sex, and sexual activity with sex workers [6].
Human T-cell lymphotropic virus type 1 (HTLV-1) is regarded as endemic in several regions of the world. In Brazil, it is estimated that approximately 2.5 million people are infected, with a mean prevalence of 0.41%. This can be higher in certain cities, as is the case of Salvador, Bahia, where the rate is 1.35–1.76% [7–9]. The most alarming conditions among those who develop the disease are neurological sequelae, such as HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) and adult T cell leukemia/lymphoma (ATL) [8]. This infection is generally associated with low morbidity; more than 90% of infected individuals will remain asymptomatic and therefore unaware of their seropositive status, thus establishing a sustained pathway of silent transmission [9].
HTLV-1 transmission may occur via blood transfusions, sexual contact, vertical transmission (notably through breastfeeding) and the sharing of needles among IVDUs. Certain groups may be at greater risk, such as immigrants from endemic areas, sexual partners and offspring of infected individuals, sex workers and drug users [8,9].
Impulsiveness plays an important role in mediating risk behaviors [6,10] and may predispose individuals to adverse outcomes such as HCV or HTLV-1 infections. Impulsiveness is a complex construct associated with a rapid manifestation of a behavior not planned in advance, stemming from a sudden thought, with a strong likelihood of the individual's inability to resist the need to carry out the action [11]. It can be a reaction to internal or external stimuli, and the individual does not measure the necessary consequences of this reaction to him/herself or to others [12].
Impulsiveness usually has a social connotation of a prejudicial and negative nature and has been associated with such deviant behaviors as aggression, suicide attempts, disturbing drug consumption and other risk behaviors. It is therefore considered to be a personality trait of substantial social relevance [13,14]. Several psychiatric disorders, such as bipolar disorder, attention deficit hyperactivity disorder, substance use disorders and personality disorders (borderline and antisocial), commonly progress with elevated levels of impulsiveness [11,15].
The objective of this study was to evaluate and compare the frequency of engagement in risk behaviors and impulsiveness measures between groups of HCV-infected individuals and HTLV-1-infected individuals in the city of Salvador, Brazil. The importance of comparing these two groups of patients stems from the fact that both viruses are associated with risk behaviors; moreover, previous studies have reported that patients with HCV have higher levels of impulsiveness compared to other populations, including those infected with hepatitis B virus (HBV) [6,14,16,17]. However, it is unknown whether this pattern of behavior is comparable to or different from those with HTLV-1. Inserting HTLV-1 in this differentiation broadens comparisons of these aspects between HCV and other viruses, considering the uniqueness of comparison between these two viruses. On the other hand, it includes the evaluation of important aspects of human behavior in the context of HTLV-1 infection, which is widely neglected from the scientific and medical points of view and from health policies; even more so when compared with infection by HCV, which relies on more robust policies and funding. Understanding the patterns of these 2 groups with regard to risk behaviors and impulsiveness patterns may be of help in preparing better clinical approaches and in the development of more effective public policies in order to interrupt the transmission network for each of these pathogens.
Motivated by the results of high levels of impulsiveness among HCV-infected individuals in previous studies, it was hypothesized that this variable might also be more elevated in this group when compared with individuals infected by HTLV-1.
2Material and methods2.1Study designThis study employed a quantitative, observational and comparative cross-sectional design in order to compare aspects of impulsiveness and risk behavior engagement among HCV- or HTLV-1-infected individuals receiving follow-up care at the University Hospital Complex Professor Edgard Santos – Com-HUPES (Federal University of Bahia). Data collection was carried out between 2010 and 2014.
2.2Ethical considerationsThis study was approved by the Research Ethics Committee of the Maternity Hospital Climério de Oliveira at the Federal University of Bahia (MCO-UFBA – process number 14/2002) and is in accordance with the Declaration of Helsinki on human research, version 2013. All participating patients read and signed appropriate informed consent forms.
2.3SubjectsAll individuals infected by HCV or HTLV-1 who received assistance at the referred health services and who were identified by the research group were invited to participate in the study. A convenience sample of 171 HCV-infected individuals and 119 HTLV-1-infected individuals was selected to participate in this study, and the final sample population was determined based on various inclusion and exclusion criteria.
2.3.1Group 1: HCV-infected individuals171 HCV-infected patients who were treated at Com-HUPES. Inclusion criteria: (a) age greater than 18 years; (b) diagnosis of chronic HCV infection, as determined by a positive anti-HCV test by enzyme-linked immunosorbent assay III and confirmed by qualitative determination of the presence of HCV RNA. Exclusion criteria: (a) coinfection with HBV (8 patients excluded), human immunodeficiency virus (HIV) (2 patients excluded) or HTLV-1 (1 patient excluded); (b) current treatment with interferon alpha – considering that this drug is strongly associated with psychiatric changes and that it could modify the levels of the subjects’ impulsiveness and behavior patterns, thus acting as a confounding factor (17 patients excluded); (c) missing data (2 patients excluded). Ultimately, the HCV-infected population comprised 141 subjects. In this group, there were 89 (63.1%) patients on the waiting list for liver transplantation and 52 (36.9%) who did not have indications for hepatic transplantation (e.g., controlled infection without expressive damage or had contraindication to this procedure; of these, only 14.3% presented, upon liver biopsy, the absence of inflammatory activity and no fibrosis by way of the METAVIR classification, besides showing transaminases at insignificantly elevated levels) at the moment of the interview.
2.3.2Group 2: HTLV-1 infected individuals119 HTLV-1-infected patients treated at Com-HUPES. Inclusion criteria: (a) age greater than 18 years; (b) infection with HTLV-1, as determined by serological diagnosis performed by ELISA (Cambridge Biotech Corp., Worcester, MA, USA) and confirmed by Western Blot (HTLV blot 2.4, Genelabs, Singapore). Exclusion criteria: (a) coinfection with HCV (5 patients excluded), HBV (1 patient excluded) or HIV (0 patients excluded); (b) missing data (0 patients excluded). Ultimately, the HTLV-1-infected population comprised 113 subjects. In this group, there were 43 (38.1%) asymptomatic patients, 36 (31.9%) patients with an overactive bladder and 34 (30.1%) with HAM/TSP.
2.4Study instruments(a) Sociodemographic questionnaire: designed by the research team itself, it includes questions regarding sociodemographic data and several risk behaviors throughout life (e.g., sex with a sex worker, having more than 3 sexual partners in the previous year, use of inhaled or injection drugs and use of condoms during sexual activity).
(b) Barratt Impulsiveness Scale (BIS-11): validated Brazilian version [18] of the 30-item (4-point Likert-type scale) self-evaluation scale used to measure total impulsiveness (TI) and 3 impulsiveness domains: (a) attentional impulsiveness (AI): difficulty related to concentration or paying attention; (b) motor impulsiveness (MI): spur-of-the-moment reactions and/or restlessness, related to nonrestraint of incoherent responses to context demands; (c) nonplanning impulsiveness (NP): orientation directed to the present instead of the future [19,20]. BIS-11 is one of the most important instruments used to measure impulsiveness and has been widely employed in scientific studies, thus allowing for the comparison with several other studies.
Interviews were carried out by researchers properly trained on the application of the study instruments. The patients were identified by the outpatient clinic staff and referred to the research group, at which time they underwent application of the instruments.
2.5Data analysisThe data were analyzed using the statistical software R (R Development Core Team, 2011). Categorical variables were shown as frequencies and percentages; variables with normal distribution were represented by means and standard deviations. Normality was defined through graphical analysis and the Shapiro-Wilk test. Since the data had a normal distribution, the bivariate comparisons were conducted using Student's t-test. For categorical variables, the Chi-squared test or Fisher's test was used when necessary. The tests were performed with a significance level of p<0.05.
Variables were considered for multivariate analysis according to the biological plausibility reported in the literature to the work's main hypothesis and/or when the bivariate tests showed values of p<0.25, as per the algorithm proposed by Hosmer and Lemeshow (2000) [21]. An analysis of hierarchical logistic regression was conducted, sequentially to evaluate the power of the variables related to impulsiveness and HCV infection through the biologic plausibility model to increase the predictive power of the model in relation to the sociodemographic aspects, such as the initial independent variables. For the multivariate analysis, the model quality was evaluated by the Akaike Information Criteria (AIC), Nagelkerke R², Omnibus test for coefficients and the Hosmer-Lemeshow test.
3ResultsWith regard to sociodemographic data, there was a higher number of male individuals in the HCV group (68.8% vs. 28.3%; p<0.001). In both groups, most of the infected individuals were married or in a stable relationship (58.4% HTLV-1 and 66% HCV) (Table 1).
Comparison of sociodemographic aspects among HCV-infected and HTLV-1-infected groups.
| Total | HTLV-1 | HCV | p Value | |
|---|---|---|---|---|
| (n=254) | (n=113) | (n=141) | ||
| Agea | 56.0 (49.0–63.0) | 55.0 (45.0–64.0) | 57.0 (51.0–63.0) | 0.165 |
| Male gender | 129 (50.8) | 32 (28.3) | 97 (68.8) | <0.001 |
| Marital status | 0.132 | |||
| Single | 41 (16.1) | 24 (21.2) | 17 (12.1) | |
| Married/stable relationship | 159 (62.6) | 66 (58.4) | 93 (66.0) | |
| Divorced | 33 (13.0) | 12 (10.6) | 21 (14.9) | |
| Widower | 20 (7.9) | 11 (9.7) | 9 (6.4) | |
All data are presented as n (%).
The patients were evaluated with regard to some risk behaviors linked to sexual habits and drug use. The rates considering the behaviors were higher in the HCV group (p<0.05), either in combination or separately (i.e., sex with a sex worker at least once in their life, having more than 3 sex partners in the previous year and use of injection or inhaled drugs at least once in their life) (Table 2).
Comparison of risk behaviors and associated negative outcomes between HCV-infected and HTLV-1-infected groups.
| Total | HTLV-1 | HCV | p Value | |
|---|---|---|---|---|
| (n=254) | (n=113) | (n=141) | ||
| Risk behaviors | 96 (37.8) | 18 (15.9) | 78 (55.3) | <0.001 |
| Sexual | ||||
| Sex with sex worker | 75 (29.5) | 14 (12.4) | 61 (43.3) | <0.001 |
| More than 3 sexual partners in the last year | 23 (9.1) | 4 (3.5) | 19 (13.5) | 0.011 |
| Drug Use | ||||
| Injection drug | 20 (7.9) | 1 (0.9) | 19 (13.5) | 0.001 |
| Inhaled drug | 27 (10.6) | 2 (1.8) | 25 (17.7) | <0.001 |
| Condom use | 0.723 | |||
| Always | 69 (27.2) | 31 (27.4) | 38 (27.0) | |
| Almost Always | 27 (10.6) | 10 (8.8) | 17 (12.1) | |
| Rarely | 41 (16.1) | 16 (14.2) | 25 (17.7) | |
| Never | 112 (44.1) | 55 (48.7) | 57 (40.4) | |
| Sexually transmitted diseases (STDs) | 64 (25.2) | 26 (23.0) | 38 (26.9) | 0.778 |
All data are presented as n (%).
We did not find any statistically significant difference related to the incidence of sexually transmitted diseases (STD). Furthermore, there were no significant differences with respect to the use of condoms during sexual activities. We highlight the finding that only approximately 27% of the subjects used condoms in all sexual activities and that 40.4% (HCV) and 48.7% (HTLV-1) never used this important element of health protection against STDs and other negative outcomes (Table 2).
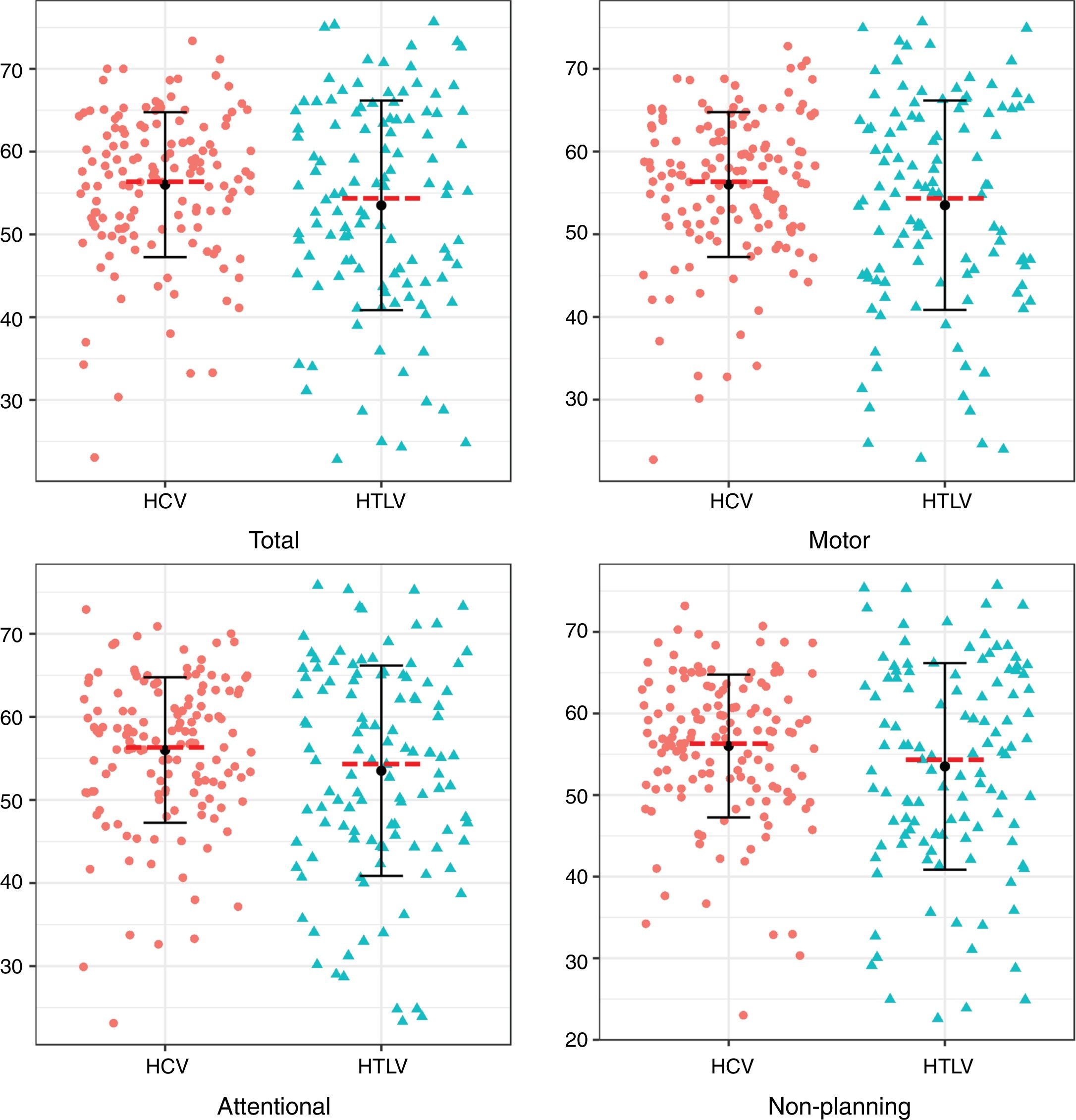
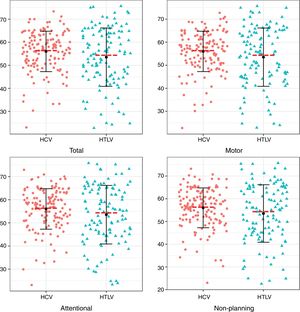
Regarding the impulsiveness data, we verified that there were significantly higher levels among patients with HCV (p<0.05) in the NP domain and in the total impulsiveness score according to the BIS-11 (Table 3). Less dispersion was identified among patients with HCV in the TI and in all domains together (NP, MI and AI) (Fig. 1).
Comparison of impulsiveness based on BIS-11 scale between HCV-infected and HTLV-1-infected groups.
| Total | HTLV-1 | HCV | p Value | |
|---|---|---|---|---|
| (n=254) | (n=113) | (n=141) | ||
| Impulsiveness (BIS-11)a | ||||
| Nonplanning | 21.5 (18.0–26.0) | 20.0 (17.0–24.0) | 23.0 (18.0–27.0) | 0.002 |
| Motor | 19.0 (16.0–22.0) | 18.0 (16.0–21.0) | 19.0 (15.0–22.0) | 0.450 |
| Attentional | 18.0 (16.0–21.0) | 18.0 (15.0–20.0) | 18.0 (16.8–21.0) | 0.130 |
| Total | 58.0 (52.0–66.0) | 56.0 (51.0–64.0) | 60.5 (53.0–68.0) | 0.006 |
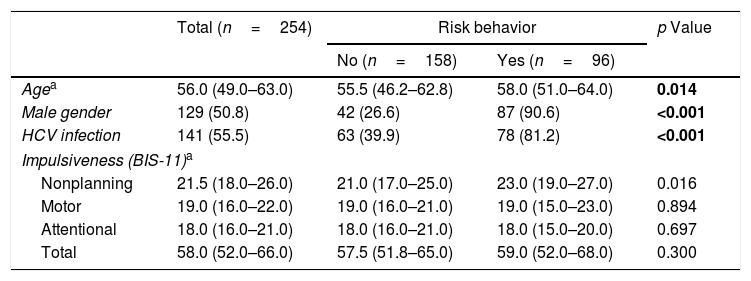
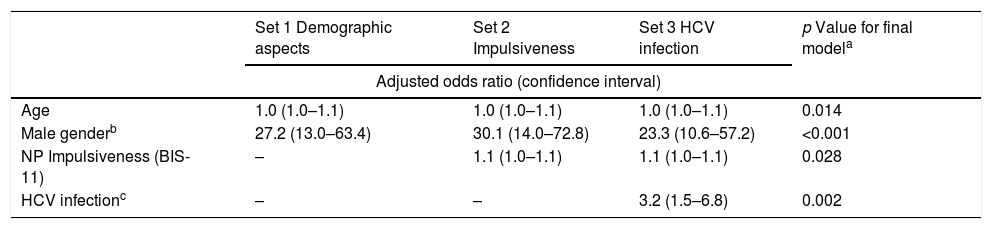
Through bivariate analysis as well as multivariate analysis (hierarchical logistic regression), several variables were shown to be associated with risk behaviors (dichotomous variable): (a) more advanced age; (b) male gender; (c) presenting higher levels of the NP domain of impulsiveness (BIS-11); and (d) infection with HCV (Tables 4 and 5).
Sociodemographic data, type of infection and impulsiveness – risk behavior as a dichotomous variable.
| Total (n=254) | Risk behavior | p Value | ||
|---|---|---|---|---|
| No (n=158) | Yes (n=96) | |||
| Agea | 56.0 (49.0–63.0) | 55.5 (46.2–62.8) | 58.0 (51.0–64.0) | 0.014 |
| Male gender | 129 (50.8) | 42 (26.6) | 87 (90.6) | <0.001 |
| HCV infection | 141 (55.5) | 63 (39.9) | 78 (81.2) | <0.001 |
| Impulsiveness (BIS-11)a | ||||
| Nonplanning | 21.5 (18.0–26.0) | 21.0 (17.0–25.0) | 23.0 (19.0–27.0) | 0.016 |
| Motor | 19.0 (16.0–22.0) | 19.0 (16.0–21.0) | 19.0 (15.0–23.0) | 0.894 |
| Attentional | 18.0 (16.0–21.0) | 18.0 (16.0–21.0) | 18.0 (15.0–20.0) | 0.697 |
| Total | 58.0 (52.0–66.0) | 57.5 (51.8–65.0) | 59.0 (52.0–68.0) | 0.300 |
All data are presented as n (%), unless otherwise specified.
Multivariate analysis by hierarchical logistic regression. Risky behavior as a dichotomous variable.
| Set 1 Demographic aspects | Set 2 Impulsiveness | Set 3 HCV infection | p Value for final modela | |
|---|---|---|---|---|
| Adjusted odds ratio (confidence interval) | ||||
| Age | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.014 |
| Male genderb | 27.2 (13.0–63.4) | 30.1 (14.0–72.8) | 23.3 (10.6–57.2) | <0.001 |
| NP Impulsiveness (BIS-11) | – | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) | 0.028 |
| HCV infectionc | – | – | 3.2 (1.5–6.8) | 0.002 |
This is the first study to compare data on risk behaviors and impulsiveness between HCV- and HTLV-1-infected individuals. Among patients with HCV, significantly higher rates have been reported for important risk behaviors related to sexual practices and drug use, as well as higher total scores of impulsiveness and the NP domain. Multivariate analysis showed that being male, being older, having greater nonplanning impulsiveness and having HCV were all independently associated with the presence of risk behavior engagement. Regarding the impulsiveness, less dispersion pointed out that HCV+ individuals are more homogenous among themselves in this behavior aspect compared to HTLV-1+ individuals.
Previous studies have shown high rates of seropositivity for HCV among IVDUs, between 50% and 90% [22], while the seroprevalence of HTLV-1 among IVDUs in Salvador is reportedly 35.2% [23]. Between 58% and 78% of patients with HCV have a current or past history of a disturbing level of consumption of psychoactive substances in general [24].
The use of drugs, associated with the higher rates of TI and NP among HCV patients, may have predisposed these individuals to higher rates of risky sexual behaviors. In their review article, Lucaciu and Dumitrascu [25] commented on the presence of risk behaviors linked to injection drug use, alcohol consumption, and risky sexual behaviors among individuals with HCV, consistent with the results of our study. The domain NP has a close connection with the risk behaviors insofar as they signal action directed at the present without much concern for future consequences.
Although the age variable was evaluated and was considered significant in the multivariate analysis, these data must be interpreted with caution, as the mean ages of the groups with or without risk behavior were very close and do not represent relevantly different age groups.
The use of condoms was analyzed separately, despite it being a risk behavior as well. We consider that additional factors different from the other hazardous behaviors linked to impulsiveness could motivate this behavior, such as the decision not to use a condom due to, for example, being married or in a stable relationship (which represents the majority of the study participants) or for religious reasons. Even so, it is risk behavior that is worth mentioning.
Dantas-Duarte et al. [6], comparing patients infected with HCV and patients with liver disease from other etiologies, found higher levels of impulsiveness in the HCV-infected patients (all domains) (p<0.001), also finding an association between impulsiveness and risk behavior engagement.
Through neuropsychological tests and comparing to a population without hepatitis C, it was shown that HCV-infected adults were more predisposed to choosing immediate rewards rather than long-term rewards, which was related to their performance in executive function tasks. The authors suggested that this alteration occurred after infection. Thus, a circular phenomenon is established: impulsive individuals are more prone to being infected with HCV, and the chronic infection leads to cognitive impairment, which in turn predisposes individuals to making changes in decision making [17].
Fábregas et al. [14], comparing populations comprising individuals with and without HCV, found higher scores in TI and in the domain AI in patients with HCV. Giotakos et al. [16], in a sample of incarcerated rapists/child molesters, found higher levels of TI among anti-HCV positive subjects compared to those without viral infections or to those infected with HBV.
The set of studies above match the findings of the present study, where higher rates of impulsiveness were found among HCV-infected individuals compared to people without viral infections or other types of chronic infections, like HBV [6,14,16,17]. The current study further expands the range of viral conditions associated with lower rates of impulsiveness compared to HCV, providing foundational information regarding HTLV-1 for comparative analyses regarding this aspect of human behavior.
A possible explanation for higher levels of impulsiveness in this group is the evidence of the influence of HCV infection on the central nervous system with alterations in behavior patterns, and the presence of subjects in the sample who were perhaps contaminated through the use of injectable drugs. By contrast, the lack of consistent evidence that HTLV-1 may in and of itself foster behavior changes, notably impulsiveness, and the possibility of contamination of some individuals not through risk behaviors, but through breastfeeding, may have generated lower levels of impulsiveness and risk behaviors in the results. However, more studies need to be developed in order to have better clarifications with regard to these explanations.
This study had limitations. A convenience sample was used, making it not possible to extrapolate the data to other populations. Due to the transversal study design, it was impossible to establish a cause and effect relationship between impulsiveness and risk behavior engagement. Furthermore, it was not possible to separate the individuals into subgroups based on the severity of organic impairment caused by the virus, as the limited sample sizes would have led to the loss of statistical significance important to many of the analyses. There was no comparison with groups of carriers of other viruses (such as HIV and HBV). Lastly, the prior history of other situations that might have been the means of contamination for any one of the viruses was not ascertained (blood transfusions and other medical procedures, sharing tools of personal hygiene and breastfeeding).
5ConclusionsThis study demonstrates that individuals with HCV show more important risk behaviors and higher rates of impulsiveness when compared to those infected by HTLV-1, confirming what previous studies have shown with regard to other populations, including individuals infected with HBV. Low rates of condom use and a notable incidence of STDs were observed in both groups. The present study compares HCV-infected individuals directly with HTLV-1-infected individuals for the first time. HCV infection, along with NP impulsiveness and male gender, were independently associated with the presence of risk behavior engagement. Future longitudinal studies with probability samples, patient stratification according to clinical characteristics and inserting in the comparisons groups of individuals infected by other viruses will be welcomed, as they will likely provide a broader assessment of the issues addressed in the present study.AbbreviationsHCV
hepatitis C virus
IVDUintravenous drug user
HTLV-1human T-lymphotropic virus 1
HAM/TSPmyelopathy/tropical spastic paraparesis
ATLadult T cell leukemia/lymphoma
HBVhepatitis B virus
Com-HUPESUniversity Hospital Complex Professor Edgard Santos
HIVhuman immunodeficiency virus
BIS-11Barratt Impulsiveness Scale
TItotal impulsiveness
AIattentional impulsiveness
MImotor impulsiveness
NPnonplanning impulsiveness
AICAkaike Information Criteria
STDsexually transmitted diseases
Financial supportThis project was partially supported by the National Council of Technological and Scientific Development (CNPq): 462014/2014-2 – Edital Universal MCT/CNPQ 2014. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors thank Gabriel B. Z. Fontes for technical assistance.